Thalassaemia and Aberrations of Growth and Puberty
Andreas Kyriakou, MD and Nicos Skordis, MD
Pediatric Endocrine Unit, Dept. of Pediatrics, Makarios Hospital, Nicosia, Cyprus.
Correspondence
to:
Nicos Skordis, M.D. Pediatric Endocrine Unit, Makarios Hospital Nicosia
1474, Cyprus. Tel: +357 22405000, Fax: +357 22305072. E-mail: nskordis@cytanet.com.cy
Published: July 27, 2009
Received: June 7, 2009
Accepted: July 26, 2009
Medit J of Hemat Infect Dis 2009, 1(1): e2009003 DOI 10.4084/MJHID.2009.003
This article is available from: http://www.mjhid.org/article/view/4612
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Endocrine
dysfunction in Thalassaemia major (TM) is a common and disturbing
complication, which requires prompt recognition and treatment. The
contribution of the underlying molecular defect in TM to the
development of endocrinopathies is significant because the patients
with the more severe genetic defects have a greater rate of iron
loading through higher red cell consumption. TM patients frequently
present delay of growth and puberty with reduction of final height. The
pathogenesis of growth failure is multifactorial and is mainly due to
chronic anemia and hypoxia, chronic liver disease, zinc and folic acid
deficiency, iron overload, intensive use of chelating agents, emotional
factors, and endocrinopathies (hypogonadism, delayed puberty,
hypothyroidism) and GH-IGF-1 axis dysregulation. Although appropriate
iron chelation therapy can improve growth and development, TM children
and adolescents treated intensively with desferrioxamine remain short
as well, showing body disproportion between the upper and lower body
segment. Body disproportion is independent of pubertal or prepubertal
period of greater height gain. Treatment with recombinant GH (rhGH) is
recommended when GH deficiency is established, and even so, the
therapeutic response is often non satisfactory. Growth acceleration is
mostly promoted with sex steroids in children with associated pubertal
delay. Sexual complications in TM, which include Delayed Puberty,
Arrested Puberty and Hypogonadism, present the commonest endocrine
complication. Iron deposition on gonadotroph cells of the pituitary
leads to disruption of gonadotrophin production which is proven by the
poor response of FSH and LH to GnRH stimulation. In the majority of
patients gonadal function is normal as most women with Amenorrhea are
capable of achieving pregnancy with hormonal treatment and similarly
men with azoospermia become fathers. Secondary Hypogonadism appears
later in life, and is manifested in women as
Secondary Amenorrhea and in men as decline in sexual drive and
azzoospermia. The damage to the hypothalamus and pituitary is
progressive, even when intensive chelating therapy is given and the
appearance of Hypogonadism in both sexes is often unavoidable. Close
follow up and proper management is crucial for every patient with TM.
Early recognition of growth disturbance and prevention of hypogonadism
by early and judicious chelation therapy is mandatory for the
improvement of their quality of life. Patients with TM can now live a
better life due to modern advances in their medical care and our better
understanding in the pathogenesis, manifestation and prevention of
endocrine complications.
Introduction
Growth:
Protocol for investigation of Thalassaemic children:
Puberty
Treatment
of beta-Thalassaemia Major (TM) is based on regular blood transfusions
to maintain the pre-transfusional Hb level above 9 gr/dl and
appropriate chelation therapy to avoid the consequences of iron
overload. The metabolically active iron catalyses the formation of free
radicals, which damage membrane lipids leading to cell death and
eventually organ failure. The endocrine glands are particularly
vulnerable to the excess iron, so that the appearance of endocrine
dysfunction in Thalassaemia major is a common and disturbing
complication, which requires prompt recognition and treatment. The
contribution of the underlying molecular defect in TM to the
development of endocrinopathies and particularly Hypogonadotrophic
Hypogonadism is significant because the patients with the more severe
genetic defects have a greater rate of iron loading through higher red
cell consumption [1].
Growth:
TM patients frequently present
delay of growth and puberty with
reduction of final height. Growth failure in TM has been recognised for
many years, and has persisted despite major treatment advances. The
child with TM has a particular growth pattern, which is relatively
normal until age 9-10 years; after this age a slowing down of growth
velocity and a reduced or absent pubertal growth spurt are observed.
The growth plate fusion is usually delayed until the end of the second
decade of life [2]. The pathogenesis of growth
failure is multifactorial [3,4]
and is mainly due to chronic anemia and hypoxia, chronic liver disease,
zinc and folic acid deficiency, iron overload, intensive use of
chelating agents, emotional factors, endocrine-pathies (hypogonadism,
delayed puberty, hypo-thyroidism) and GH-IGF-1 axis dysregulation.
Chronic hypoxia is no longer a
contributing factor nowadays in properly
treated children. Linear growth in childhood is disrupted only in a
small percentage of children due to anemia, ineffective erythropoiesis
and iron overload. During the first decade of life the maintenance of
haemoglobin levels above 9 g/dL together with adequate iron chelation
therapy makes the children with TM indistinguishable from their non
thalassaemic peers [5,6].
Zinc deficiency is probably a concomitant factor in growth failure. The effects of zinc supplementation on growth velocity were assessed in 22 patients with biochemical evidence of zinc deficiency. The mean height velocity of zinc supplemented children was significantly greater that of normal children [7]. The crucial role of zinc deficiency however was not confirmed by other studies [8,9].
It is well known that high serum ferritin levels during the first decade of life are associated with final short stature [10,11], indicating that appropriate iron chelation therapy can prevent or limit this complication. However, several studies showed high prevalence of short stature in TM children and adolescence treated intensively with desferrioxamine (DFX)4,[12,13]. In addition, premature chelating therapy, between ages of 2-5 years, may have deleterious effects on growth [12,14,15]. DFX is proven to inhibit cell proliferation, DNA synthesis, collagen formation and trace mineral deposition such as cooper and zinc. Mineral depletion may result in a decrease of alkaline phosphatase activity. This complex mechanism results in platyspondylosis with flattening of the vertebral bodies and consequent shortening of the spinal height, resulting in truncal shortening , also in the presence of normal stature[1,2,15-20].
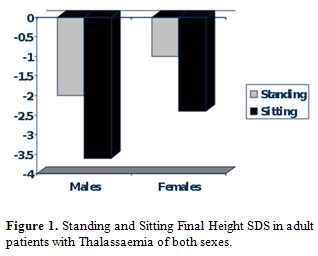
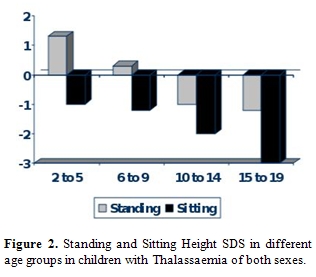
Body disproportion between the upper (short) and lower body segment (normal) is observed in approximately 15-40% of TM patients [18]. Spinal growth impairment starts during infancy and deteriorates progressively. Most Thalassaemics, especially males do not reach their target height and their Sitting height is more severely affected (Figure 1). During puberty hypogonadism further impairs spinal growth (Figure 2).
Zinc deficiency is probably a concomitant factor in growth failure. The effects of zinc supplementation on growth velocity were assessed in 22 patients with biochemical evidence of zinc deficiency. The mean height velocity of zinc supplemented children was significantly greater that of normal children [7]. The crucial role of zinc deficiency however was not confirmed by other studies [8,9].
It is well known that high serum ferritin levels during the first decade of life are associated with final short stature [10,11], indicating that appropriate iron chelation therapy can prevent or limit this complication. However, several studies showed high prevalence of short stature in TM children and adolescence treated intensively with desferrioxamine (DFX)4,[12,13]. In addition, premature chelating therapy, between ages of 2-5 years, may have deleterious effects on growth [12,14,15]. DFX is proven to inhibit cell proliferation, DNA synthesis, collagen formation and trace mineral deposition such as cooper and zinc. Mineral depletion may result in a decrease of alkaline phosphatase activity. This complex mechanism results in platyspondylosis with flattening of the vertebral bodies and consequent shortening of the spinal height, resulting in truncal shortening , also in the presence of normal stature[1,2,15-20].
Body disproportion between the upper (short) and lower body segment (normal) is observed in approximately 15-40% of TM patients [18]. Spinal growth impairment starts during infancy and deteriorates progressively. Most Thalassaemics, especially males do not reach their target height and their Sitting height is more severely affected (Figure 1). During puberty hypogonadism further impairs spinal growth (Figure 2).


Sex steroids replacement
therapy cannot adversely affect body
disproportion, as no difference has been observed in pubertal growth
and final height between treated Hypogonadal patients compared to those
with spontaneous puberty and truncal shortening at final height is
evident in patients with either spontaneous or induced puberty [17].
Body disproportion therefore is independent of pubertal or prepubertal
period of greater height gain. Decrease in upper/lower segment ratio
has been reported in patients who have been poorly chelated during
childhood and adolescence, so that other contributing factors besides
chelation therapy or the disease itself influence spinal growth [21].
The hormonal cause of growth retardation in TM children is complex. Besides hypogonadism and hypothyroidism it has been apparent that GH-IGF-1 axis plays a role in their abnormal growth [22]. Evaluation of GH-IGF-1 axis has given contradictory results. The response of GH to stimulation tests has been found to be normal [23,24] or reduced with a variable prevalence [4,25-33] in a number of TM patients with short stature. De Sanctis et al reported 3.1% of thalassemics to have GHRH-GH-IGF axis dysfunction [21]. The presence of GH neurosecretory dysfunction is supported by the impaired 24 hour GH secretion [29,34-37] due to hypothalamic or pituitary dysfunction. The anterior pituitary is particularly sensitive to free radical oxidative stress. MRI of pituitary shows that even a modest amount of iron deposition within the anterior pituitary can interfere with its function [38]. Sex hormones play an important role in pubertal growth spurt and TM patients with delayed puberty do not exhibit normal growth spurt; their GH peak amplitude is reduced as well as their nocturnal GH levels [26,34,39]. Several authors have reported normal GH and GHBP levels but low levels of IGF-1 and IGFBP-3 which are not properly increased with IGF-1 generation test, suggesting that insensitivity to GH action may be the cause of abnormal growth [20,25,28,32,40-42]. However, a lack of correlation between IGF-1, IGFBP-3 and height SDS in TM children with growth failure may indicate that growth failure is not specifically related to GH-IGF-1 axis. There is a possibility that DFX and iron loading at the growth plate have a deleterious effect in local IGF-1 production and growth regulation [43,44].
Can children with TM attain normal stature and develop normally with early and reasonable DFX treatment? Although iron chelation can decrease the frequency of endocrinopathies, early DFX treatment may result in growth impairment. On the other hand poor compliance with DFX may eventually lead to severe iron burden, gonadal dysfunction and eventually growth failure. The benefits of treatment should be weighted against the potential adverse effects and the caring physician should balance between the efficacy and the injudicious use of DFX. An ideal therapeutic regimen, which will avoid the toxic effects of iron overload and that of continuous subcutaneous chelation therapy, has yet to be found. It is therefore recommended that growth in both standing and sitting position should be assessed at 6-month intervals in order to detect early growth failure. Long-term observations on the effect of therapy are needed before this mysterious puzzle is solved. Alternative oral chelation agents are often an option in cases of DFX toxicity, although some bone lesions remain irreversible. Prevention of growth retardation is essential. Monitoring growth in all children by using growth charts for both standing and sitting height is mandatory. The mean hemoglobin levels must be kept near 9 gr/dl. Prompt initiation of iron chelation therapy prevents pituitary haemosiderosis, which is the main cause of GH insufficiency.
Treatment with recombinant GH (rhGH) is recommended when GH deficiency is established.Therapeutic response with rhGH administration in cases with GH deficiency, is often non satisfactory. In poor responders such treatment should be discontinued. Treatment with rhGH for 1 year seems to be effective in increasing growth velocity without causing adverse effects on bone maturation, glucose tolerance, serum lipids and blood pressure [45-52]. The encouraging results described during the first year of rhGH treatment do not persist during the second and the third years. This is because increase in bone age with continued treatment is equal to or slightly greater than the height age increase [49]. Prolonged therapy with rhGH could not improve final height; on the contrary a negative effect may be hypothesized [52]. Growth acceleration is mostly promoted with sex steroids in children with pubertal delay as sexual complications present a significant issue in Thalassaemics.
The hormonal cause of growth retardation in TM children is complex. Besides hypogonadism and hypothyroidism it has been apparent that GH-IGF-1 axis plays a role in their abnormal growth [22]. Evaluation of GH-IGF-1 axis has given contradictory results. The response of GH to stimulation tests has been found to be normal [23,24] or reduced with a variable prevalence [4,25-33] in a number of TM patients with short stature. De Sanctis et al reported 3.1% of thalassemics to have GHRH-GH-IGF axis dysfunction [21]. The presence of GH neurosecretory dysfunction is supported by the impaired 24 hour GH secretion [29,34-37] due to hypothalamic or pituitary dysfunction. The anterior pituitary is particularly sensitive to free radical oxidative stress. MRI of pituitary shows that even a modest amount of iron deposition within the anterior pituitary can interfere with its function [38]. Sex hormones play an important role in pubertal growth spurt and TM patients with delayed puberty do not exhibit normal growth spurt; their GH peak amplitude is reduced as well as their nocturnal GH levels [26,34,39]. Several authors have reported normal GH and GHBP levels but low levels of IGF-1 and IGFBP-3 which are not properly increased with IGF-1 generation test, suggesting that insensitivity to GH action may be the cause of abnormal growth [20,25,28,32,40-42]. However, a lack of correlation between IGF-1, IGFBP-3 and height SDS in TM children with growth failure may indicate that growth failure is not specifically related to GH-IGF-1 axis. There is a possibility that DFX and iron loading at the growth plate have a deleterious effect in local IGF-1 production and growth regulation [43,44].
Can children with TM attain normal stature and develop normally with early and reasonable DFX treatment? Although iron chelation can decrease the frequency of endocrinopathies, early DFX treatment may result in growth impairment. On the other hand poor compliance with DFX may eventually lead to severe iron burden, gonadal dysfunction and eventually growth failure. The benefits of treatment should be weighted against the potential adverse effects and the caring physician should balance between the efficacy and the injudicious use of DFX. An ideal therapeutic regimen, which will avoid the toxic effects of iron overload and that of continuous subcutaneous chelation therapy, has yet to be found. It is therefore recommended that growth in both standing and sitting position should be assessed at 6-month intervals in order to detect early growth failure. Long-term observations on the effect of therapy are needed before this mysterious puzzle is solved. Alternative oral chelation agents are often an option in cases of DFX toxicity, although some bone lesions remain irreversible. Prevention of growth retardation is essential. Monitoring growth in all children by using growth charts for both standing and sitting height is mandatory. The mean hemoglobin levels must be kept near 9 gr/dl. Prompt initiation of iron chelation therapy prevents pituitary haemosiderosis, which is the main cause of GH insufficiency.
Treatment with recombinant GH (rhGH) is recommended when GH deficiency is established.Therapeutic response with rhGH administration in cases with GH deficiency, is often non satisfactory. In poor responders such treatment should be discontinued. Treatment with rhGH for 1 year seems to be effective in increasing growth velocity without causing adverse effects on bone maturation, glucose tolerance, serum lipids and blood pressure [45-52]. The encouraging results described during the first year of rhGH treatment do not persist during the second and the third years. This is because increase in bone age with continued treatment is equal to or slightly greater than the height age increase [49]. Prolonged therapy with rhGH could not improve final height; on the contrary a negative effect may be hypothesized [52]. Growth acceleration is mostly promoted with sex steroids in children with pubertal delay as sexual complications present a significant issue in Thalassaemics.
Protocol for investigation of Thalassaemic children:
- Measure current height both standing and sitting and plot on the growth chart. Calculate the target height based on parental heights. Compare with previous measurement to estimate the growth velocity. Examine pubertal status. Note any physical disproportion. Review emotional and social status.
- Assess bone maturation
- Routine blood tests including liver function tests, ferritin, biochemical profile, and zinc
- Urine analysis
- Thyroid function tests (Free T4, TSH), IFG-1 and IGFBP-3
Stimulation tests to assess GH
secretion, where at least two tests are
required. Priming with sex steroids (testosterone depot 100 mg IM in
boys and Ethinyl Estradiol 10 mcg orally for 3 days in girls 72hrs
before test may be necessary in children who are prepubertal and
have a bone age of 10 years.
IGF-1 generation test in patients with low levels of IFG-1 and IFGBP-3 and normal GH secretion to exclude GH resistance.
IGF-1 generation test in patients with low levels of IFG-1 and IFGBP-3 and normal GH secretion to exclude GH resistance.
Puberty
Is the period of life that
leads to adulthood through dramatic
physiologic and psychologic changes. It is the period during which
physical and hormonal changes occur such that the capability of sexual
reproduction is attained. This biological phenomenon, which is the
result of the activation of the Hypothalamic – Pituitary – Gonadal axis
and clinically manifested by the appearance of sexual characteristics
comprises this unique and integrated transition from childhood to young
adulthood.
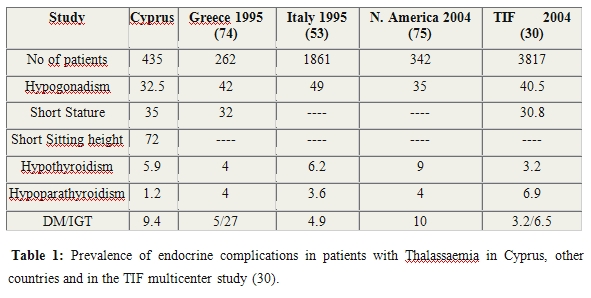
Sexual complications in TM
present the commonest endocrine complication in almost all studies (Table 1).
These include: Delayed Puberty, Arrested Puberty and Hypogonadism.
Delayed puberty is defined as the absence of any pubertal sign
in girls (breast enlargement) and in boys (testicular
enlargement) by the age of 13 and 14 years respectively. Delayed
puberty in TM is almost always due to Hypogonadotrophic Hypogonadism,
which still remains the most stressful complication [53].
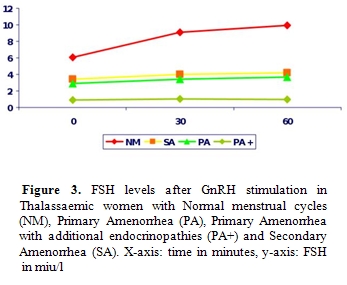
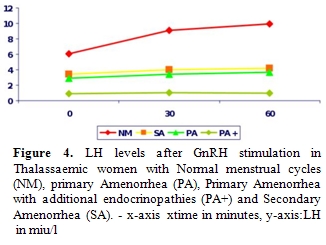
Iron deposition
on gonadotrophic cells of the pituitary leads to disruption of
gonadotrophin production which is proven by the poor response of FSH
and LH to GnRH stimulation and clinically manifested as
Hypogonadotrophic Hypogonadism (Figures
3 and 4).
Women have Primary Amenorrhea and additional endocrinopathies have a
completely blunted response to GnRH test indicating the severity of
haemosiderosis. Anterior pituitary function correlates well with tissue
iron deposition in the pituitary gland, as quantitatively determined by
MRI measurements (T2*) [38, 54].
However, there is no correlation
between MRI measurements, the GnRH stimulation test and the clinical
status of the patients [38].



The association of
susceptibility to develop Hypogonadotrophic
Hypogonadism with the genotype has already been proven [1,55,56]. The contribution of the
underlying molecular defect in TM to
the development of endocrinopathies in TM and particularly
Hypogonatotrophic Hypogonadism is significant, because the patients
with the more severe defects have a greater rate of iron loading
through higher red cell consumption and probably a different
vulnerability to free radical damage [1,55].
Hypogonatotrophic hypogonadism in Thalassaemia is related not only to iron toxicity on gonadotroph cells but also to iron toxicity on the adipose tissue thus changing the physiological role of leptin in sexual maturation and fertility. Leptin is a polypeptide hormone that is produced in fat cells due to the expression of the ob gene. In girls leptin levels increase dramatically as puberty develops and stimulate the Hypothalamic-Pituitary-Gonadal axis [57,58]. There is evidence that this hormone acts as a permissive signal allowing puberty to precede. The impaired synthesis of leptin in Thalassaemic patients seems to be related to transferrin receptor levels and therefore iron toxicity but further research will be required to elucidate this critical issue [59,60]. Gonadal iron deposition occasionally occurs. The iron deposition on the gonads is a rarer condition. In the majority of patients gonadal function is normal as most women with Amenorrhea are capable of ovulating with hormonal treatment and similarly men with azoospermia become fathers.
Arrested puberty is defined as the absence of further pubertal progression -once puberty has started -for more than one year, where testicular volume in boys never exists 6 to 8 ml and breast size in girls remains unchanged. Failure of sexual development by the age of 15 to 16 years in both sexes is defined as Hypogonadism. Secondary Hypogonadism appears later in life, and is manifested in women as Secondary Amenorrhea and in men as decline in sexual drive and azzoospermia.
Adolescent girls with TM often present with Primary Amenorrhea and boys fail to become well virilized. The damage to the hypothalamus and pituitary is progressive, even when intensive chelating therapy is given and the appearance of Hypogonadism in both sexes is often unavoidable [1,55,61]. Most women with TM manifest Secondary Amenorrhea at some stage in their life and men develop hypogonadism in their 3rd decade after being normal for some years and even becoming fathers [55,62,63].
The overall frequency of bone disease in hypogonadal patients with TM is increased compared with those of normal gonadal function [1,64]. Sex steroids regulate skeletal maturation and preservation in both men and women, therefore the impact of gonadal insufficiency on skeletal integrity has been widely recognised in both genders. BMD normally rises at a steady rate throughout childhood until around the age of 12, and then there is a sudden acceleration of bone mineral accretion which coincides with the onset of puberty and the pubertal growth spurt. Failure to progress normally through puberty is associated with failure of achievement of peak bone mass, which is a contributing factor to the ultimate bone disease in Thalassaemia [44,65,66].
Hypogonatotrophic hypogonadism in Thalassaemia is related not only to iron toxicity on gonadotroph cells but also to iron toxicity on the adipose tissue thus changing the physiological role of leptin in sexual maturation and fertility. Leptin is a polypeptide hormone that is produced in fat cells due to the expression of the ob gene. In girls leptin levels increase dramatically as puberty develops and stimulate the Hypothalamic-Pituitary-Gonadal axis [57,58]. There is evidence that this hormone acts as a permissive signal allowing puberty to precede. The impaired synthesis of leptin in Thalassaemic patients seems to be related to transferrin receptor levels and therefore iron toxicity but further research will be required to elucidate this critical issue [59,60]. Gonadal iron deposition occasionally occurs. The iron deposition on the gonads is a rarer condition. In the majority of patients gonadal function is normal as most women with Amenorrhea are capable of ovulating with hormonal treatment and similarly men with azoospermia become fathers.
Arrested puberty is defined as the absence of further pubertal progression -once puberty has started -for more than one year, where testicular volume in boys never exists 6 to 8 ml and breast size in girls remains unchanged. Failure of sexual development by the age of 15 to 16 years in both sexes is defined as Hypogonadism. Secondary Hypogonadism appears later in life, and is manifested in women as Secondary Amenorrhea and in men as decline in sexual drive and azzoospermia.
Adolescent girls with TM often present with Primary Amenorrhea and boys fail to become well virilized. The damage to the hypothalamus and pituitary is progressive, even when intensive chelating therapy is given and the appearance of Hypogonadism in both sexes is often unavoidable [1,55,61]. Most women with TM manifest Secondary Amenorrhea at some stage in their life and men develop hypogonadism in their 3rd decade after being normal for some years and even becoming fathers [55,62,63].
The overall frequency of bone disease in hypogonadal patients with TM is increased compared with those of normal gonadal function [1,64]. Sex steroids regulate skeletal maturation and preservation in both men and women, therefore the impact of gonadal insufficiency on skeletal integrity has been widely recognised in both genders. BMD normally rises at a steady rate throughout childhood until around the age of 12, and then there is a sudden acceleration of bone mineral accretion which coincides with the onset of puberty and the pubertal growth spurt. Failure to progress normally through puberty is associated with failure of achievement of peak bone mass, which is a contributing factor to the ultimate bone disease in Thalassaemia [44,65,66].
Protocol
for investigation of pubertal disorder:
The absence of any clinical pubertal signs in a boy (testicular enlargement) older that 14 years and in a girl (breast development) older than 13 years requires investigation.
Measure Testosterone in the boy and Oestradiol in the girl. DHEA-S in both sexes is often helpful
Perform the GnRH test to evaluate the pituitary capacity to secrete the gonadotropins FSH and LH, where the response in Hypogonadism is low
Bone age is helpful for the treatment decision options.
The absence of any clinical pubertal signs in a boy (testicular enlargement) older that 14 years and in a girl (breast development) older than 13 years requires investigation.
Measure Testosterone in the boy and Oestradiol in the girl. DHEA-S in both sexes is often helpful
Perform the GnRH test to evaluate the pituitary capacity to secrete the gonadotropins FSH and LH, where the response in Hypogonadism is low
Bone age is helpful for the treatment decision options.
Therapeutic
approach
In delayed puberty should mimic biological and biochemical pubertal changes, aiming on promotion of linear growth as well [67-70].
Induction of puberty in boys can be achieved with Testosterone depot IM 25-50mg monthly for 6 months and reassessment. Pubic hair will appear and penile size will increase. Increase in testicular volume indicates activation of the axis and release of Gonadotrophins (FSH and LH), where no further treatment is needed except for close observation. In case where testicular size is unchanged, then treatment is continued for 6 months and subsequently the dose is increased to 100 mg monthly for one year. Therapeutic schedule is determined by the growth potential, clinical response and emotional factors. For testicular enlargement, the therapeutic regime is altered to the combination of hCG and hMG or recFSH, both of which mimic the pituitary Gonadotrophins. The final adult dose of Testosterone depot, is always individualized and usually 50mg/weekly IM or alternatively transdermally in patches 5 mg/daily. The oral route (testosterone undeconate) should be avoided due to liver toxicity.
For pubertal induction in girls oral Ethinylestradiol is preferred at the dose 100 ng/kg/d for 6 months, where increase in breast size and growth acceleration is noted. This dose is continued for additional 6 months and increased to 200 ng/kg/d for the subsequent year. Therapeutic schedule is determined by the same factors as in boys. The adult dose is 400 ng/kg/d, where the uterine size is satisfactorily increased for the induction of menarche. Induction of puberty can be successfully achieved by the transdermal use of Estrogens.
Menarche is achieved by the addition of Medroxyprogesterone 10 mg/d for 10 days when the size of the uterus exceeds 5 cm. When menstrual bleeding occurs spontaneously during Estrogen treatment, the regime should be adjusted. For maintenance of the menstrual cycle the use of Estrogens (Conjugated Estrogens 0.625 ή 1.25 mg, Ethinyl Estradiol 20 μg) from day 1st to 25th and Progesterone from day 14th to 25th is required. The transdermal use of Estradiol και Norethisterone is advantageous due to decreased liver toxicity and preferred in most cases.
Normal sexual activity and reproductive capacity have become demanding tasks for women with TM. Despite the presence of Hypogonadotrophic Hypogonadism and severe iron deposition, ovarian function may be preserved, as they are still able to increase oestradiol level following gonadotrophin stimulation test and produce ova [71,72]. Women with TM who are regularly transfused and are well chelated become able to conceive after a closely monitored treatment [63,73]. Males who have normal gonadal function maintain their spermatogenic ability and therefore, frequently become fathers. On the other side of the spectrum, in cases where impaired spermatogenesis is present, a combination treatment with hCG and hMG/ recFSH has proven to be beneficial in improving their reproductive capacity [70].
In delayed puberty should mimic biological and biochemical pubertal changes, aiming on promotion of linear growth as well [67-70].
Induction of puberty in boys can be achieved with Testosterone depot IM 25-50mg monthly for 6 months and reassessment. Pubic hair will appear and penile size will increase. Increase in testicular volume indicates activation of the axis and release of Gonadotrophins (FSH and LH), where no further treatment is needed except for close observation. In case where testicular size is unchanged, then treatment is continued for 6 months and subsequently the dose is increased to 100 mg monthly for one year. Therapeutic schedule is determined by the growth potential, clinical response and emotional factors. For testicular enlargement, the therapeutic regime is altered to the combination of hCG and hMG or recFSH, both of which mimic the pituitary Gonadotrophins. The final adult dose of Testosterone depot, is always individualized and usually 50mg/weekly IM or alternatively transdermally in patches 5 mg/daily. The oral route (testosterone undeconate) should be avoided due to liver toxicity.
For pubertal induction in girls oral Ethinylestradiol is preferred at the dose 100 ng/kg/d for 6 months, where increase in breast size and growth acceleration is noted. This dose is continued for additional 6 months and increased to 200 ng/kg/d for the subsequent year. Therapeutic schedule is determined by the same factors as in boys. The adult dose is 400 ng/kg/d, where the uterine size is satisfactorily increased for the induction of menarche. Induction of puberty can be successfully achieved by the transdermal use of Estrogens.
Menarche is achieved by the addition of Medroxyprogesterone 10 mg/d for 10 days when the size of the uterus exceeds 5 cm. When menstrual bleeding occurs spontaneously during Estrogen treatment, the regime should be adjusted. For maintenance of the menstrual cycle the use of Estrogens (Conjugated Estrogens 0.625 ή 1.25 mg, Ethinyl Estradiol 20 μg) from day 1st to 25th and Progesterone from day 14th to 25th is required. The transdermal use of Estradiol και Norethisterone is advantageous due to decreased liver toxicity and preferred in most cases.
Normal sexual activity and reproductive capacity have become demanding tasks for women with TM. Despite the presence of Hypogonadotrophic Hypogonadism and severe iron deposition, ovarian function may be preserved, as they are still able to increase oestradiol level following gonadotrophin stimulation test and produce ova [71,72]. Women with TM who are regularly transfused and are well chelated become able to conceive after a closely monitored treatment [63,73]. Males who have normal gonadal function maintain their spermatogenic ability and therefore, frequently become fathers. On the other side of the spectrum, in cases where impaired spermatogenesis is present, a combination treatment with hCG and hMG/ recFSH has proven to be beneficial in improving their reproductive capacity [70].
Conclusion
Several
endocrine glands may be affected in patients with TM in childhood,
adolescence and adulthood. Pituitary damage due to iron overload is the
underlying pathogenetic factor in hypogonadism and partly contributes
to poor growth. Luckily these complications do not adversely affect the
life expectancy of these individuals. Close follow up and proper
management is crucial for every patient. Early recognition of growth
disturbance and prevention of hypogonadism by early and judicious
chelation therapy is mandatory for the improvement of their quality of
life. Patients with TM can now live a better life due to modern
advances in their medical care and our better understanding in the
pathogenesis, manifestation and prevention of endocrine complications.
References
- Skordis N, Michaelidou M, Savva SC,
Ioannou Y, Rousounides A, Kleanthous M, Skordos G, Christou S.
The impact of Genotype on Endocrine complications in Thalassaemia
major. Eur J Haematol. 2006 Aug;77(2):150-6. Epub 2006.

- Rodda CP, Reid ED, Johnson S, Doery J,
Matthews R, Bowden DK. Short stature in homozygous beta-thalassaemia is
due to disproportionate truncal shortening. Clin Endocrinol (Oxf). 1995
Jun; 42(6):587-92.

- Skordis N. The growing child with
Thalassaemia. J Pediatr Endocrinol Metab 2006; 19: 467-9.

- De Sanctis V, Roos M, Gasser T, Fortini M,
Raiola G, Galati MC; Italian Working Group on Endocrine Complications
in Non-Endocrine Diseases. Impact of long-term iron chelation therapy
on growth and endocrine functions in Thalassaemia. J Pediatr Endocrinol
Metab. 2006 Apr;19(4):471-80.

- Kattamis C, Liakopoulou T, Kattamis A.
Growth and development in children with thalassaemia major. Acta
Paediatr Scand (Suppl.) 1990; 366:111-7; discussion 118.

- Spiliotis BE. Beta-thalassemia and normal
growth: are they compatible? Eur J Endocrinol. 1998 Aug;139(2):143-4.

- Arcasoy A, Cavdar A, Cin S, Erten J,
Babacan E, Gözdasoglu S, Akar N. Effects of zinc supplementation on
linear growth in beta-thalassemia (a new approach). Am J Hematol.
1987 Feb; 24(2):127-36.

- Eshghi P, Alavi S, Ghavami S, Rashidi A.
Growth impairment in beta-thalassemia major: the role of trace element
deficiency and other potential factors. J Pediatr Hematol Oncol. 2007
Jan;29(1):5-8.

- Mehdizadeh M, Zamani G, Tabatabaee S. Zinc
status in patients with major beta-thalassemia. Pediatr Hematol Oncol.
2008 Jan-Feb;25(1):49-54.

- Shalitin S, Carmi D, Weintrob N, Phillip
M, Miskin H, Kornreich L, Zilber R, Yaniv I, Tamary H. Serum ferritin
level as a predictor of impaired growth and puberty in thalassemia
major patients. Eur J Haematol. 2005 Feb;74(2):93-100.

- García-Mayor RV, Andrade Olivie A,
Fernández Catalina P, Castro M, Rego Iraeta A, Reparaz A. Linear growth
in thalassemic children treated with intensive chelation therapy. A
longitudinal study. Horm Res. 1993;40(5-6):189-93.

- De Virgiliis S, Congia M, Frau F, Argiolu
F, Diana G, Cucca F, Varsi A, Sanna G, Podda G, Fodde M, et al.
Deferoxamine-induced growth retardation in patients with thalassemia
major. J Pediatr. 1988 Oct;113(4):661-9.

- Olivieri NF, Koren G, Harris J, Khattak S,
Freedman MH, Templeton DM, Bailey JD, Reilly BJ. Growth failure and
bony changes induced by deferoxamine. Am J Pediatr Hematol Oncol. 1992
Spring;14(1):48-56.

- De Luca F, Simone E, Corona G, Pandullo E,
Siracusano MF, Arrigo T. Adult height in thalassaemia major without
hormonal treatment. Eur J Pediatr. 1987 Sep;146(5):494-6.

- De Sanctis V, Katz M, Vullo C, Bagni B,
Ughi M, Wonke B. Effect of different treatment regimes on linear growth
and final height in beta-thalassaemia major. Clin Endocrinol (Oxf).
1994 Jun;40(6):791-8.

- Skordis N, Endocrine complications in Cypriot Thalassaemic patients. In: S. Ando and C. Brancati (eds): Endocrine Disorders in Thalassaemia. Heidenberg: Springer Verlag Publ.1995:83-89.
- Filosa A, Di Maio S, Baron I, Esposito G,
Galati MG. Final height and body disproportion in Thalassaemic boys and
girls with spontaneous or induced puberty. Acta Paediatr 2000;
89:1295-1301

- Caruso-Nicoletti M, De Sanctis V, Capra M,
Cardinale G, Cuccia L, Di Gregorio F, Filosa A, Galati MC, Lauriola A,
Malizia R, Mangiagli A, Massolo F, Mastrangelo C, Meo A, Messina MF,
Ponzi G, Raiola G, Ruggiero L, Tamborino G, Saviano A. Short stature
and body proportion in thalassaemia. J Pediatr Endocrinol Metab.
1998;11 Suppl 3:811-6.

- Caruso-Nicoletti M, Di Bella D, Pizzarelli
G, Leonardi C, Sciuto C, Coco M, Di Gregorio F. Growth failure and bone
lesions due to desferrioxamine in thalassaemic patients. J Pediatr
Endocrinol Metab. 1998;11 Suppl 3:957-60.

- Low CK, Kwan YW, Cheung PT, Li MC, Ha SY,
Lau YL, Karlberg J.The effect of platyspondyly and pubertal growth
spurt on the stature of patients with beta-thalassaemia major. Chin Med
J (Engl). 1998 Aug;111(8):731-5.

- De Sanctis V. Growth and puberty and its
management in Thalassaemia. Horm Res 2002; 58(S1): 72-79.

- Toumba M, Sergis A, Kanaris C, Skordis N.
Endocrine complications in patients with Thalassaemia major. Pediatric
Endocrine Reviews 2007;5:642-648.

- Karydis I, Karagiorga-Lagana M,
Nounopoulos C, Tolis G.Basal and stimulated levels of growth hormone,
insulin-like growth factor-I (IGF-I), IGF-I binding and IGF-binding
proteins in beta-thalassemia major. J Pediatr Endocrinol Metab. 2004
Jan;17(1):17-25.

- Leger J, Girot R, Crosnier H, Poste-Vinay
MC, Rappaport R. Normal growth hormone (GH) response to GH-releasing
hormone in children with thalassaemia major before puberty: a possible
age related effect. J Clin Endocrinol Metab 1989; 69:453-456.

- Soliman T, EL Banna N, Ansari M. GH
response to provocation and circulating IGF-1 and IGF-binding protein-3
concentrations, the IGF-1 generation test and clinical response to GH
therapy in children with beta-thalassaemia. Eur J Endocrinol 1998;
138:394-400.

- De Luca G, Maggiolini M, Bria M,
Caracciolo M, Giorno A, Salemo M, Marisco S, Lanzino M, Brancati C,
Ando S. GH secretion in thalassaemia patients with short stature. Horm
Res 1995; 44: 158-163.

- Pérignon F, Brauner R, Souberbielle JC, de
Montalembert M, Girot R. Growth and endocrine function in major
thalassemia. Arch Fr Pediatr. 1993 Oct;50(8):657-63.

- Cavallo L, Gurrado R, Gallo F, Zacchino C,
De Mattia D, Tatň L. Growth deficiency in polytransfused
beta-thalassaemia patients is not growth hormone dependent. Clin
Endocrinol (Oxf). 1997 Jun;46(6):701-6.

- Soliman AT, elZalabany MM, Mazloum Y,
Bedair SM, Ragab MS, Rogol AD, Ansari BM. Spontaneous and provoked
growth hormone (GH) secretion and insulin-like growth factor I (IGF-I)
concentration in patients with beta thalassaemia and delayed growth. J
Trop Pediatr. 1999 Dec;45(6):327-37.

- De Sanctis V, Eleftheriou A, Malaventura
C. Prevalence of endocrine complications and short stature in patients
with thalassaemia major: a multicenter study by the Thalassaemia
International Federation (TIF). Thalassaemia International Federation
Study Group on Growth and Endocrine Complications in Thalassaemia.
Pediatr Endocrinol Rev. 2004 Dec;2 Suppl 2:249-55.

- Chrysis DC, Alexandrides TK, Koromantzou
E, Georgopoulos N, Vassilakos P, Kiess W, Kratsch J, Beratis NG,
Spiliotis BE. Novel application of IGF-I and IGFBP-3 generation tests
in the diagnosis of growth hormone axis disturbances in children with
beta-thalassaemia. Clin Endocrinol (Oxf). 2001 Feb;54(2):253-9.

- Moayeri H, Oloomi Z. Prevalence of growth
and puberty failure with respect to growth hormone and gonadotropins
secretion in beta-thalassemia major. Arch Iran Med. 2006
Oct;9(4):329-34.

- Masala A, Meloni T, Gallisai D, Alagna S,
Rovasio PP, Rassu S, Milia AF.Endocrine functioning in multitransfused
prepubertal patients with homozygous beta-thalassemia. J Clin
Endocrinol Metab. 1984 Apr;58(4):667-70.

- Shehadeh N, Hazani A, Rudolf MCJ, Peleg I,
Benderly A, Hochberg Z. Neurosecretory dysfunction of growth hormone
secretion in thalassaemia major. Acta Paediatr Scand 1990; 79: 790-795.

- Katzos G, Harsoulis F, Papadopoulou M,
Athanasiou M, Sava K. Circadian growth hormone secretion in short
multitransfused prepubertal children with thalassaemia major. Eur J
Pediatr. 1995 Jun;154(6):445-9.

- Chatterjee R, Katz M, Cox T, Bantock H.
Evaluation of growth hormone in thalassaemic boys with failed puberty:
spontaneous versus provocative test. Eur J Pediatr. 1993
Sep;152(9):721-6.

- Roth C, Pekrun A, Bartz M, Jarry H, Eber
S, Lakomek M, Schröter W. Short stature and failure of pubertal
development in thalassaemia major: evidence for hypothalamic
neurosecretory dysfunction of growth hormone secretion and defective
pituitary gonadotropin secretion. Eur J Pediatr. 1997
Oct;156(10):777-83.

- Berkovitch M, Bistritzer T, Milone SD,
Perlman K, Kucharczyk W, Olivieri NF.Iron deposition in the anterior
pituitary in homozygous beta-thalassemia: MRI evaluation and
correlation with gonadal function. J Pediatr Endocrinol Metab. 2000
Feb;13(2):179-84.

- Leheup BP, Cisternino M, Bozzola M,
Donsset B, Marvadi PL, Antoniazzi F, Tato L, Severi F, Sommelet D,
Pierson M. Growth hormone response following growth hormone releasing
hormone injection in thalassaemia major: influence of pubertal
development. J Endocrinol Invest 1991; 14:37-40.

- Oerter KE, Kamp GA, Munson PJ, Nienhuis
AW, Cassorla FG, Manasco PK. Multiple hormone deficiencies in children
with haemochromatosis. J Clin Endocrinol Metab 1993; 76: 357-361.

- Karamifar H, Karimi M, Amirhakimi G,
Sharbatialaei M, De Sanctis V. Reduced insulin growth factor I
concentrations in iron-overloaded beta thalassaemic patients with
normal growth hormone secretion and liver function. Pediatr Endocrinol
Rev 2004; 2 (Suppl 2):256-258.

- Scacchi M, Danesi L, Cattaneo A, Valassi
E, Pecori Giraldi F, Argento C, D'Angelo E, Mirra N, Carnelli V,
Zanaboni L, Cappellini MD, Cavagnini F. Growth hormone deficiency
(GHD) in adult thalassaemic patients. Clin Endocrinol (Oxf). 2007;
67(5):790-795.

- Tiosano D, Hochberg Z. Endocrine
complications of thalassaemia. J Endocrinol 2001; 24:716-723.

- Skordis N, Efstathiou E, Kyriakou A,
Toumba M. Hormonal dysregulation and bones in thalassaemia--an
overview. Pediatr Endocrinol Rev. 2008 Oct;6 Suppl 1:107-15.

- Arcasoy A, Ocal G, Kemahli S, Berberoğlu
M, Yildirmak Y, Canatan D, Akçurin S, Akar N, Uysal Z, Adiyaman P,
Cetinkaya E. Recombinant human growth hormone treatment in children
with thalassemia major. Pediatr Int. 1999 Dec;41(6):655-61.

- Katzos G, Papakostantinou-Athanasiadou E,
Athanasiou-Metaxa M, Harsoulis F. Growth hormone treatment in short
children with beta-thalassemia major. J Pediatr Endocrinol Metab. 2000
Feb;13(2):163-70.

- Sartorio A, Conte G, Conti A, Masala A,
Alagna S, Rovasio P, Faglia G. Effects of 12 months rec-GH therapy on
bone and collagen turnover and bone mineral density in GH deficient
children with thalassaemia major. J Endocrinol Invest. 2000
Jun;23(6):356-61.

- Kwan EY, Tam SC, Cheung PT, Low LC. The
effect of 3 years of recombinant growth hormone therapy on glucose
metabolism in short Chinese children with beta-thalassemia major. J
Pediatr Endocrinol Metab. 2000 May;13(5):545-52.

- Cavallo L, Acquafredda A, Zecchino C, De
Sanctis V, Cisternino M, Caruso Nicoletti M, Galati M, Massolo F.
Recombinant growth hormone treatment in short patients with thalassemia
major: results after 24 and 36 months. J Pediatr Endocrinol Metab. 2001
Sep-Oct;14(8):1133-7.

- Masala A, Atzeni MM, Alagna S, Gallisai D,
Burrai C, Mela MG, Rovasio PP, Gallo P. Growth hormone secretion in
polytransfused prepubertal patients with homozygous beta-thalassemia.
Effect of long-term recombinant GH (recGH) therapy. J Endocrinol
Invest. 2003 Jul;26(7):623-8.

- Wu KH, Tsai FJ, Peng CT. Growth hormone
(GH) deficiency in patients with beta-
thalassemia major and the efficcy of recombinant GH treatment. Ann
Hematol. 2003 Oct;82(10):637-40. Epub 2003 Jul 31.

- Cavallo L, De Sanctis V, Cisternino M,
Caruso Nicoletti M, Galati MC, Acquafredda A, Zecchino C, Delvecchio M.
Final height in short polytransfused thalassemia major patients treated
with recombinant growth hormone. J Endocrinol Invest. 2005
Apr;28(4):363-6.

- Italian Working Group on Endocrine
Complications in Non-Endocrine Diseases. Multicentre study on
prevalence of endocrine complications in Thalassaemia major. Clin
Endocrinol 1995; 42: 581-586.

- Christoforidis A, Haritandi A, Perifanis
V, Tsatra I, Athanassiou-Metaxa M, Dimitriadis AS. MRI for the
determination of pituitary iron overload in children and young adults
with beta-thalassaemia major. Eur J Radiol. 2007 Apr;62(1):138-42. Epub
2006 Dec 11.

- Skordis N , Gourni M, Kanaris C, Toumba M,
Kleanthous M, Karatzia N, Pavlides N, Angastiniotis M. The
impact of iron overload and genotype on gonadal function in women with
thalassaemia major. Pediatr Endocrinol Rev 2004;2(Suppl2):292-5.

- Chern JP, Lin KH, Tsai WY, Wang SC, Lu MY,
Lin DT, Lin KS, Lo SH. Hypogonadotropic hypogonadism and hematologic
phenotype in patients with transfusion-dependent beta-thalassemia. J
Pediatr Hematol Oncol. 2003 Nov;25(11):880-4.

- Kiess W, Reich A, Meyer K, Glasow A,
Deutscher J, Klammt J, Yang Y, Müller G, Kratzsch J. A role for leptin
in sexual maturation and puberty? Horm Res 1999; 51 (S3): 55-63.

- Teirmaa T, Luukkaa V, Rourou J, Koulu M,
Huupponen R. Correlation between circulating leptin and LH during the
menstrual cycle in normal-weight women. Eur J Endocrinol 1998; 139:
190-194.

- Perrone L, Perrotta S, Raimondo P,
Mucerino J, De Rosa C, Siciliani MC, Santoro N, Del Giudice EM.
Inappropriate leptin secretion in thalassaemia: A potential cofactor of
pubertal timing derangement. J Pediatr Endocrinol Metab 2003; 16:
877-881.

- Dedousis GVZ, Kyrtsonis MC, Andrikopoulos
NE, Voskaridou E, Loutradis A. Inverse correlation of plasma leptin and
soluble transferrin receptor levels in β-thalassaemia patients. Ann
Hematol 2002; 81: 543-547.

- Chatterjee R, Katz M, Cox TF, Porter JB.
Prospective study of the hypothalamic-pituitary axis in thalassaemic
patients who developed secondary amenorrhoea. Clin Endocrinol (Oxf).
1993 Sep;39(3):287-96.

- De Sanctis V, Vullo C, Katz M, Wonke B,
Tanaw R, Bagni B. Gonadal function in patients with B –
Thalassaemia Major. J Clin Pathol 1988; 41:133-137.

- Skordis N, Petrikkos L, Toumba M,
Hadjigavriel M, Sitarou M, Kolnakou A, Skordos G, Pangalou E, Christou
S. Update on fertility in thalassaemia major. Pediatr Endocrinol Rev.
2004 Dec;2 Suppl 2:296-302.

- Kyriakou A, Savva SC, Savvides I, Pangalou
E, Ioannou YS, Christou S, Skordis N. Gender differences in the
prevalence and severity of bone disease in thalassaemia. Pediatr
Endocrinol Rev. 2008 Oct;6 Suppl 1:116-22.

- Bielinski K, Darbyshire J, Mathers L,
Crabtree J, Kirk M, Stirling F, Shaw J. Impact of disordered puberty on
bone density in beta-thalassaemia major. Br J Haematol 2003;
120:353-358.

- Anapliotou M.L, Kastanias I.T, Psara P,
Evangelou E.A, Liparaki M., Dimitriou, P. The contribution of
hypogonadism to the development of osteoporosis in thalassaemia major:
new therapeutic approaches. Clin Endocrinol. 1995; 42: 279–287.

- Pozo J, Argente J. Ascertainment and
treatment of delayed puberty. Horm Res. 2003; 60 (S3):35-48.

- Mac Gillivray MH. Induction of puberty in
Hypogonadal children. J Pediatr Endocrinol Metab 2004;17(S4):1277-1287.

- Caruso –Nicoletti M, De Sanctis V, Cavallo
L, Raiola G, Ruggiero L,Skordis N, Wonke B. Manegement of puberty for
optimal Auxological results. J Pediatr Endocrinol Metab
2001;14(S2):939-944.

- De Sanctis V, Vullo C, Katz M, Wonke B,
Nannetti C, Bagni B. Induction of spermatogenesis in
Thalassaemia. Fertility Sterility 1998; 50: 969-975.

- Skordis N, Christou S, Koliou M, Pavlides
N, Angastiniotis M. Fertility in female patients with thalassemia. J
Pediatr Endocrinol Metab. 1998;11 Suppl 3:935-43.

- Jensen CE, Tuck SM, Wonke B. Fertility in
beta thalassaemia major: a report of 16 pregnancies, preconceptual
evaluation and a review of the literature.
Br J Obstet Gynaecol. 1995 Aug;102(8):625-9.

- Toumba M, Kanaris C, Simamonian K, Skordis
N. Outcome and management of pregnancy in women with thalassaemia in
Cyprus. East Mediterr Health J. 2008 May-Jun;14(3):628-35.

- Kattamis CA, Kattamis AC. Management of
thalassaemia: growth and development, hormone substitution, vitamin
supplementation and vaccination. Sem Hematol 1995; 32:269-279.

- Cunningham M, Macklin E, Neufeld E, Cohen
A, for the Thalassemia Clinical Research Network. Complications of
thalassemia major in North America. Blood 2004; 104: 34-39.
