Cardiac Function and Iron Chelation in Thalassemia Major and Intermedia: a Review of the Underlying Pathophysiology and Approach to Chelation Management
Athanasios Aessopos and Vasilios Berdoukas
First Dept. of Internal Medicine, University of Athens Medical School, Laiko Hospital, Athens, Greece
Correspondence
to:
Athanasios Aessopos, MD, PhD; Laiko Hospital, 17 Ag Thoma St, Athens
11527, Greece; Tel: +306944473215; Fax: +302104619778; Email: aaisopos@cc.uoa.gr
Published: July 18, 2009
Received: June 21, 2009
Accepted: July 17, 2009
Medit J Hemat Infect Dis 2009, 1(1): e2009002 DOI 10.4084/MJHID.2009.002
This article is available from: http://www.mjhid.org/article/view/4574
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Heart
disease is the leading cause of mortality and one of the main causes of
morbidity in beta-thalassemia. Patients with homozygous thalassemia may
have either a severe phenotype which is usually transfusion dependent
or a milder form that is thalassemia intermedia. The two main factors
that determine cardiac disease in homozygous β thalassemia are the high
output state that results from chronic tissue hypoxia, hypoxia-induced
compensatory reactions and iron overload. The high output state
playing a major role in thalassaemia intermedia and the iron load being
more significant in the major form. Arrhythmias, vascular involvement
that leads to an increased pulmonary vascular resistance and an
increased systemic vascular stiffness and valvular abnormalities also
contribute to the cardiac dysfunction in varying degrees according to
the severity of the phenotype. Endocrine abnormalities,
infections, renal function and medications can also play a role in the
overall cardiac function. For thalassaemia major, regular and
adequate blood transfusions and iron chelation therapy are the
mainstays of management. The approach to thalassaemia intermedia,
today, is aimed at monitoring for complications and initiating, timely,
regular transfusions and/or iron chelation therapy. Once the
patients are on transfusions, then they should be managed in the same
way as the thalassaemia major patients. If cardiac manifestations
of dysfunction are present in either form of thalassaemia, high pre
transfusion Hb levels need to be maintained in order to reduce cardiac
output and appropriate intensive chelation therapy needs to be
instituted. In general recommendations on chelation, today, are
usually made according to the Cardiac Magnetic Resonance findings, if
available. With the advances in the latter technology and the
ability to tailor chelation therapy according to the MRI findings as
well as the availability of three iron chelators, together with
increasing the transfusions as need, it is hoped that the incidence of
cardiac dysfunction in these syndromes will be markedly reduced. This
of course depends very much on the attention to detail with the
monitoring and the cooperation of the patient with both the recommended
investigations and the prescribed chelation.
Introduction
Common mechanisms of heart injury in Thalassemia (Th)
Cardiovascular consequences
Chelation treatment for prevention and treatment of iron induced heart disease.
β-thalassemia is an inherited
hemoglobin disorder resulting from either homozygous or double
heterozygous inheritance of two abnormal genes from the β–globin locus,
leading to impaired synthesis of the β-globin chain and resulting in
chronic dyserythropoietic anemia [1]. Depending on
the clinical severity,
two forms, thalassemia major (ΤΜ) and thalassemia intermedia, (ΤΙ) are
distinguished [1]. The majority of
patients have TM but up to 25% may have
TI1. TM is rapidly fatal unless adequate transfusions, in conjunction
with intensive iron chelation therapy, are started sufficiently early [2].
In contrast, TI is generally characterized by a mild clinical picture,
has a better prognosis and survival and requires therapeutic
interventions only later in life, if at all [3]. The
clinical course of
both forms of thalassemia (Th), if they remain untreated, is
complicated by the multiple effects of chronic anaemia and of the
resultant tissue hypoxia as well as by their compensatory reactions,
including increased erythropoiesis with bone marrow expansion and
increased intestinal iron absorption1. Those manifestations are
completely or partially inhibited nowadays in TM patients, due to the
early application of regular transfusion-chelation therapy at the cost
of chronic iron overload and the need for iron chelation therapy, while
in TI patients these features are still present in varying degrees.
Cardiovascular involvement represents a well-known complication and
remains the primary cause of mortality both in TM and in TI [2-4].
As discussed below, it seems quite different in the two forms of the
disease. Despite the fact that both forms share common basic underlying
pathophysiological mechanisms that affect the heart, the different
degree of contribution of these mechanisms in TM and in TI, result in a
variety of left and right heart involvement, which ultimately lead to
congestive heart failure. Knowledge of the complexity of the underlying
mechanisms in thalassaemia may help to prevent or to treat the heart
injury.
Common mechanisms of heart injury in Thalassemia (Th)
Cardiac structure and function
in Th are mainly affected by two factors: iron load and increased
cardiac output. Additional factors are involved and will be
discussed.
The cardiac iron load:
Iron overload results from two main mechanisms. In both TM and
TI, it is associated with red cell transfusion and increased intestinal
iron absorption [1]. The iron overload in TM is
dominated by the
transfusion iron, while in TI the absorption is the greatest source.
Furthermore, the disease itself, including ineffective erythropoiesis
as well as peripheral haemolysis results in selective tissue iron
deposition. Therefore, although iron overload is mainly a problem of TM
patients, it also exists to a lesser extent in TI. The heart, along
with liver and endocrine glands, is one of the main organs where iron
deposition causes severe complications [5]. Iron
overload interferes in
the cardiomyocytes’ capacity to catalyze the formation of deleterious
oxygen free radicals [5]. The
quantification of myocardial iron content is
not generally easy and only T2* CMR has allowed a reliable estimation
in a large number of TM patients [6]. There are two
mechanisms of iron
related injury; these are direct and indirect.
Direct Iron related
injury:
In TM survival was dependent on regular transfusion. Patients receive
between 0.3-0.5 mg/Kg/day of iron through transfusions. The average
daily losses are less than 1mg in males and 2 mgs in menstruating
females. There are no other physiological mechanisms for effecting body
iron reduction; therefore the body stores the iron. Before the
availability of iron chelation therapy, the majority of transfused TM
patients died, usually in the second and third decade of life, from
cardiac failure that was due to iron overload. In TI the increased
gastro-intestinal absorption of iron, which is much higher than that in
normal individuals is most likely due to a paradoxical suppression of
hepcidin [7-9]. In dyserythropoietic anaemias,
this
suppression has recently been found to be induced by
Matriptase-2, a transmembrane serine protease [10].
Hepcidin
interferes in iron homeostasis by inhibiting iron absorption from
duodenal enterocytes, iron release from hepatocytes and from
macrophages that recycle iron from senescent
erythrocytes [7,8]. In Th, the accumulated
iron, is thought to
saturate liver firstly, and then to accumulate in other organs.
Therefore In the less loaded TI, the absorbed iron seems to accumulate
mainly in the liver and less frequently involves the heart. A
number of studies using CMR T2* have demonstrated this finding.
One study in 31 TI patients revealed that 23% of cases had cardiac iron
overload, defined as a T2* value <20 msec [11-13].
The usual management
of TI is clinical observation with occasional transfusions and
intervention with regular transfusions and iron chelation therapy if
indicated.In histological examination of the heart in patients with TM,
the iron accumulates in all four chambers, papillary muscles and the
electrical conduction system, including the sinoatrial and
atrioventricular nodes. In the free wall of the left ventricle
there is more iron concentrated in the epicardial layers than in the
endocardial and middle third [14]. Iron is stored in
cells, including
myocytes, in the form of ferritin, haemosiderin and free iron. The
latter is referred as the labile cellular iron (LCI) [15].
There is
a significant flux between the three forms, with haemosiderin being the
least soluble and accessible. The LCI is the most toxic form as it
stimulates the formation of free radicals (Fenton Reactions), which
results in peroxidative damage of membrane lipids and proteins
provoking cellular injury. In heart, this leads to impaired function of
the mitochondrial respiratory chain and is clinically manifested by
reduction of cardiac muscular contractility and CCF
development [16]. To date, at least 90 genes that
control iron
metabolism have been identified [17]. In each
individual therefore,
it is highly likely that the handling of iron and the action of iron
chelators will be different. These concepts fit in well with the wide
range of reported different clinical cardiac courses seen mainly in TM
patients who have followed similar life-time, well accepted
treatment [18]. Knowledge derived by recent MRI
studies which also
assessed cardiac function, showed that all patients with reduced LV
function had cardiac iron overload and in many cases this was
severe [6,19,20]. This strongly
suggests that in addition to the damage
caused by the accumulated iron, excessive iron in the myocytes results
in greater amounts of LCI leading to free radical formation that
overwhelms the antioxidant mechanisms and ultimately precipitates
cardiac dysfunction. On the other hand, in the above MRI studies,
despite heavy iron load, many TM patients maintained normal cardiac
function, albeit perhaps temporarily, and, as discussed above, this may
be related to their intracellular iron metabolism, in particular their
handling of oxidants. It has been shown that TM individuals who had the
genetic factor apo-lipoprotein E4 are at greater risk for LV
dysfunction than those with other alleles such as apo E2 and apo E 3
because of reduced ability to handle oxidative stress [21,22].
Indirect iron
related injury: All
the following factors related to indirect iron related cardiac injury
are more common in TM than TI. However, they are relevant to
both.
Infections: Any significant infection may precipitate cardiac failure particularly in the presence of other underlying cardiac pathology. Immune competence in beta-thalassemia is impaired [23-26] and patients are more vulnerable to infections. Furthermore, siderophore bacteria, such as yersinia and klebsiella, rely on iron for multiplication and grow well in the microenvironment of transfusion iron loaded patients [25].Iron overload is considered to be the main etiologic factor that can disturb the immune balance in favour of the growth of infectious organisms [24]. This may also be affected by differences in the existing immunogenetic profile in Th [26] especially with respect to viral infections. Two severe cardiac complications, pericarditis and myocarditis, are linked to iron load induced viral infection susceptibility.Pericarditis was frequently seen in Th. In TM patients with poor or no chelation in the past [27] it was quite frequent (50%). Today, with the use of chelation therapy, it is very rare (5%) [18]. Similarly, the reported myocarditis in TM with decreased LV function [28], seems most likely to be related to iron load. Even though there may be histological evidence of infections, as demonstrated by lymphocytic infiltration, recent CMR evidence shows that LV failure only occurs in the presence of excessive iron [6,19 ,29]. Viral myocarditis without iron in the heart may be rare and may follow similar outcomes to those of the normal population.
Arhythmias: The iron induced cardiac toxicity is often complicated by arrhythmias such as extra atrial and ventricular beats, paroxysmal atrial tachycardia, flutter or fibrillation. The high output state may also be related to the incidence of arrhythmias to a lesser extent. Life threatening ventricular tachycardia is rare and often associated with reduced LV function. Short runs of non specific ventricular tachycardia are quite common and are more common with elevated cardiac iron [30]. Atrial arrhythmias occur more frequently in both TI and TM. These are more clinically relevant and difficult to treat. They do not necessarily relate to the degree of cardiac iron load at the time of onset, but may result from past damage caused by the iron load or high cardiac output. Some of these arrhythmias can also be triggering factors for CCF or reduced cardiac function in TM patients without previous obvious LV dysfunction.
Endocrine abnormalities: Endocrine abnormalities occur in Th but with greater frequency in TM. Iron toxicity may also indirectly affect heart function by damaging other organs in varying degrees. The endocrine abnormalities hypothyroidism and diabetes mellitus can have a significant impact on cardiac function [31]. Hypothyroidism can precipitate pericardial effusion, decreased LV function, bradycardia and increased peripheral vascular resistance. The onset of diabetes is often associated with the presentation of cardiac dysfunction. This correlates with a recent finding that pancreatic iron correlates well with cardiac iron and not with hepatic iron [32]. Chronic hyperglycaemia is an oxidative stress on many organs, particularly the heart. Hypocalcaemia associated with occult or overt hypoparathyroidism can precipitate heart dysfunction.
Medications: Vitamin C has been given to patients with in order to enhance their iron excretion when they are on chelation therapy. There have been case reports of TM who developed sudden acute cardiac failure with a fatal outcome that had been precipitated by the administration of Vitamin C possibly by releasing free iron that is toxic [33].
Vascular Involvement (After load): Systemic arterial involvement in Th, has been observed recently through clinical, functional [34] and anatomical [35] studies, and plays a role in the development of cardiac dysfunction by affecting heart after load. Vascular involvement starts early in life and becomes obvious in the older patients [36], principally in TI. Haemolysis participates in this injury as does iron overload, most likely through the effect of the labile plasma iron (LPI). The other contributory mechanisms will be discussed in detail below in the section on elastic tissue abnormalities.
Infections: Any significant infection may precipitate cardiac failure particularly in the presence of other underlying cardiac pathology. Immune competence in beta-thalassemia is impaired [23-26] and patients are more vulnerable to infections. Furthermore, siderophore bacteria, such as yersinia and klebsiella, rely on iron for multiplication and grow well in the microenvironment of transfusion iron loaded patients [25].Iron overload is considered to be the main etiologic factor that can disturb the immune balance in favour of the growth of infectious organisms [24]. This may also be affected by differences in the existing immunogenetic profile in Th [26] especially with respect to viral infections. Two severe cardiac complications, pericarditis and myocarditis, are linked to iron load induced viral infection susceptibility.Pericarditis was frequently seen in Th. In TM patients with poor or no chelation in the past [27] it was quite frequent (50%). Today, with the use of chelation therapy, it is very rare (5%) [18]. Similarly, the reported myocarditis in TM with decreased LV function [28], seems most likely to be related to iron load. Even though there may be histological evidence of infections, as demonstrated by lymphocytic infiltration, recent CMR evidence shows that LV failure only occurs in the presence of excessive iron [6,19 ,29]. Viral myocarditis without iron in the heart may be rare and may follow similar outcomes to those of the normal population.
Arhythmias: The iron induced cardiac toxicity is often complicated by arrhythmias such as extra atrial and ventricular beats, paroxysmal atrial tachycardia, flutter or fibrillation. The high output state may also be related to the incidence of arrhythmias to a lesser extent. Life threatening ventricular tachycardia is rare and often associated with reduced LV function. Short runs of non specific ventricular tachycardia are quite common and are more common with elevated cardiac iron [30]. Atrial arrhythmias occur more frequently in both TI and TM. These are more clinically relevant and difficult to treat. They do not necessarily relate to the degree of cardiac iron load at the time of onset, but may result from past damage caused by the iron load or high cardiac output. Some of these arrhythmias can also be triggering factors for CCF or reduced cardiac function in TM patients without previous obvious LV dysfunction.
Endocrine abnormalities: Endocrine abnormalities occur in Th but with greater frequency in TM. Iron toxicity may also indirectly affect heart function by damaging other organs in varying degrees. The endocrine abnormalities hypothyroidism and diabetes mellitus can have a significant impact on cardiac function [31]. Hypothyroidism can precipitate pericardial effusion, decreased LV function, bradycardia and increased peripheral vascular resistance. The onset of diabetes is often associated with the presentation of cardiac dysfunction. This correlates with a recent finding that pancreatic iron correlates well with cardiac iron and not with hepatic iron [32]. Chronic hyperglycaemia is an oxidative stress on many organs, particularly the heart. Hypocalcaemia associated with occult or overt hypoparathyroidism can precipitate heart dysfunction.
Medications: Vitamin C has been given to patients with in order to enhance their iron excretion when they are on chelation therapy. There have been case reports of TM who developed sudden acute cardiac failure with a fatal outcome that had been precipitated by the administration of Vitamin C possibly by releasing free iron that is toxic [33].
Vascular Involvement (After load): Systemic arterial involvement in Th, has been observed recently through clinical, functional [34] and anatomical [35] studies, and plays a role in the development of cardiac dysfunction by affecting heart after load. Vascular involvement starts early in life and becomes obvious in the older patients [36], principally in TI. Haemolysis participates in this injury as does iron overload, most likely through the effect of the labile plasma iron (LPI). The other contributory mechanisms will be discussed in detail below in the section on elastic tissue abnormalities.
Increased Cardiac
Output (CO) effect:
Disease related increased CO, resulting in increased workload on the
heart, contributes to the development of cardiac dysfunction in Th
patients. Anemia together with marrow expansion leads to volume
overload that then demands increased contractility. (Starling’s Law).In
normal individuals. Hb levels between 80-100 g/l do not have any effect
on the resting cardiac output [37,38]. TM
patients, however, even
those well transfused (pre transfusion Hb level > 95 g/l) with
excellent suppression of marrow activity and with a mean Hb level of
113 g/l, demonstrate some degree of high cardiac output (Cardiac Index
4.3±0.9/3.in TM cf. 3.8±0.8 P<.01 in normal
individuals)18. In patients with high output state, the heart‘s
systolic function index
and ejection fraction is expected to be higher than in normal subjects.
Thus, for Th patients, even well transfused TM, it has been recommended
that a normal LVEF should be above 60% [39,40] and
the degree of CO
increase should be taken into account when assessing EF in each
individual patient [41]. In those TM who are poorly
transfused the
increased cardiac output will be greater. In TI, with minimal to
no transfusion, the increased cardiac output represents one of the
basic pathophysiologic mechanisms of cardiovascular involvement and is
a constant finding. [42,43,44,45]. More
specifically, echocardiographic
measurements reveal an almost two-fold increase in cardiac output
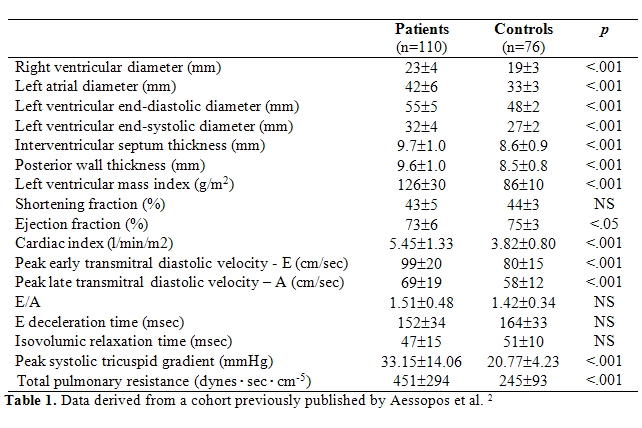
levels, compared to normal subjects (Table
1) [42].

Indications of the presence of
high output state were also derived by a cardiac magnetic resonance
imaging (CMR) study in TI patients. [9] Chronic
hemolytic anemia, resulting
from ineffective erythropoiesis, is the hallmark of all thalassemia
syndromes [1]. In TI, chronic anemia, however, is not
always severe
(hemoglobin levels range usually between 70 and 110 g/l) and apparently
is not the only cause of high output state in these patients. Besides
the overall hemoglobin level, the proportions of the different
hemoglobin types, especially the high percentage of fetal hemoglobin
(HbF), are also important. More specifically, HbF reduces tissue oxygen
delivery due to its increased oxygen affinity [46].
Thus, both chronic
anemia and increased HbF percentage result in prolonged tissue hypoxia.
This in turn, leads to a compensatory bone marrow expansion, with
extramedullary haemopoiesis, splenomegaly and hepatomegaly, all of
which also contribute to the high output state through peripheral
vasodilatation and shunt development [42, 46-48]. Similarly and more
impressively, compensatory mechanisms also occur in TM who are not
adequately transfused. In addition, vessels in Th are more
susceptible to pulse pressure-driven dilatation, due to a co-existent
elastic tissue injury, which is discussed in details below. Liver iron
load or viral induced hepatic injury can also contribute, as cirrhosis
can increase CO significantly [49]. The contribution
of peripheral
vasodilatation and intramedullary shunting seems to play an important
role in the high output state. Indeed, it has been shown that the
abolition of splenic shunting and the increase in hemoglobin level
following splenectomy are not sufficient to counteract the preexistent
high cardiac output levels in Th [47, 48].
Additonal Factors that
impact on Cardiac Injury
Haemolysis-induced tissue injury – Vascular involvement and elastic tissue abnormalities: Chronic haemolysis and iron overload, are currently considered as sources of strong oxidative stress. Reports have shown that the free haeme and the red cell membrane elements that are produced during haemolysis have a negative effect on nitric oxide and arginine availability, which in turn promotes vasoconstriction [50]. At the same time, they lead to further endothelial dysfunction, resulting in a more pronounced nitric oxide reduction, as well as to a diffuse elastic tissue injury. The presence of such an elastic tissue defect has been described with a high prevalence in patients with haemoglobinopathies, especially in those with Th [36,51]. The defect resembles hereditary pseudoxanthoma elasticum (PXE), a rare (1:70000 to 1:160000) connective tissue disorder, and covers the whole clinical spectrum of PXE, which consists mainly of skin (small yellowish papules or larger coalescent plaques), ocular and vascular manifestations (degeneration of the elastic lamina of the arterial wall, often with calcification) [36,51]. Endocardium, cardiac valves and pericardium may also be involved [36,42]. As the clinical expression of the elastic tissue injury is age-related, TI patients are more affected by PXE lesions due to their prolonged survival. Thus, it has been shown that TI patients aged >30 years (mean age 41.4 years) presented a 55% occurrence of tibial artery calcification as part of elastic tissue abnormalities [52]. Interestingly, histopathological studies in TI have shown it to be present in removed spleens even from the first decade of life [35]. On the other hand, the degenerative arterial lesions observed in the elastic lamina and adventitia render vessels more susceptible to dilatation by pulse pressure increase. Finally, the functional component of the arterial involvement was recently studied in TM, sickle-cell anemia and sickle-thalassemia patients. Increased arterial stiffness along with endothelial dysfunction was encountered and attributed to the two common pathogenic mechanisms, namely haemolysis and iron load [34,53].
Valvular involvement: Although valvular involvement is present in Th, it is more pronounced in TI. Endocardial degenerative lesions, in the form of thickening and calcification, affect the cardiac valves, mitral annulus and papillary muscles and this is often followed by moderate valvular regurgitation and occasionally by aortic stenosis. These findings were described echocardiographically with a high frequency in a large group of 110 TI patients [42]. More specifically, leaflet thickening was present in 48% of patients, endocardial calcification in 21%, mitral regurgitation in 47%, aortic regurgitation in 15%, while there were 3 cases with mild to moderate aortic stenosis. The hyperkinetic state due to the high output, the iron overload and primarily the aforementioned elastic tissue abnormalities have been suggested as the responsible pathogenic mechanisms [42,43]. Although the haemodynamic consequences of the above mild or moderate valvular abnormalities are not usually significant, they may have an additive effect when associated with the other pathogenic mechanisms in the development of heart disease. Moreover, atrioventricular conduction disturbances as well as the risk of cerebrovascular thrombotic events, in the context of a coexistent hypercoagulable state, may also play a role [42,54].
Hypercoagulability: Is a well-described entity in Th [55]. A number of pathogenic mechanisms have been discussed in relation to the underlying genetic defect and its consequences, namely haemolysis and iron overload and the resulting oxidative tissue damage. More specifically, the free α-globin chains that result from the decreased synthesis of the β-chains, along with the free iron provoke oxidative damage to the red blood cell membrane proteins; these changes result in the exposure of negatively charged phospholipids, which create a pre-coagulant surface [55,56]. Moreover, data derived from TM and sickling syndromes, as described above, showed that endothelial function is also impaired [34,53]. Oxidative damage, resulting once again from the two common mechanisms, haemolysis and iron load, leads to an increase expression of adhesion molecules ICAM and VCAM and impaired NO bioavailability, hence provoking hypercoagulability and decreasing NO-dependent, flow-mediated dilatation [34,57]. Furthermore, platelets are activated with enhanced aggregation, while splenectomy increases platelet counts and induces membranes abnormalities that enhanced the already increased platelet aggregation. In parallel, the observed deficiency of the coagulation inhibitors, protein C and protein S, the elevated levels of thrombin-ATIII complex due to splenectomy and/or liver dysfunction as well as the co-inheritance of several coagulation defects, such as factor V (Leiden) and factor ΙΙ deficiency, may also contribute to the pathogenesis of hypercoagulability in thalassemia [55,58]. Finally, a strong inflammatory reaction has been noticed, expressed by the elevated circulating levels of cytokines and adhesion molecules, and the monocyte and neutrophil activation, hence promoting hypercoagulability [57].
Haemolysis-induced tissue injury – Vascular involvement and elastic tissue abnormalities: Chronic haemolysis and iron overload, are currently considered as sources of strong oxidative stress. Reports have shown that the free haeme and the red cell membrane elements that are produced during haemolysis have a negative effect on nitric oxide and arginine availability, which in turn promotes vasoconstriction [50]. At the same time, they lead to further endothelial dysfunction, resulting in a more pronounced nitric oxide reduction, as well as to a diffuse elastic tissue injury. The presence of such an elastic tissue defect has been described with a high prevalence in patients with haemoglobinopathies, especially in those with Th [36,51]. The defect resembles hereditary pseudoxanthoma elasticum (PXE), a rare (1:70000 to 1:160000) connective tissue disorder, and covers the whole clinical spectrum of PXE, which consists mainly of skin (small yellowish papules or larger coalescent plaques), ocular and vascular manifestations (degeneration of the elastic lamina of the arterial wall, often with calcification) [36,51]. Endocardium, cardiac valves and pericardium may also be involved [36,42]. As the clinical expression of the elastic tissue injury is age-related, TI patients are more affected by PXE lesions due to their prolonged survival. Thus, it has been shown that TI patients aged >30 years (mean age 41.4 years) presented a 55% occurrence of tibial artery calcification as part of elastic tissue abnormalities [52]. Interestingly, histopathological studies in TI have shown it to be present in removed spleens even from the first decade of life [35]. On the other hand, the degenerative arterial lesions observed in the elastic lamina and adventitia render vessels more susceptible to dilatation by pulse pressure increase. Finally, the functional component of the arterial involvement was recently studied in TM, sickle-cell anemia and sickle-thalassemia patients. Increased arterial stiffness along with endothelial dysfunction was encountered and attributed to the two common pathogenic mechanisms, namely haemolysis and iron load [34,53].
Valvular involvement: Although valvular involvement is present in Th, it is more pronounced in TI. Endocardial degenerative lesions, in the form of thickening and calcification, affect the cardiac valves, mitral annulus and papillary muscles and this is often followed by moderate valvular regurgitation and occasionally by aortic stenosis. These findings were described echocardiographically with a high frequency in a large group of 110 TI patients [42]. More specifically, leaflet thickening was present in 48% of patients, endocardial calcification in 21%, mitral regurgitation in 47%, aortic regurgitation in 15%, while there were 3 cases with mild to moderate aortic stenosis. The hyperkinetic state due to the high output, the iron overload and primarily the aforementioned elastic tissue abnormalities have been suggested as the responsible pathogenic mechanisms [42,43]. Although the haemodynamic consequences of the above mild or moderate valvular abnormalities are not usually significant, they may have an additive effect when associated with the other pathogenic mechanisms in the development of heart disease. Moreover, atrioventricular conduction disturbances as well as the risk of cerebrovascular thrombotic events, in the context of a coexistent hypercoagulable state, may also play a role [42,54].
Hypercoagulability: Is a well-described entity in Th [55]. A number of pathogenic mechanisms have been discussed in relation to the underlying genetic defect and its consequences, namely haemolysis and iron overload and the resulting oxidative tissue damage. More specifically, the free α-globin chains that result from the decreased synthesis of the β-chains, along with the free iron provoke oxidative damage to the red blood cell membrane proteins; these changes result in the exposure of negatively charged phospholipids, which create a pre-coagulant surface [55,56]. Moreover, data derived from TM and sickling syndromes, as described above, showed that endothelial function is also impaired [34,53]. Oxidative damage, resulting once again from the two common mechanisms, haemolysis and iron load, leads to an increase expression of adhesion molecules ICAM and VCAM and impaired NO bioavailability, hence provoking hypercoagulability and decreasing NO-dependent, flow-mediated dilatation [34,57]. Furthermore, platelets are activated with enhanced aggregation, while splenectomy increases platelet counts and induces membranes abnormalities that enhanced the already increased platelet aggregation. In parallel, the observed deficiency of the coagulation inhibitors, protein C and protein S, the elevated levels of thrombin-ATIII complex due to splenectomy and/or liver dysfunction as well as the co-inheritance of several coagulation defects, such as factor V (Leiden) and factor ΙΙ deficiency, may also contribute to the pathogenesis of hypercoagulability in thalassemia [55,58]. Finally, a strong inflammatory reaction has been noticed, expressed by the elevated circulating levels of cytokines and adhesion molecules, and the monocyte and neutrophil activation, hence promoting hypercoagulability [57].
Cardiovascular consequences
Vascular manifestations:
The combination of hypercoagulability and haemolysis-related elastic
tissue abnormalities may lead to a wide spectrum of vascular
complications. The elastic tissue abnormalities, on one hand, have been
associated with a number of vascular complications, which have been
sporadically observed in Th patients. These findings include fatal
cerebral haemorrhages, anginal symptoms, ascending aorta aneurysm
formation and gastrointestinal bleeding [54,59,60]. Elastic tissue
abnormalities may also contribute to the frequently encountered leg
ulcerations in TI patients and may explain the observed development of
transfusion-induced arterial hypertension in sickle cell anaemia and -thalassemia patients [61,62]. On the other hand, the thalassemia-related
hypercoagulability, sometimes in combination with the elastic tissue
defects, has been held responsible for a high frequency of
thromboembolic complications. Thromboembolic events were encountered in
two large cohorts of thalassemia patients, including both TM and TI,
with a frequency of 4.3% και 5.2%, respectively [63,64].
It is noteworthy
that the prevalence of such events was higher in splenectomised
patients than in non-splenectomised ones. In particular, thromboembolic
complications were even more frequent in transfusion-independent
splenectomised TI (29%), compared to regularly transfused TM (2%), a
finding that emphasizes the role of transfusion therapy in the
inhibition of hypercoagulability in thalassemia patients [65].
Such events
comprised deep vein thrombosis (40%), portal vein thrombosis (19%),
pulmonary thromboembolism (12%), cerebral thrombosis (9%) as well as
recurrent arterial occlusion and others (20%). A recently published
multinational cohort comprising 8,860 thalassaemia patients from the
Mediterranean region and Iran showed that thromboembolic events were
4.38 times more frequent in TI than in TM, and were particularly
prevalent in splenectomised patients and patients with profound anaemia
(haemoglobin level <90 g/l) [66]. Ischemic strokes
have also been
described in combination with cardiac valvular lesions – a consequence
of the elastic tissue defect and/or atrial fibrillation [54]
on a
background of hypercoagulability. At the same time, thrombosis may be a
silent, subclinical process, as autopsy findings of thrombi in the
microvasculature of lungs and brain have been described in the absence
of clinical manifestations or other known risk factors [67].
Right heart involvement:
Right sided heart involvement in Th may result from both pulmonary
hypertension (PHT) and severe iron overload. In well transfused
and chelated TM, PHT is rare. It is however, presenting with
increasing frequency in TM, particularly those who are poorly
transfused and chelated, even at younger ages [68].
Those, who are
adequately transfused but who are poorly chelated, may present with
dominant right sided heart involvement [69] with
hepatic distension and
pain with minimal dyspnoea, without evidence of PHT. PHT represents a
prominent complication in TI. Almost 60% of cases in a large cohort of
110 adult TI patients had developed PHT [42]. More
specifically, peak
systolic tricuspid gradient values >30mmHg, indicative of pulmonary
hypertension, were present in 59.1% of TI that was age related, while
values >50 mmHg were present in 7.3% of cases. Additional reports
confirmed the above finding [70-72], while a recent
study that compared TI
with TM showed that PHT is a typical feature of non-transfused TI and
not a simple age-related effect due to their prolonged survival [43]. PHT
seems to be the leading cause of congestive heart failure in TI, due to
the subsequent right heart insufficiency usually with maintenance of LV
function. The combination of high output state and increased pulmonary
vascular resistance has been held responsible for the development of
PHT [42,43]. It is more pronounced in TI than in well
transfused TM. The
increased pulmonary vascular resistance in -thalassemia is
multifactorial. The fact that most subtypes of chronic haemolytic
anemia may develop pulmonary hypertension suggests that there is a
pathogenic link between the two conditions [71]. The
role of chronic
haemolysis in the development of PHT through the induction of nitric
oxide and arginine deficiency, which promotes vasoconstriction, has
been recently stressed [50]. At the same time, as
stated above, haemolysis
has also been associated with the coexistent diffuse elastic tissue
defect. Degenerative elastic tissue lesions have been encountered in
pulmonary autopsies in patients with haemoglobinopathies, such as
sickle cell disease [73]. Moreover, endothelial
dysfunction promotes
hypercoagulability and in situ thrombus formation within the pulmonary
vascular bed. In -thalassemia,
in particular, the oxidative stress
resulting from chronic haemolysis is enhanced by the presence of iron
overload and free-radical formation and the expected effect seem to be
more pronounced. In addition, iron overload is associated with
interstitial pulmonary fibrosis and may affect pulmonary vascular
resistance [72]. Hypercoagulability, as discussed
above, is a
well-described co-morbid state in -thalassemia, especially in
non-transfused TI patients. Extensive thromboembolic lesions have been
found in the pulmonary arterioles of splenectomised thalassaemics in
post-mortem autopsies, leading to the reduction of the total pulmonary
vascular bed [67]. Lung infections, chest deformities
intrathoracic
extramedullary haemopoietic masses and transient LV dysfunction may
also contribute to pulmonary vascular resistance [42].
All the mechanisms
for PHT development can be inhibited by adequate transfusion and
chelation therapy and explain why that finding is a rare phenomenon in
TM.
Left ventricular
involvement:
The main mechanism of left ventricular involvement in Th is iron
overload and secondarily the increased cardiac output. The
reduction in LVEF is a major element in TM for cardiomyopathy and the
worst prognostic feature with respect to patient survival. In well
transfused TM (pre transfusion Hb > 95g/l) the iron overload
predominates and in TI the increased cardiac output is prominent.
Furthermore, the resulting elevation of systemic impedance that is
presented to left ventricle leads to a less favourable interaction
between left ventricular ejection and systemic arterial compliance,
which contributes to left ventricular impairment [74,75].
These changes
are aggravated by the advancing age. Besides peripheral vascular
disorders, the coexistence of coronary artery involvement, infections
related to iron load, endocrine abnormalities, arrhythmias and
valvular lesions, render left ventricular function more
susceptible to decompensation. Th are more likely to present with overt
cardiac dysfunction in situations of stress, such as excessive physical
activity or other conditions requiring increase cardiac work load as
fever or significant anaemia. In situations of increased stress,
particularly in TI, LV cardiac decompensation may present with sudden
worsening of preexisting PHT due to further increase in pulmonary
vascular resistance.
Chelation treatment for prevention and treatment of iron induced heart disease.
Chelation therapy:
Comprehensive treatment of both TM and TI is beyond the scope of this
review. The approach to prevention and reversal of cardiac
disease is principally based on TM. However for both TM and TI (once
the decision to transfuse is made), it is important to minimize the
cardiac output with adequate levels of pre-transfusion Hb (> 95g/l
in general and higher if there is evidence of PHT or marginal cardiac
function) and to remove the iron. The monitoring to determine the
degree of cardiac iron overload is by Cardiac Magnetic Resonance (CMR)
T2* assessment. Chelation treatment today should be guided by MRI
findings, if the technique is available. We are in a transient
phase of knowledge with the availability of MRI and new chelating
agents. Important questions with respect to best management to
avoid iron induced cardiac disease remain to be elucidated. Optimal
management may be clarified from results of different trials
and current ongoing follow up studies from many subgroups of patients
using different regimes. In the presence of excess cardiac and or
hepatic iron, treatment strategies include increase of the dose and/or
frequency of desferrioxamine, switch to oral chelators (deferiprone or
deferasirox) or to the combination of deferiprone with desferrioxamine,
provided there are no contraindications to their use [76,77].
Combination
of the two iron chelators (desferrioxamine and deferiprone) seems to
maximize the efficacy producing additive and synergistic effects in
iron excretion [78,79]. It seems that each of those
two agents chelates
iron from different pools and there is at least an additive effect when
combined treatment is administered80. Available evidence now
suggests that combined therapy should be the treatment of choice for
patients with established cardiac failure. Continuous desferrioxamine
infusions alone, have been shown to improve cardiac function and
salvage patients81 and is the treatment of choice if combination
therapy is contraindicated. We have reported two cases with
severe CCF who reversed with intensive combination therapy [82,83]
and we have at least 8 more patients with similar outcome.
Two other studies show similar responses [84,85].
In a recent study
with combined treatment, apart from significant reduction in ferritin,
cardiac and liver iron and improvement in cardiac function, the
absolute endothelial function was also improved [77].
Furthermore,
improvement with glucose tolerance with the use of combination therapy
has been reported [86,87] as well as anecdotal
reports of improvement in
other endocrine functions.
With respect to hepatic iron removal, the efficacy of the two oral chelators is at least equal to the standard doses of desferrioxamine [20,88,89]. Recent and ongoing studies have demonstrated that deferiprone, a small molecule that permeates all tissues, is more efficient in removing cardiac iron and improving cardiac function than desferrioxamine [20,90,91]. Some preliminary clinical and laboratory observations with deferasirox are encouraging with respect to removal of cardiac iron [92,93]. As yet, there are no studies with combinations of deferasirox and desferrioxamine so this therapeutic regime cannot be recommended at this stage. According to the current knowledge and based on the CMR findings , the suggested chelation regimes are as follows.
With respect to hepatic iron removal, the efficacy of the two oral chelators is at least equal to the standard doses of desferrioxamine [20,88,89]. Recent and ongoing studies have demonstrated that deferiprone, a small molecule that permeates all tissues, is more efficient in removing cardiac iron and improving cardiac function than desferrioxamine [20,90,91]. Some preliminary clinical and laboratory observations with deferasirox are encouraging with respect to removal of cardiac iron [92,93]. As yet, there are no studies with combinations of deferasirox and desferrioxamine so this therapeutic regime cannot be recommended at this stage. According to the current knowledge and based on the CMR findings , the suggested chelation regimes are as follows.
Acceptable Cardiac
Iron: For
patients with T2* > 20 ms., the therapeutic strategy should be
continuation of monotherapy with either desferrioxamine or either
of the available oral chelators (deferiprone and desferasirox)
with regular follow-up. For the patient’s convenience, desferrioxamine
administration may be converted to either of the two oral chelators. If
there has been iron overload in the past that was attributed to
desferrioxamine therapy and that was subsequently cleared with
intensification of chelation therapy, then monotherapy with
desferrioxamine is not recommended.
Mild to Moderate Cardiac
Iron Loading:
T2* values between 10-20ms are considered to reflect a mild to
moderately iron loaded myocardium. Bearing in mind that the patients
may be at risk of developing cardiac problems under stress such as
infections, clearing myocardial tissue from iron seems to be a rational
target. Therefore, combined treatment for these patients should not be
a priori excluded. Patients have presented with LV dysfunction at
levels of T2* of 15 msec, without any precipitating factors [19,77]. Therefore, if T2* is ≤
15 msec, combination chelation therapy
is recommended76. However, questions still exist, regarding the
frequency and the amount of desferrioxamine administration that is
appropriate in a combined regimen. A dose of 35-40mg/kg/day three-four
times weekly combined with deferiprone at a dose of 75mg/kg/day seems
to be reasonable. In patients with T2* 15-20 ms,
monotherapy with deferiprone and deferasirox are available
options [20,88]. However, in
this circumstance close monitoring is
necessary. Patients treated up to the time of the MRI with
desferrioxamine in this category and who availed themselves of that
treatment satisfactorily, in general should not be on monotherapy with
that compound, as desferrioxamine was inadequate at preventing
the iron accumulation in the heart and may indicate some type of
resistance to its efficacy within that patient. If however, the
patient’s adherence to treatment with it was poor, then it may be
appropriate in higher doses and frequency, provided the patient can be
convinced to use it.
Heavy Cardiac Iron Load:
Patients with T2* <10msec are considered to have severe iron
overload and this category includes most patients with reduced left
ventricular (LV) function. Even those patients with normal ejection
fraction in this category are considered to have a significant risk of
developing cardiac dysfunction. Thus all patients in this category have
a strong indication for combined chelation treatment. The doses
of the two medications should be similar to those described for
patients with CCF (see below) but with the desferrioxamine being given
as a subcutaneous infusion. If deferiprone is
contraindicated, then intensive intravenous continuous desferrioxamine
infusions are the treatment of choice.
Heart Failure: For patients with heart failure desferrioxamine should be administrated at a dose of 60-80mg/kg/day intravenously and deferiprone at a dose of 75-100mg/kg/day in three divided doses. If deferiprone is contraindicated, the patient should be managed with continuous desferrioxamine infusions, which usually require the placement of an indwelling catheter [94]. It seems however, that the rate of removal of iron with such therapy is much slower than with combination therapy [77]. Caution should be taken with the 24h desferrioxamine infusion to avoid fluid overload especially when intravenous antibiotics and anti arrhythmic agents are also indicated.
Heart Failure: For patients with heart failure desferrioxamine should be administrated at a dose of 60-80mg/kg/day intravenously and deferiprone at a dose of 75-100mg/kg/day in three divided doses. If deferiprone is contraindicated, the patient should be managed with continuous desferrioxamine infusions, which usually require the placement of an indwelling catheter [94]. It seems however, that the rate of removal of iron with such therapy is much slower than with combination therapy [77]. Caution should be taken with the 24h desferrioxamine infusion to avoid fluid overload especially when intravenous antibiotics and anti arrhythmic agents are also indicated.
Treatment Modifications:
Any treatment modification should be followed by close monitoring.
Should any serious adverse effect present as a consequence of the
administration of a particular chelator, appropriate guidelines as to
its continued use should be followed. If treatment has ultimately
modified the MRI patient’s classification then, it may be adjusted as
discussed above according to the changes in MRI values. In all of the
above, hepatic iron and endocrine status should also be considered and
modification to the recommended regimes should be made in order to
achieve normal hepatic iron levels in the long term.
Guidelines if MRI is not available: In situations in which MRI is not available, then all the patients’ traditional parameters need to be analysed, (ferritins, liver iron concentrations) as well as ECG and echocardiogram. These may serve as a guide to treatment. Furthermore, according to knowledge from MRI studies, all patients with reductions in LVEF have excessive cardiac iron load. Any echocardiographic evidence of reduce cardiac function should be considered as being associated with excessive cardiac iron load and should be managed accordingly. In countries where follow up of patients has become available and who had been treated with desferrioxamine, up to 65% of patients have cardiac iron load. In Sardinia, 13% had severe cardiac iron overload29. In our study 48% of patients have T2* < 15 ms [19]. In countries were patients’ compliance to treatment is inadequate, there was poor availability of chelation and/or the follow up was not well organized, the percentage of cardiac iron loaded patients is likely to be higher. Therefore, for patients who have never had optimal care, it is very likely the patients will have cardiac iron load and intensive combination chelation is the treatment of choice. In patients who have been poorly chelated, the risk of chelation toxicity is minimal and would only be likely to occur after prolonged therapy, however, it is important to be vigilant for such complications. If compliance with desferrioxamine has been an issue, as evidenced by high ferritin or hepatic iron, then either of the two available oral chelators is appropriate therapy. MRI is more necessary for those patients, who have had good chelation therapy with desferrioxamine but who are at risk of chelation inadequacy with respect to the heart and for those who have had treatment modification in order to follow the efficacy of the changed chelation regime.
Guidelines if MRI is not available: In situations in which MRI is not available, then all the patients’ traditional parameters need to be analysed, (ferritins, liver iron concentrations) as well as ECG and echocardiogram. These may serve as a guide to treatment. Furthermore, according to knowledge from MRI studies, all patients with reductions in LVEF have excessive cardiac iron load. Any echocardiographic evidence of reduce cardiac function should be considered as being associated with excessive cardiac iron load and should be managed accordingly. In countries where follow up of patients has become available and who had been treated with desferrioxamine, up to 65% of patients have cardiac iron load. In Sardinia, 13% had severe cardiac iron overload29. In our study 48% of patients have T2* < 15 ms [19]. In countries were patients’ compliance to treatment is inadequate, there was poor availability of chelation and/or the follow up was not well organized, the percentage of cardiac iron loaded patients is likely to be higher. Therefore, for patients who have never had optimal care, it is very likely the patients will have cardiac iron load and intensive combination chelation is the treatment of choice. In patients who have been poorly chelated, the risk of chelation toxicity is minimal and would only be likely to occur after prolonged therapy, however, it is important to be vigilant for such complications. If compliance with desferrioxamine has been an issue, as evidenced by high ferritin or hepatic iron, then either of the two available oral chelators is appropriate therapy. MRI is more necessary for those patients, who have had good chelation therapy with desferrioxamine but who are at risk of chelation inadequacy with respect to the heart and for those who have had treatment modification in order to follow the efficacy of the changed chelation regime.
Conclusions on Heart Disease
This formerly catastrophic genetic defect has been revolutionized with the availability of adequate chelation therapy and more recently with other important advances particularly MRI . Iron related heart failure is reversible in TM provided appropriate interventions are made in a timely manner. It should no longer be considered a terminal event and intensive attention to the parameters mentioned above can result in complete reversal with markedly improved quality of life.It remains important, practically, to aim to maintain low LIC’s and ferritin levels in Th (both TM and TI), particularly as the latter are easily accessible and assessable. Similarly, echocardiography should remain a routine tool as it does have some predictive value and can also be used to monitor patients in whom intensification of chelation therapy has been instituted.CMR can be particularly helpful in identifying all TM patients at risk of developing heart disease by assessing the cardiac iron load. Chelation therapy can be tailored to remove the excess heart iron. Attention to patient’s continuous compliance with adequate chelation is mandatory.The definite ability to know and reduce cardiac iron as well as improvement in cardiac function that can be achieved by appropriate chelation, should certainly lead to even further significant reduction in cardiac mortality and morbidity.
This formerly catastrophic genetic defect has been revolutionized with the availability of adequate chelation therapy and more recently with other important advances particularly MRI . Iron related heart failure is reversible in TM provided appropriate interventions are made in a timely manner. It should no longer be considered a terminal event and intensive attention to the parameters mentioned above can result in complete reversal with markedly improved quality of life.It remains important, practically, to aim to maintain low LIC’s and ferritin levels in Th (both TM and TI), particularly as the latter are easily accessible and assessable. Similarly, echocardiography should remain a routine tool as it does have some predictive value and can also be used to monitor patients in whom intensification of chelation therapy has been instituted.CMR can be particularly helpful in identifying all TM patients at risk of developing heart disease by assessing the cardiac iron load. Chelation therapy can be tailored to remove the excess heart iron. Attention to patient’s continuous compliance with adequate chelation is mandatory.The definite ability to know and reduce cardiac iron as well as improvement in cardiac function that can be achieved by appropriate chelation, should certainly lead to even further significant reduction in cardiac mortality and morbidity.
References
- Rund D, Rachmilewitz E. Beta-thalassemia. N
Engl J Med. 2005;353:1135-1146.

- Borgna-Pignatti C, Rugolotto S, De Stefano
P, et al. Survival and complications in patients with thalassemia major
treated with transfusion and deferoxamine. Haematologica.
2004;89:1187-1193.

- Camaschella C, Cappellini MD. Thalassemia
intermedia. Haematologica. 1995;80:58-68.

- Cunningham MJ, Macklin EA, Neufeld EJ,
Cohen AR. Complications of beta-thalassemia major in North America.
Blood. 2004;104:34-39.

- Hershko C, Link G, Cabantchik I.
Pathophysiology of iron overload. Ann N Y Acad Sci. 1998;850:191-201.

- Anderson LJ, Holden S, Davis B, et al.
Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis
of myocardial iron overload. Eur Heart J. 2001;22:2171-2179.

- Nemeth E, Ganz T. Hepcidin and iron-loading
anemias. Haematologica. 2006;91:727-732.

- Papanikolaou G, Tzilianos M, Christakis JI,
et al. Hepcidin in iron overload disorders. Blood. 2005;105:4103-4105.

- Gardenghi S, Marongiu MF, Ramos P, et al.
Ineffective erythropoiesis in beta-thalassemia is characterized by
increased iron absorption mediated by down-regulation of hepcidin and
up-regulation of ferroportin. Blood. 2007;109:5027-5035.

- Silvestri L, Guillem F, Pagani A, et al.
Molecular mechanisms of the defective hepcidin inhibition in TMPRSS6
mutations associated with iron-refractory iron deficiency anemia.
Blood. 2009;113:5605-5608.

- Pepe A CE, Santarelli MF, et al. Magnetic Resonance characterization of Thalassemia Intermedia patients confronted with Thalassemia Major patients. J Am Coll Cardiol 2006;47:136A.
- Origa R, Barella S, Argiolas GM, Bina P,
Agus A, Galanello R. No evidence of cardiac iron in 20 never- or
minimally-transfused patients with thalassemia intermedia.
Haematologica 2008;93:1095-1096.

- Ramazzotti A, Pepe A, Positano V, et al.
Standardized T2* map of a normal human heart to correct T2* segmental
artefacts; myocardial iron overload and fibrosis in thalassemia
intermedia versus thalassemia major patients and electrocardiogram
changes in thalassemia major patients. Hemoglobin. 2008;32:97-107.

- Modell CB. Haemoglobinopathies. The
pathophysiology of beta-thalassaemia major. J Clin Pathol Suppl (R Coll
Pathol). 1974;8:12-18.

- Esposito BP, Breuer W, Sirankapracha P,
Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron
overload: redox activity and susceptibility to chelation. Blood.
2003;102:2670-2677.

- Glickstein H, El RB, Link G, et al. Action
of chelators in iron-loaded cardiac cells: Accessibility to
intracellular labile iron and functional consequences. Blood.
2006;108:3195-3203.

- Trinder D, Fox C, Vautier G, Olynyk JK.
Molecular pathogenesis of iron overload. Gut. 2002;51:290-295.

- Aessopos A, Farmakis D, Hatziliami A, et
al. Cardiac status in well-treated patients with thalassemia major. Eur
J Haematol. 2004;73:359-366.

- Aessopos A, Fragodimitri C, Karabatsos F,
et al. Cardiac magnetic resonance imaging R2* assessments and analysis
of historical parameters in patients with transfusion-dependent
thalassemia. Haematologica. 2007;92:131-132.

- Pennell DJ, Berdoukas V, Karagiorga M, et
al. Randomized controlled trial of deferiprone or deferoxamine in
beta-thalassemia major patients with asymptomatic myocardial siderosis.
Blood. 2006;107:3738-3744.

- Ferrara M, Matarese SM, Francese M, et al.
Role of apolipoprotein E (APOE) polymorphism on left cardiac failure in
homozygous beta thalassaemic patients. Br J Haematol. 2001;114:959-960.

- Economou-Petersen E, Aessopos A, Kladi A,
et al. Apolipoprotein E epsilon4 allele as a genetic risk factor for
left ventricular failure in homozygous beta-thalassemia. Blood.
1998;92:3455-3459.

- Farmakis D, Giakoumis A, Polymeropoulos E,
Aessopos A. Pathogenetic aspects of immune deficiency associated with
beta-thalassemia. Med Sci Monit. 2003;9:RA19-22.

- Walker EM, Jr., Walker SM. Effects of iron
overload on the immune system. Ann Clin Lab Sci. 2000;30:354-365.

- Lesic B, Foulon J, Carniel E. Comparison
of the effects of deferiprone versus deferoxamine on growth and
virulence of Yersinia enterocolitica. Antimicrob Agents Chemother.
2002;46:1741-1745.

- Kremastinos DT, Flevari P, Spyropoulou M,
Vrettou H, Tsiapras D, Stavropoulos-Giokas CG. Association of heart
failure in homozygous beta-thalassemia with the major
histocompatibility complex. Circulation. 1999;100:2074-2078.

- Jessup M, Manno CS. Diagnosis and
management of iron-induced heart disease in Cooley's anemia. Ann N Y
Acad Sci. 1998;850:242-250.

- Kremastinos DT, Tsetsos GA, Tsiapras DP,
Karavolias GK, Ladis VA, Kattamis CA. Heart failure in beta
thalassemia: a 5-year follow-up study. Am J Med. 2001;111:349-354.

- Tanner MA, Galanello R, Dessi C, et al.
Myocardial iron loading in patients with thalassemia major on
deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8:543-547.

- Lekawanvijit S, Chattipakorn N. Iron
overload thalassemic cardiomyopathy: iron status assessment and
mechanisms of mechanical and electrical disturbance due to iron
toxicity. Can J Cardiol. 2009;25:213-218.

- Tsironi M, Korovesis K, Farmakis D,
Deftereos S, Aessopos A. Hypocalcemic heart failure in thalassemic
patients. Int J Hematol. 2006;83:314-317.

- Au WY, Lam WW, Chu W, et al. A T2*
magnetic resonance imaging study of pancreatic iron overload in
thalassemia major. Haematologica. 2008;93:116-119.

- Nienhuis AW. Vitamin C and iron. N Engl J
Med. 1981;304:170-171.

- Cheung YF, Chan GC, Ha SY. Arterial
stiffness and endothelial function in patients with beta-thalassemia
major. Circulation. 2002;106:2561-2566.

- Tsomi K, Karagiorga-Lagana M, Karabatsos
F, et al. Arterial elastorrhexis in beta-thalassaemia intermedia,
sickle cell thalassaemia and hereditary spherocytosis. Eur J Haematol.
2001;67:135-141.

- Aessopos A, Farmakis D, Loukopoulos D.
Elastic tissue abnormalities resembling pseudoxanthoma elasticum in
beta thalassemia and the sickling syndromes. Blood. 2002;99:30-35.

- Aessopos A, Deftereos S, Farmakis D, et
al. Cardiovascular adaptation to chronic anemia in the elderly: an
echocardiographic study. Clin Invest Med. 2004;27:265-273.

- Varat MA, Adolph RJ, Fowler NO.
Cardiovascular effects of anemia. Am Heart J. 1972;83:415-426.

- Pepe A, Positano V, Santarelli MF, et al.
Multislice multiecho T2* cardiovascular magnetic resonance for
detection of the heterogeneous distribution of myocardial iron
overload. J Magn Reson Imaging. 2006;23:662-668.

- Westwood MA, Anderson LJ, Maceira AM, et
al. Normalized left ventricular volumes and function in thalassemia
major patients with normal myocardial iron. J Magn Reson Imaging.
2007;25:1147-1151.

- Aessopos A, Deftereos S, Tsironi M, et al.
Predictive echo-Doppler indices of left ventricular impairment in
B-thalassemic patients. Ann Hematol. 2007;86:429-434.

- Aessopos A, Farmakis D, Karagiorga M, et
al. Cardiac involvement in thalassemia intermedia: a multicenter study.
Blood. 2001;97:3411-3416.

- Aessopos A, Farmakis D, Deftereos S, et
al. Thalassemia heart disease: a comparative evaluation of thalassemia
major and thalassemia intermedia. Chest. 2005;127:1523-1530.

- Vaccari M, Crepaz R, Fortini M, et al.
Left ventricular remodeling, systolic function, and diastolic function
in young adults with beta-thalassemia intermedia: a Doppler
echocardiography study. Chest. 2002;121:506-512.

- Ferrara M, Matarese SM, Borrelli B, et al.
Cardiac involvement in beta-thalassemia major and beta-thalassemia
intermedia. Hemoglobin. 2004;28:123-129.

- Galanello R, Barella S, Turco MP, et al.
Serum erythropoietin and erythropoiesis in high- and low-fetal
hemoglobin beta-thalassemia intermedia patients. Blood. 1994;83:561-565.

- Aessopos A, Farmakis D, Tsironi M, et al.
Hemodynamic assessment of splenomegaly in beta-thalassemia patients
undergoing splenectomy. Ann Hematol. 2004;83:775-778.

- Aessopos A, Farmakis D, Deftereos S, et
al. Cardiovascular effects of splenomegaly and splenectomy in
beta-thalassemia. Ann Hematol. 2005;84:353-357.

- Murray JF, Dawson AM, Sherlock S.
Circulatory changes in chronic liver disease. Am J Med. 1958;24:358-367.

- Vichinsky EP. Pulmonary hypertension in
sickle cell disease. N Engl J Med. 2004;350:857-859.

- Aessopos A, Farmakis D, Loukopoulos D.
Elastic tissue abnormalities in inherited haemolytic syndromes. Eur J
Clin Invest. 2002;32:640-642.

- Aessopos A, Samarkos M, Voskaridou E, et
al. Arterial calcifications in beta-thalassemia. Angiology.
1998;49:137-143.

- Aessopos A, Farmakis D, Tsironi M, et al.
Endothelial function and arterial stiffness in sickle-thalassemia
patients. Atherosclerosis. 2007;191:427-432.

- Aessopos A, Farmakis D, Karagiorga M,
Rombos I, Loucopoulos D. Pseudoxanthoma elasticum lesions and cardiac
complications as contributing factors for strokes in beta-thalassemia
patients. Stroke. 1997;28:2421-2424.

- Eldor A, Rachmilewitz EA. The
hypercoagulable state in thalassemia. Blood. 2002;99:36-43.

- Borenstain-Ben Yashar V, Barenholz Y,
Hy-Am E, Rachmilewitz EA, Eldor A. Phosphatidylserine in the outer
leaflet of red blood cells from beta-thalassemia patients may explain
the chronic hypercoagulable state and thrombotic episodes. Am J
Hematol. 1993;44:63-65.

- Aggeli C, Antoniades C, Cosma C, et al.
Endothelial dysfunction and inflammatory process in
transfusion-dependent patients with beta-thalassemia major. Int J
Cardiol. 2005;105:80-84.

- Giordano P, Del Vecchio GC, Altomare M, et
al. Resistance to activated protein C in thalassaemic patients: an
underlying cause of thrombosis. Eur J Haematol. 1998;61:123-127.

- Farmakis D, Moyssakis I, Perakis A, et al.
Unstable angina associated with coronary arterial calcification in a
thalassemia intermedia patient with a pseudoxanthoma elasticum-like
syndrome. Eur J Haematol. 2003;70:64-66.

- Farmakis D, Vesleme V, Papadogianni A,
Tsaftaridis P, Kapralos P, Aessopos A. Aneurysmatic dilatation of
ascending aorta in a patient with beta-thalassemia and a pseudoxanthoma
elasticum-like syndrome. Ann Hematol. 2004;83:596-599.

- Aessopos A, Kati M, Tsironi M, Polonifi E,
Farmakis D. Exchange blood transfusions for the treatment of leg
ulcerations in thalassemia intermedia. Haematologica. 2006;91:ECR11.

- Wasi P, Na-Nakorn S, Pootrakul P, Sonakul
D, Piankijagum A, Pacharee P. A syndrome of hypertension, convulsion,
and cerebral haemorrhage in thalassaemic patients after multiple
blood-transfusions. Lancet. 1978;2:602-604.

- Borgna Pignatti C, Carnelli V, Caruso V,
et al. Thromboembolic events in beta thalassemia major: an Italian
multicenter study. Acta Haematol. 1998;99:76-79.

- Moratelli S, De Sanctis V, Gemmati D, et
al. Thrombotic risk in thalassemic patients. J Pediatr Endocrinol
Metab. 1998;11 Suppl 3:915-921.

- Cappellini MD, Robbiolo L, Bottasso BM,
Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and
hypercoagulability in splenectomized patients with thalassaemia
intermedia. Br J Haematol. 2000;111:467-473.

- Taher A, Isma'eel H, Mehio G, et al.
Prevalence of thromboembolic events among 8,860 patients with
thalassaemia major and intermedia in the Mediterranean area and Iran.
Thromb Haemost. 2006;96:488-491.

- Sonakul D, Fucharoen S. Pulmonary
thromboembolism in thalassemic patients. Southeast Asian J Trop Med
Public Health. 1992;23 Suppl 2:25-28.

- Aessopos A, Farmakis D. Pulmonary
hypertension in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:342-349.

- Aessopos A, Berdoukas V, Tsironi M. The
heart in transfusion dependent homozygous thalassaemia
today--prediction, prevention and management. Eur J Haematol.
2008;80:93-106.

- Aessopos A, Stamatelos G, Skoumas V,
Vassilopoulos G, Mantzourani M, Loukopoulos D. Pulmonary hypertension
and right heart failure in patients with beta-thalassemia intermedia.
Chest. 1995;107:50-53.

- Derchi G, Forni GL, Formisano F, et al.
Efficacy and safety of sildenafil in the treatment of severe pulmonary
hypertension in patients with hemoglobinopathies. Haematologica.
2005;90:452-458.

- Zakynthinos E, Vassilakopoulos T, Kaltsas
P, et al. Pulmonary hypertension, interstitial lung fibrosis, and lung
iron deposition in thalassaemia major. Thorax. 2001;56:737-739.

- Haque AK, Gokhale S, Rampy BA, Adegboyega
P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell
hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol.
2002;33:1037-1043.

- London GM, Cohn JN. Prognostic application
of arterial stiffness: task forces. Am J Hypertens. 2002;15:754-758.

- Safar ME, Levy BI, Struijker-Boudier H.
Current perspectives on arterial stiffness and pulse pressure in
hypertension and cardiovascular diseases. Circulation.
2003;107:2864-2869.

- Tanner MA, Galanello R, Dessi C, et al.
Combined chelation therapy in thalassemia major for the treatment of
severe myocardial siderosis with left ventricular dysfunction. J
Cardiovasc Magn Reson. 2008;10:12.

- Tanner MA, Galanello R, Dessi C, et al. A
randomized, placebo-controlled, double-blind trial of the effect of
combined therapy with deferoxamine and deferiprone on myocardial iron
in thalassemia major using cardiovascular magnetic resonance.
Circulation. 2007;115:1876-1884.

- Grady R BV, Rachmielewitz EA, Giardina PJ. Optimising chelation therapy: Combining deferiprone and deferoxamine. Blood. 2000;96:604a.
- Wonke B, Wright C, Hoffbrand AV. Combined
therapy with deferiprone and desferrioxamine. Br J Haematol.
1998;103:361-364.

- Kontoghiorghes GJ. Future chelation
monotherapy and combination therapy strategies in thalassemia and other
conditions. comparison of deferiprone, deferoxamine, ICL670, GT56-252,
L1NAll and starch deferoxamine polymers. Hemoglobin. 2006;30:329-347.

- Davis BA, Porter JB. Long-term outcome of
continuous 24-hour deferoxamine infusion via indwelling intravenous
catheters in high-risk beta-thalassemia. Blood. 2000;95:1229-1236.

- Tsironi M, Deftereos S, Andriopoulos P,
Farmakis D, Meletis J, Aessopos A. Reversal of heart failure in
thalassemia major by combined chelation therapy: a case report. Eur J
Haematol. 2005;74:84-85.

- Tsironi M, Polonifi K, Deftereos S, et al.
Transfusional hemosiderosis and combined chelation therapy in sickle
thalassemia. Eur J Haematol. 2005;75:355-358.

- Tavecchia L, Masera N, Russo P, et al.
Successful recovery of acute hemosiderotic heart failure in
beta-thalassemia major treated with a combined regimen of
desferrioxamine and deferiprone. Haematologica. 2006;91:ECR19.

- Wu KH, Chang JS, Tsai CH, Peng CT.
Combined therapy with deferiprone and desferrioxamine successfully
regresses severe heart failure in patients with beta-thalassemia major.
Ann Hematol. 2004;83:471-473.

- Farmaki K, Angelopoulos N, Anagnostopoulos
G, Gotsis E, Rombopoulos G, Tolis G. Effect of enhanced iron chelation
therapy on glucose metabolism in patients with beta-thalassaemia major.
Br J Haematol. 2006;134:438-444.

- Christoforidis A, Perifanis V,
Athanassiou-Metaxa M. Combined chelation therapy improves glucose
metabolism in patients with beta-thalassaemia major. Br J Haematol.
2006;135:271-272.

- Peng CT, Chow KC, Chen JH, Chiang YP, Lin
TY, Tsai CH. Safety monitoring of cardiac and hepatic systems in
beta-thalassemia patients with chelating treatment in Taiwan. Eur J
Haematol. 2003;70:392-397.

- Cappellini MD, Cohen A, Piga A, et al. A
phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator,
in patients with beta-thalassemia. Blood. 2006;107:3455-3462.

- Piga A, Gaglioti C, Fogliacco E, Tricta F.
Comparative effects of deferiprone and deferoxamine on survival and
cardiac disease in patients with thalassemia major: a retrospective
analysis. Haematologica. 2003;88:489-496.

- Borgna-Pignatti C, Cappellini MD, De
Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or
deferiprone-treated patients with thalassemia major. Blood.
2006;107:3733-3737.

- Eleftheriou P TM, Pennell D, Porter J. . Response of myocardial T2* to oral deferasirox monotherapy for 1 year in 29 patients with transfusion-dependent anaemias; A subgroup analysis. Vol. 91: Haematologica 2006:(Suppl 1) abst 999.
- Wood J TA, Paley C et al. . MRI T2* demonstrates reduced cardiac iron burden following moderate –to high- dose desferasirox treatment in chronically transfused β-thalassaemia patients. . Vol. 110: Blood (ASH Annual Meeting Abstracts), ; Nov 2007: 2781
- Anderson LJ, Westwood MA, Holden S, et al.
Myocardial iron clearance during reversal of siderotic cardiomyopathy
with intravenous desferrioxamine: a prospective study using T2*
cardiovascular magnetic resonance. Br J Haematol. 2004;127:348-355.
