Alexandre
E. Malek1*, Cristina Gutierrez2*,
Victor E. Mulanovich1, Joshua Botdorf2,
Roy F. Chemaly1, Shivan Shah1,
Brandi M. McCall2, Judd T. Melancon2,
Kelly K. McConn1, Jovan Borjan3,
Issam I. Raad1, Jan A. Burger4,
Guillermo Garcia-Manero4 and Javier A. Adachi1..
1
Department
of Infectious Diseases, Infection Control and Employee Health. The
University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd.,
Houston, TX 77030, USA.
2 Department of Critical Care
Department; The University of Texas MD Anderson Cancer Center, 1515
Holcombe Blvd., Houston, TX 77030, USA.
3 Division of Pharmacy; The University of Texas
MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA.
4 Department of Leukemia; The University of
Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX
77030, USA.
* Alexandre E. Malek and Cristina Gutierrez are
first Co-authors.
Correspondence to: Javier A. Adachi. Department of Infectious Diseases,
Infection Control and Employee Health; The University of Texas MD
Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA.
E-mail:
jaadachi@mdanderson.org
Published: July 1, 2020
Received: May 13, 2020
Accepted: June 15, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020044 DOI
10.4084/MJHID.2020.044
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
emergence and spread of 2019 novel coronavirus have led to an
unprecedented public health crisis around the globe, threatening the
lives of millions of people. We report a severe case of COVID-19 in a
patient with chronic lymphocytic leukemia and describe primarily the
clinical presentation and the challenges encountered in the COVID-19
diagnosis, treatment, and specimens sampling pitfalls. This case
highlights the importance of a comprehensive diagnostic approach of
pneumonia in immunocompromised hosts, including timely and safe
bronchoscopy, because of the broad differential diagnosis, more
challenging with the current outbreak of COVID-19.
|
Introduction
What
began as a cluster of pneumonia cases in Wuhan, China, in December 2019
is now known as the worldwide pandemic of the novel coronavirus disease
2019, coined COVID-19.[1] The first case of COVID-19 in the United
States was on January 20, 2020, and as of May 4, 2020, more than 3.6
million infections have been reported globally, accounting for a death
toll of more than 252,000 persons.[1,2] This disease is caused by the
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and a
subpopulation may present with extrapulmonary symptoms nausea,
vomiting, abdominal pain and diarrhea,[3] leading to a potential delay
in diagnosis.
While currently, around 15 million people are
living with cancer,[4] little is known about the burden of COVID-19 in
cancer patients who are at increased risk of worse outcomes.[5] Here we
present a case of severe COVID-19 in a patient with chronic lymphocytic
leukemia (CLL) who initially evaded a timely diagnosis, but she
successfully recovered after 17 days of intubation.
Case Presentation
A
41-year-old morbidly obese female with a diagnosis of B-cell type CLL,
RAI stage 0, with 13q deletion and mutated immunoglobulin heavy chain,
on active surveillance without chemotherapy, presented to an urgent
care facility for four days of nausea, vomiting, and diarrhea with a
low-grade fever of 100.8 °F (38.2 °C).
She was treated with intravenous (IV) fluids and anti-emetics and
discharged home with the presumptive diagnosis of acute
gastroenteritis. Over the following week, her gastrointestinal (GI)
symptoms improved but did not resolve fully.
Ten days after
symptoms onset, she presented to the Emergency Department (ED) with two
days of dry cough, shortness of breath, myalgias, and persistent fevers
of 102 °F (38.9 °C). The signs
and symptoms are outlined in Figure
1.
She denied recent international or domestic travel or contact with
known or suspected COVID-19 cases. In the ED, she was hypoxic with SpO2
85% on 6 L/min nasal cannula. She was placed on a non-rebreather mask
(15 L/min of oxygen) and was later electively intubated and placed on
mechanical ventilation with a lung-protective strategy. Empiric therapy
with cefepime 2 g IV Q 12 hours, linezolid 600 mg IV Q 12h and
doxycycline were initiated. A posteroanterior chest radiograph showed
bilateral diffuse lung opacities (Figure
2, panel a).
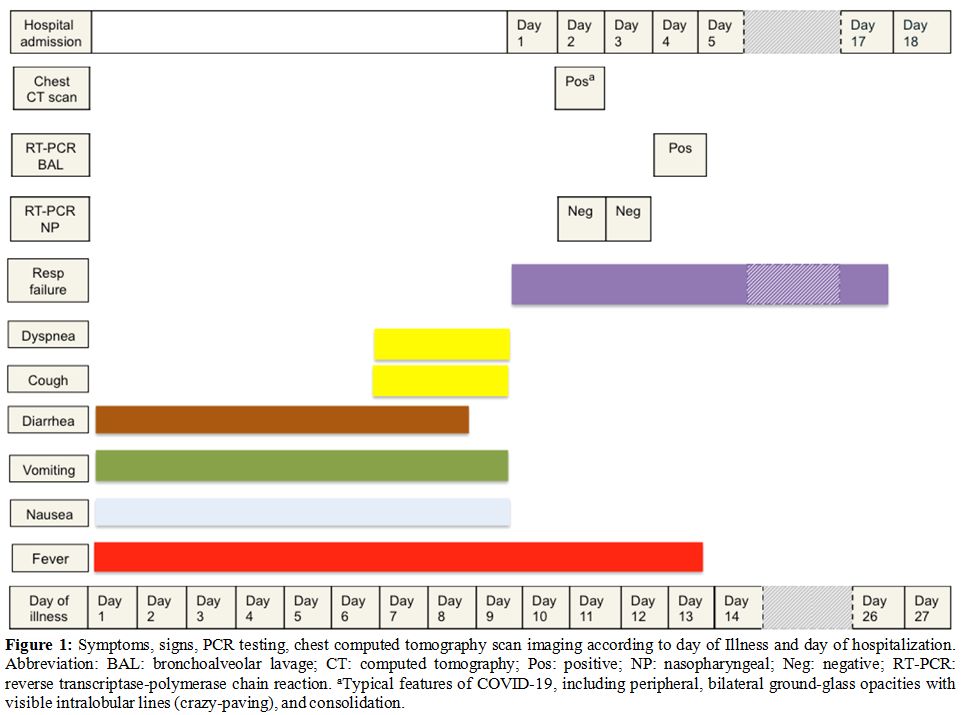 |
Figure 1.
Symptoms, signs, PCR testing,
chest computed tomography scan imaging according to day of Illness and
day of hospitalization. Abbreviation: BAL: bronchoalveolar lavage; CT:
computed tomography; Pos: positive; NP: nasopharyngeal; Neg: negative;
RT-PCR: reverse transcriptase-polymerase chain reaction. aTypical
features of COVID-19, including peripheral, bilateral ground-glass
opacities with visible intralobular lines (crazy-paving), and
consolidation. |
 |
Figure 2. Panel
a: Posteroanterior chest
radiograph, (illness day 10, hospital day 1), showing bilateral lungs
opacities and infiltrates. Panel b: A chest computed tomography scan,
(illness day 11, hospital day 2), revealing a bilateral multi-segmental
ground glass and consolidative opacities (centrally and mainly
peripherally). |
Her
laboratory studies were notable for: white blood cell count 11,700 /µl
with 61% neutrophils and 35% lymphocytes, a relative lymphocytopenia in
the setting of CLL. Troponin level <6 ng/L and total
immunoglobulin
G, 839 mg/dL. The remaining laboratory results are summarized in Table 1.
A nasopharyngeal (NP) swab specimen was negative for common respiratory
viruses. This specimen was collected in accordance with the Centers for
Disease Control and Prevention and was negative for reverse
transcriptase (rRT)-polymerase-chain-reaction (PCR) for SARS-CoV-2.
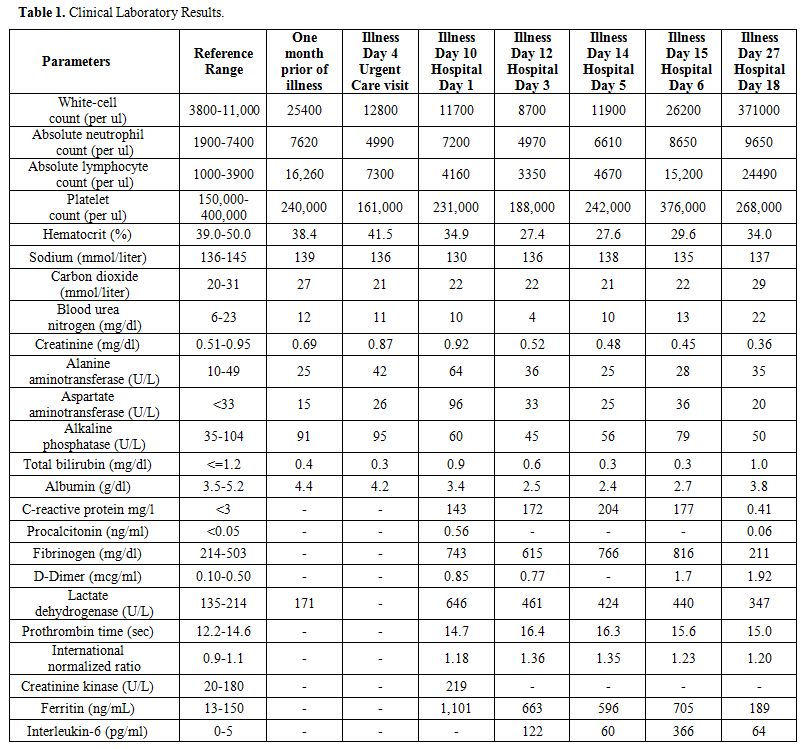 |
Table
1. Clinical Laboratory Results. |
A
chest computed tomography (CT) scan was performed on hospital day (HD)
2 and showed bilobar multi-segmental ground-glass opacities (GGO)
located both centrally and peripherally (Figure 2, panel b).
Despite negative testing, COVID-19 pneumonia was highly
suspected. A second NP swab specimen for SARS-CoV-2 was
performed
and reported negative. Blood cultures, serum Aspergillus
antigen, βeta-(1,3)-d-glucan,
and urine legionella antigen were all negative.
In
view of the second negative COVID testing, and the patient’s risk
factors for opportunistic infections, a bronchoscopy with
bronchoalveolar lavage (BAL) was performed. Precautions to avoid
generating aerosolized particles were taken, such as the use of
personal protective aerosolized equipment (PAPR) and paralyzing the
patient during the procedure. The BAL specimen was negative for
aspergillus antigen, pneumocystis
jirovecii PCR, cytomegalovirus PCR,
bacterial and fungal cultures. However, the BAL rRT-PCR for SARS-CoV-2
was positive on HD 4. The treatment was transitioned to
hydroxychloroquine 400 mg twice daily for two doses, then 200 mg twice
daily combined with azithromycin 500 mg first dose, then 250 mg once
daily for a total of 5 days. Additionally, two doses of tocilizumab of
8 mg/kg every 12 hours were administered on HD 4 with one infusion of
immunoglobulins (30 g). The patient developed acute respiratory
distress syndrome (ARDS), and she was dependent on mechanical
ventilation thereafter. On HD12, a short course of high dose
intravenous methylprednisolone 1 mg/kg per day was administered and
which resulted in a gradual improvement of the patient’s respiratory
status. Five days after the initiation of corticosteroids (HD17), the
patient was successfully extubated. Before discharge, a repeat
SARS-CoV-2 PCR from NP specimen remained negative. She responded well
to skilled occupational therapy exercises and, on HD 28, she was
discharged home on room air, with stable conditions, and without
sequelae. After one month of discharge, serologic testing for COVID-19
(Viracor Eurofins) showed positive IgG, 56.6 Units (normal
range, ≤ 9.0
Units).
Discussion
To
our knowledge, this is a unique case of severe COVID-19 in a patient
with CLL that illustrates several aspects of this novel infection that
are not yet fully understood, and the PCR testing including specimen
collection as sensitivity and specificity of the test may vary in
accordance to affected organs. Of note, four cases of mild COVID-19
cases in CLL patients have been reported,[6] and no standardized COVID-19 treatment in patients with hematological malignancies is available.
Our
patient initially reported GI symptoms in the absence of respiratory
symptoms, which did not develop until a week into the illness. The GI
manifestations of COVID-19 have been described in 2 to 10% in cases
series and an observational study (N=1099) reported the presence of
nausea or vomiting (5.0%) and diarrhea (3.8%) in infected patients.[7]
However, other studies showed that up to 11% of patients had on
admission at least one GI symptom, and around 50% of patients developed
GI symptoms during the hospitalization.[8,9] Early
nonspecific symptoms of COVID-19 can lead to diagnostic difficulty in
distinguishing between other common infectious diseases.
The
SARS-CoV-2 has been detected in nasopharyngeal, oropharyngeal, sputum,
and BAL specimens in COVID-19. BAL samples are the most accurate but
involve dedicated personnel and invasive procedures for the collection.[10] NP swab is the recommended test for suspected COVID-19 as it is safe and well-tolerated by patients.[11–13]
However, false negatives (20-40% in NP swab) can occur due to viral
load variability throughout stages of the disease, or due to poor
technique and this could result in missed diagnosis.[13–16]
The positivity of PCR varies depending on the specimens, with higher
positive rates on BAL (93%) and sputum (72%) when compared to
nasopharyngeal swabs (63%).[10] Despite these
findings, in suspected COVID-19 cases, the use of bronchoscopy has been
limited, and not recommended routinely, due to the risk it poses to
medical staff.[17,18] However, in immunocompromised
patients, the diagnosis of COVID-19 can be obscured by other etiologies
such as CMV and PJP pneumonia. In such cases, protocols within the
institutions on how to perform bronchoscopies safely should be in
place; some considerations might include performing BAL immediately
after endotracheal intubation. Moreover, as evidenced in this case,
highly suspected COVID-19 cases should lead to discussions to safely
pursue a diagnosis while also being able to rule out other common
causes of respiratory failure in cancer patients.
A chest CT
scan has a high sensitivity for COVID-19 and may be considered as a
primary tool for COVID-19 detection in highly epidemic areas.[19]
Given the lack of clear data regarding the sensitivity of rRT-PCR NP
swab in patients with GI manifestations early on in the disease,
further study is needed to assess the impact of early chest CT scan on
COVID-19-related outcomes.
Conclusions
This
case highlights the importance of clinicians relying on indirect
markers of COVID-19, such as characteristic clinical, radiographic and
laboratory findings in patients seeking medical care. Improved
understanding of the variety of clinical manifestation is critical for
prompting appropriate specimen collection, timely diagnosis, and
treatment initiation. It remains uncertain whether the combination of
anti-IL-6, corticosteroids, and immunoglobulins could work
synergistically in patients with chronic lymphocytic leukemia with
severe COVID-19 and ARDS. Further studies are needed to define the
optimal treatment of COVID-19 in cancer patients as early treatment
will likely prevent further complications and improve outcomes.
References
- https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19.pdf?sfvrsn=7b8629bb_4
- Holshue
ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First
case of 2019 novel coronavirus in the United States. N Engl J Med.
2020; https://doi.org/10.1056/NEJMoa2001191 PMid:32004427 PMCid:PMC7092802
- Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020; https://doi.org/10.1053/j.gastro.2020.02.054 PMid:32142785 PMCid:PMC7130192
- Howlader
N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer
Statistics Review, 1975-2016, National Cancer Institute. Bethesda, https://seer.cancer.gov/csr/1975_2016/ A 2019. No Title.
- Liang
W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in
SARS-CoV-2 infection: a nationwide analysis in China. The Lancet
Oncology. 2020. https://doi.org/10.1016/S1470-2045(20)30096-6
- Baumann T, Delgado J, Montserrat E. CLL and COVID-19 at the Hospital Clinic of Barcelona: an interim report. Leukemia. 2020; https://doi.org/10.1038/s41375-020-0870-5 PMid:32433507 PMCid:PMC7237061
- W.-J.
G, Z.-Y. N, Y. H, W.-H. L, C.-Q. O, J.-X. H, et al. Clinical
Characteristics of Coronavirus Disease 2019 in China. N Engl J Med.
2020;
- Lin L, Jiang X, Zhang Z, Huang S,
Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with
SARS-CoV-2 infection. Gut. 2020; https://doi.org/10.1136/gutjnl-2020-321013 PMid:32241899
- Jin
X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological,
clinical and virological characteristics of 74 cases of
coronavirus-infected disease 2019 (COVID-19) with gastrointestinal
symptoms. Gut. 2020;
- Wang W, Xu Y, Gao
R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types
of Clinical Specimens. JAMA - Journal of the American Medical
Association. 2020. https://doi.org/10.1001/jama.2020.3786
- https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html
- Wang
D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics
of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected
Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020; https://doi.org/10.1001/jama.2020.1585 PMid:32031570 PMCid:PMC7042881
- Yu
F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al. Quantitative Detection
and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin Infect
Dis. 2020; https://doi.org/10.1093/cid/ciaa345 PMid:32221523 PMCid:PMC7184442
- Long
C, Xu H, Shen Q, Zhang X, Fan B, Wang C et al. Diagnosis of the
Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol.
2020;25:126. https://doi.org/10.1016/j.ejrad.2020.108961 PMid:32229322 PMCid:PMC7102545
- Li
Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability Issues of
RT-PCR Testing of SARS-CoV-2 for Hospitalized Patients Clinically
Diagnosed with COVID-19. J Med Virol. 2020; https://doi.org/10.1002/jmv.25786 PMid:32219885 PMCid:PMC7228231
- Li
D, Wang D, Dong J, Wang N, Huang H, Xu H, et al. False-negative results
of real-time reverse-transcriptase polymerase chain reaction for severe
acute respiratory syndrome coronavirus 2: Role of deep-learning-based
ct diagnosis and insights from two cases. Korean J Radiol. 2020; https://doi.org/10.3348/kjr.2020.0146 PMid:32174053 PMCid:PMC7082661
- Bouadma
L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2
infections: practical considerations and management strategy for
intensivists. Intensive Care Med. 2020; https://doi.org/10.1007/s00134-020-05967-x PMid:32103284 PMCid:PMC7079839
- Wahidi
MM, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et al. American
Association for Bronchology and Interventional Pulmonology (AABIP)
Statement on the Use of Bronchoscopy and Respiratory Specimen
Collection in Patients with Suspected or Confirmed COVID-19 Infection.
J Bronchology Interv Pulmonol. 2020; https://doi.org/10.1097/LBR.0000000000000681 PMid:32195687 PMCid:PMC7141581
- Ai
T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT
and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A
Report of 1014 Cases. Radiology. 2020; https://doi.org/10.1148/radiol.2020200642 PMid:32101510 PMCid:PMC7233399
TOP]


