Yumeng Zhang1, Jinming Song2, David Rutenberg1 and Lubomir Sokol2.
1 University of South Florida, Tampa, FL 33612
2 Moffitt Cancer Center, Tampa, FL 33612
Corresponding
author: Dr. Lubomir Sokol. Department of Malignant Hematology, Moffitt
Cancer Center, 12902 USF Magnolia Dr, Tampa FL 33612. E-mail:
Lubomir.Sokol@moffitt.org
Published: January 1, 2020
Received: August 14, 2019
Accepted: December 16, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020007 DOI
10.4084/MJHID.2020.007
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We
describe a case of impending acute liver failure in a patient with
Sézary syndrome (SS). The three-phase computed tomography (CT) of the
liver showed neither mass nor hepatomegaly. Liver biopsy confirmed
infiltration with malignant CD4+ clonal T-cells. Prompt administration
of combination chemotherapy, consisting of gemcitabine, dexamethasone,
and cisplatin (GDP), resulted in full recovery of liver function. To
the best of our knowledge, this is the first report of liver failure
from SS. Commercial next-generation sequencing panel identified 11
clinically relevant mutations. Interestingly, the identified ARID2
mutation, frequently observed in hepatocellular carcinoma, rarely
occurs in hematologic malignancies. Further studies are necessary to
elucidate the role of ARID2 mutations in the biological behavior of
Sezary cells, such as a propensity to infiltrate liver parenchyma.
|
Introduction
Cutaneous
T cell lymphoma (CTCL) is characterized by skin infiltration with
malignant monoclonal CD4+ T cells, or rarely CD8+ T cells. CTCL is rare
and mostly affects older patients with a median age of 60 years at
diagnosis. Early-stage mycosis fungoides (MF), manifesting with
patch/plague disease, has an indolent clinical course in contrast to
patients with skin tumors or leukemic disease. Sézary syndrome (SS) is
an aggressive leukemic form of CTCL. It presents with circulating
Sézary cells in peripheral blood, generalized erythroderma, and
frequently lymphadenopathy.[1]
Patients with
hematologic malignancies infrequently develop acute liver failure
(ALF). The main mechanisms for ALF include tumor infiltration,
drug-induced hepatotoxicity, and reactivation of viral hepatitis. ALF
has not been reported in SS. Here, we report a case of impending ALF
secondary to hepatic involvement of SS. The patient had full recovery
of the liver function after initiation of chemotherapy.
Case Presentation
A
70-year-old white man with MF on external beam radiation therapy
presented with uncontrolled pruritus, erythroderma, skin desquamation,
and rapidly enlarging lymphadenopathy of the neck, axilla, and groin
for three weeks. He also had fatigue and a 15 lb weight loss over one
month. Forty years ago, he was diagnosed with diffuse large B cell
lymphoma (DLBCL) of the right testis. The patient was successfully
treated with three cycles of cyclophosphamide, doxorubicin,
vincristine, and prednisone (CHOP), followed by methotrexate, radiation
therapy, and orchiectomy. He has not had recurrent disease since. Labs
on admission showed a white blood cell count of 20x103/µL (normal range [NR] 4.6-10.2x103/µL)
with 39% atypical lymphocytes. Liver function tests (LFT) showed an AST
of 159 IU/L (NR 10-50 IU/L), ALT of 263 IU/L (NR 0-41 IU/L), and
alkaline phosphatase (ALP) of 326 IU/L (NR 40-130 IU/L). Total
bilirubin and INR were normal. Peripheral blood flow cytometry showed
26234 Sézary cells/µL. Skin biopsy revealed a large cell transformation
of MF. Bone marrow biopsy showed mildly hypercellular marrow
infiltrated with a monoclonal CD4+ T cell population. Left axillary
lymph node biopsy showed an aberrant CD4+ T-cell population without
large-cell transformation. A high Ki-67 proliferation index (50%)
suggested an aggressive disease.
While being treated for his
symptoms, he developed worsening of transaminitis and anasarca on
hospital day (HD) 4. Despite stopping potentially hepatotoxic agents
including allopurinol and gabapentin, his LFTs rose exponentially. On
HD 6, his AST/ALT/ALP were 1570/1442/660 IU/L, respectively, total
bilirubin was 6.7 mg/dL, and INR was 1.3. Work-up included negative
infectious etiologies (hepatitis A, hepatitis B, hepatitis C,
cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, and
herpes simplex virus); negative hemophagocytic lymphohistiocytosis
markers; negative antinuclear antibody and anti-mitochondria antibody.
Anti-smooth muscle antibody was mildly elevated (38 units, normal range
0-19 units), which was more consistent with liver injury than
autoimmune hepatitis. Abdominal Doppler ultrasound and triple-phase CT
scan of the liver were negative for liver pathology or hepatomegaly (Figure 1).
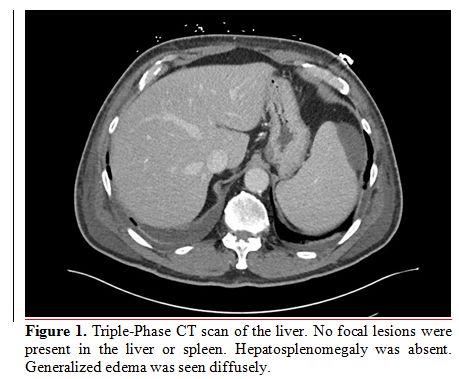 |
Figure
1. Triple-Phase CT scan of the liver. No focal lesions were present in
the liver or spleen. Hepatosplenomegaly was absent. Generalized edema
was seen diffusely. |
A
trial of steroids was initiated. However, the AST/ALT continued to
increase rapidly to the 2000s IU/ml by HD 7. His jaundice and mental
status worsened. Despite the absence of liver abnormalities on imaging
studies, ALF secondary to hepatic involvement of SS was suspected. He
was referred for diagnostic core needle liver biopsy. The final
pathology report of the liver biopsy confirmed malignant hepatic
infiltration with Sézary cells. Cytomorphology showed dense portal
infiltrate by mostly small mature atypical lymphocytes without large
cell transformation; immunohistochemical staining revealed CD4+ T cells
with loss of CD7 (Figure 2);
flow cytometry and T-cell receptor gene rearrangement studies of the
tissue confirmed monoclonal T cell population. Next-Generation
Sequencing (NGS) of peripheral blood (Foundation One Inc. Cambridge,
MA) showed a high mutation burden (25 mutations/Mb) and identified 31
genetic alternations. Seven clinically relevant mutations included
CCND3 P203L, STAT3 D661Y, ARID2 G70Fs*20, ASXL1 T822fs*11, CBL C3965,
CD58 Q32*, CDKN2a splice site 151-1G>A, CIITA Q909*, EP300 Q641*,
MAPK1 E322K, and TNFAIP3 loss.
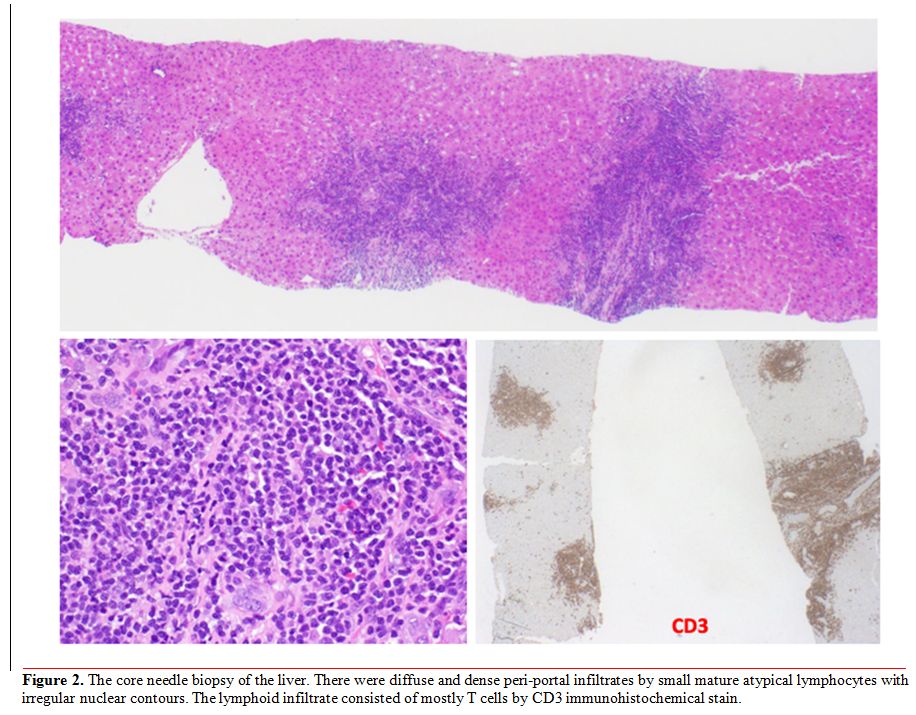 |
Figure 2. The core needle
biopsy of the liver. There were diffuse and dense peri-portal
infiltrates by small mature atypical lymphocytes with irregular nuclear
contours. The lymphoid infiltrate consisted of mostly T cells by CD3
immunohistochemical stain. |
He
was treated with a chemotherapy regimen consisting of gemcitabine,
dexamethasone, and cisplatin (GDP). The patient’s LFTs and mental
status started to improve on the second day of GDP.
His LFTs
normalized and erythroderma resolved during the 1-month follow-up. He
completed two additional cycles of GDP, followed by maintenance
gemcitabine. Restaging PET/CT showed complete remission. Unfortunately,
he passed away six months later due to myocardial infarction.
Discussion
SS
comprises approximately 5% of all CTCL cases. SS carries a poor
prognosis with a median overall survival rate of 2-3 years. SS can
involve the bone marrow, liver, lung, and gastrointestinal tract in
advanced stages.[2] Large cell transformation in
patients with MF/SS is associated with more aggressive clinical
behavior. Huberman et al. reported visceral involvement in 70-90% CTCL
patients at the time of death.[3] In a small cohort,
16% of patients with CTCL had biopsy documented hepatic infiltrates.
Clinical factors that are associated with hepatic infiltrate include
peripheral blood involvement, leukocytosis, and generalized
erythroderma. Interestingly, none of the patients had abnormal LFTs.[3]
ALF is a very poor prognostic sign with a mortality rate of 83% and a
mean survival rate of 10.7 days from the time of diagnosis in other
non-Hodgkin’s lymphomas. Remission from chemotherapy only occurs in
less than 15% of cases.[4] In the case of acute liver
failure in a Japanese patient with MF, the patient passed away within
eight weeks of visceral involvement.[5]
To the
best of our knowledge, the present case is the first report of liver
injury from malignant hepatic infiltration of SS. This case showed that
the prompt administration of chemotherapy could result in the complete
recovery of liver function and prevent progression into ALF. The
diagnostic dilemma included the lack of abnormalities in multiple
imaging modalities and concerns for recurrent disease of the previously
treated DLBCL. Homogeneous infiltration of malignant cells in the liver
occasionally makes it difficult to detect with imaging studies and can
result in a delay in diagnosis. High clinical suspicion should trigger
liver biopsy and molecular studies.
A high mutation burden in
NGS may potentially explain the aggressiveness of the disease. Among
the identified mutations, TNFAIP3 mutations have been reported in CTCL
previously.[6] CCND3, STAT3, ASXL1, CBL, CDKN2A,
EP300, and MAPK1 have been reported in adult T cell lymphoma/leukemia
and other types of mature T cell lymphomas (COSMIC 2017).
ARID2
mutation has rarely been reported in hematologic malignancy
(prevalence: 0.5%), but frequently described in hepatocellular
carcinoma (prevalence: 5-20%).[5,7] ARID2 encodes a subunit of the SWI/SNF-B (PBAF) chromatin remodeling complex, which assists in mediating gene expression[8] and double-strand DNA gene repair.[9]
ARID2 functions as a tumor suppressor gene and is associated with
loss-of-function mutations in most cases. In the absence of ARID2,
cells are sensitized to DNA damage secondary to ultraviolet light and
other carcinogens. In the present case, ARID2 mutation was a frameshift
mutation of G70fs*20 in the splice site, likely resulting in loss of
function. The ARID2 mutation possibly predisposes Sezary cells to
acquire more mutations and aggressive features. Further studies are
necessary to elucidate the role of ARID2 mutations in the biological
behavior of Sezary cells, such as a propensity to infiltrate liver
parenchyma.
References
- Wilcox, R.A., Cutaneous T-cell lymphoma: 2017
update on diagnosis, risk-stratification, and management. Am J Hematol,
2017. 92(10): p. 1085-1102. https://doi.org/10.1002/ajh.24876 PMid:28872191
- Scarisbrick,
J.J., et al., Prognostic factors, prognostic indices and staging in
mycosis fungoides and Sezary syndrome: where are we now? Br J Dermatol,
2014. 170(6): p. 1226-36. https://doi.org/10.1111/bjd.12909 PMid:24641480
- Huberman,
M.S., et al., Hepatic involvement in the cutaneous T-cell lymphomas:
results of percutaneous biopsy and peritoneoscopy. Cancer, 1980. 45(7):
p. 1683-8. https://doi.org/10.1002/1097-0142(19800401)45:7<1683::AID-CNCR2820450727>3.0.CO;2-C
- Lettieri,
C.J. and B.W. Berg, Clinical features of non-Hodgkins lymphoma
presenting with acute liver failure: a report of five cases and review
of published experience. Am J Gastroenterol, 2003. 98(7): p. 1641-6. https://doi.org/10.1111/j.1572-0241.2003.07536.x PMid:12873593
- Shibata,
S., et al., Folliculotropic mycosis fungoides with severe hepatic
failure due to hepatic involvement. Acta Derm Venereol, 2009. 89(4): p.
423-4. https://doi.org/10.2340/00015555-0665 PMid:19688164
- Braun,
F.C., et al., Tumor suppressor TNFAIP3 (A20) is frequently deleted in
Sezary syndrome. Leukemia, 2011. 25(9): p. 1494-501. https://doi.org/10.1038/leu.2011.101 PMid:21625233
- Zhao, H., et al., ARID2: a new tumor suppressor gene in hepatocellular carcinoma. Oncotarget, 2011. 2(11): p. 886-91. https://doi.org/10.18632/oncotarget.355 PMid:22095441 PMCid:PMC3259997
- Lemon,
B., et al., Selectivity of chromatin-remodelling cofactors for
ligand-activated transcription. Nature, 2001. 414(6866): p. 924-8. https://doi.org/10.1038/414924a PMid:11780067
- Kakarougkas,
A., et al., Requirement for PBAF in transcriptional repression and
repair at DNA breaks in actively transcribed regions of chromatin. Mol
Cell, 2014. 55(5): p. 723-32. https://doi.org/10.1016/j.molcel.2014.06.028 PMid:25066234 PMCid:PMC4157577
[TOP]

