Thalassaemia Intermedia: an Update
Ali T. Taher1, Khaled M. Musallam1 and Maria D. Cappellini2
1Department of Internal Medicine, Hematology-Oncology Division, American University of Beirut Medical Centre, Beirut, Lebanon
2Universitá di Milano, Policlinico Foundation IRCCS, Milan, Italy
Correspondence
to:
Ali T. Taher, MD, Professor of Medicine, Hematology-Oncology Division,
Department of Internal Medicine, American University of Beirut Medical
Center. Phone: +961-1-350000; Fax: +961-1-370814; Email: ataher@aub.edu.lb
Published: August 29, 2009
Received: August 10, 2009
Accepted: August 19, 2009
Medit J of Hemat Infect Dis 2009, 1 (1): e2009004; DOI 10.4084/MJHID.2009.004
This article is available from: http://www.mjhid.org/article/view/4709
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Our
understanding of the molecular and pathophysiological mechanisms
underlying the disease process in patients with thalassaemia intermedia
(TI) has substantially increased over the past decade. TI encompasses a
wide clinical spectrum of beta-thalassaemia phenotypes. Some TI
patients are asymptomatic until adult life, whereas others are
symptomatic from as young as 2 years of age. A number of clinical
complications commonly associated with TI are rarely seen in
thalassaemia major, including extramedullary hematopoiesis, leg ulcers,
gallstones, thrombosis and pulmonary hypertension. There are a number
of options currently available for managing patients with TI, including
transfusion therapy, iron chelation therapy, modulation of foetal
haemoglobin production and haematopoietic stem cell transplantation.
However, at present, there are no clear guidelines for an orchestrated
optimal treatment plan.
Introduction


Thalassaemia was
defined as a clinical entity in 1925 by Dr. Thomas B. Cooley at the
annual meeting of the American Pediatric Society where he presented
five young children with severe anaemia, splenomegaly, and peculiar
bone abnormalities [1]. Almost all patients with
β-thalassaemia syndromes were later classified as having thalassaemia
minor or thalassaemia major (TM). However, a small number of patients
did not fit into this categorical distribution. Sturgeon was the first
to describe this group in the American literature. He suggested the
term thalassaemia intermedia (TI) to describe patients who had clinical
manifestations that are too severe to be termed minor and too mild to
be termed major [2]. Ever since, our knowledge of the
molecular basis and pathophysiology of TI has progressed significantly
in the last decade, including an increased understanding of the genetic
mutations that lead to the TI phenotypes [3,4]. The
clinical presentation and sequelae of TI span a wide spectrum of
severity. The natural history of the disease allows the emergence of
several complications that are relatively unique to TI compared to TM5.
As such, the optimal course of management for TI patients has been hard
to identify and several controversies remain regarding the best
treatment plan6. This review summarizes molecular and clinical
characteristics of TI. Moreover, currently practiced treatment options
and insights onto future perspectives are discussed.
Molecular
Understanding
The
clinical
manifestations of thalassaemia result from defects in one of two types
of haemoglobin (Hb) polypeptide chains (alpha or beta). For Hb to
function properly, the number of alpha-chains must precisely match the
number of beta-chains; thalassaemia is caused by an imbalance in globin
chain synthesis. The beta-thalassaemias, including TI, arise from
defective gene function leading to the partial suppression of
beta-globin protein production. The extent of suppression varies from
patient to patient and dictates the clinical severity of disease. Most
TI patients are homozygotes or compound heterozygotes for
beta-thalassaemia, meaning that both beta-globin loci are affected [3]. Less commonly, only a single beta-globin locus is
affected, the other being completely normal [1].
The mild clinical characteristics of TI compared with TM major result
primarily from three different mechanisms [3,7]:
- Inheritance of a mild or silent beta-chain mutation. Rather than a complete absence of beta-chain synthesis, the level of synthesis is subnormal. This leads to a smaller imbalance between the number of alpha-and beta-chains compared with an absence of beta-chains.
- Co-inheritance of determinants associated with increased gamma-chain production. The increased number of gamma-chains helps to neutralize the large proportion of unbound alpha-chains.
- Co-inheritance of alpha-thalassaemia. This helps to suppress the synthesis of alpha-chains, causing less of an alpha/beta-chain imbalance.
The phenotype of TI may result
from the increased production of
alpha-globin chains by a triplicated or quadruplicated alpha genotype
associated with beta-heterozygosity [8,9,10]. Table 1
shows β-globin mutations in TI and TM that have a direct effect on
modifying the amount of excess alpha-chains, such as inheritance of
abnormal alpha- or gamma-chain genes. Tertiary modifiers are
polymorphisms occurring at loci involved in bone, iron and bilirubin
metabolism which can affect clinical expression, although these are
thought to be of relatively little importance. Recent studies of the
JAK2 cytoplasmic tyrosine kinase, which has a vital role in signal
transduction from several haemopoietic growth factor receptors,
revealed a V617F mutation that was implicated in a variety of diseases
mainly related to myelo-proliferative disorders including polycythaemia
vera, essential thrombocythaemia, and idiopathic myelofibrosis. TI
patients, however, do not show increased expression of this mutation[11].

Environmental factors including
social conditions, nutrition and the
availability of medical care have also been implicated as key players
in the phenotypic variability of TI [12].
A number of studies have attempted to classify patients with TI according to the severity of their condition, although these studies have had only limited success [13,14]. A recent study described the development of a phenotype scoring system that successfully sub-classified TI patients into three separate groups: mild, moderate or severe [15]. The severity of TI was graded according to a number of clinical features, such as age at presentation, severity of anaemia, extent of growth retardation and bone marrow hyperplasia, blood transfusion requirements and need for splenectomy. This classification could prove useful for relating genotype to phenotype and for developing separate treatment guidelines for different disease severities. However, further studies would be required to confirm the reliability and utility of this approach.
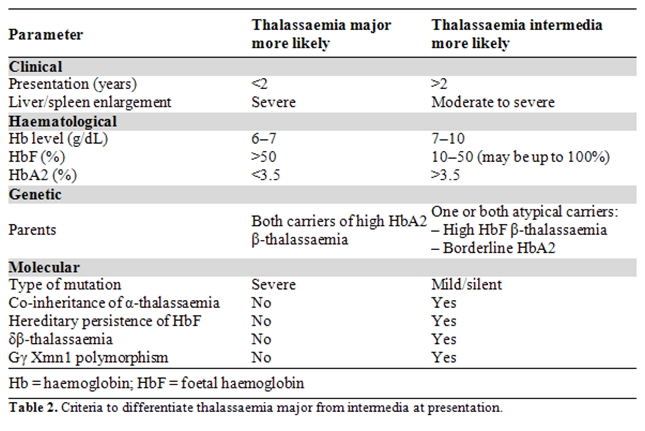
Although the clinical phenotypes of thalassaemia minor, intermedia and major differ, there are some similarities. There is an increasing awareness of the need for accurate diagnosis in order to achieve optimal patient management and to avoid over or under treatment [3,16]. The accurate identification of TI versus thalassaemia minor and TM can be difficult if based on clinical presentation alone, although certain differentiating parameters have been established. In general, TI is characterized by Hb levels maintained around 7–10 g/dL without the need for regular blood transfusions, by more severe red blood cell (RBC) abnormalities than thalassaemia minor, by a varying degree of spleen enlargement, and by skeletal changes such as expansion of the facial bones and obliteration of the maxillary sinuses, which causes protrusion of the upper jaw [5,6,16]. Criteria for differentiating TM from TI at presentation are summarised in Table 2.
A number of studies have attempted to classify patients with TI according to the severity of their condition, although these studies have had only limited success [13,14]. A recent study described the development of a phenotype scoring system that successfully sub-classified TI patients into three separate groups: mild, moderate or severe [15]. The severity of TI was graded according to a number of clinical features, such as age at presentation, severity of anaemia, extent of growth retardation and bone marrow hyperplasia, blood transfusion requirements and need for splenectomy. This classification could prove useful for relating genotype to phenotype and for developing separate treatment guidelines for different disease severities. However, further studies would be required to confirm the reliability and utility of this approach.
Although the clinical phenotypes of thalassaemia minor, intermedia and major differ, there are some similarities. There is an increasing awareness of the need for accurate diagnosis in order to achieve optimal patient management and to avoid over or under treatment [3,16]. The accurate identification of TI versus thalassaemia minor and TM can be difficult if based on clinical presentation alone, although certain differentiating parameters have been established. In general, TI is characterized by Hb levels maintained around 7–10 g/dL without the need for regular blood transfusions, by more severe red blood cell (RBC) abnormalities than thalassaemia minor, by a varying degree of spleen enlargement, and by skeletal changes such as expansion of the facial bones and obliteration of the maxillary sinuses, which causes protrusion of the upper jaw [5,6,16]. Criteria for differentiating TM from TI at presentation are summarised in Table 2.

Pathophysiology
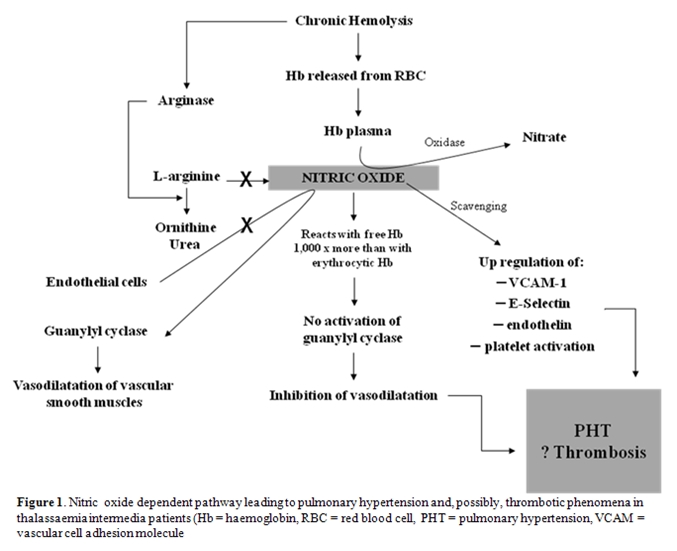
Three main factors are responsible for the clinical manifestations of TI: ineffective erythropoiesis, chronic haemolytic anaemia and iron overload. The severity of clinical sequelae primarily depends on the underlying molecular defects. Alpha-chains are highly unstable and precipitate within erythroid precursors in the bone marrow, causing membrane damage and cell death – this is ineffective erythropoiesis [17]. Hypertrophy of erythroid marrow in medullary and extramedullary sites, a consequence of severe ineffective erythropoiesis, results in characteristic deformities of the skull and face and may also cause cortical thinning and pathological fractures of long bones [16,18]. The degree of ineffective erythropoiesis is the primary determinant of the development of anaemia, while peripheral haemolysis of mature RBCs and an overall reduction in Hb synthesis are secondary. However, haemolysis per se has been linked to the development of a hypercoagulable state and pulmonary hypertension (PHT) in this patient population [19,20]. Damaged circulating RBCs expose negatively charged phosphatidyl-serine residues through the ‘Flip-Flop’ phenomenon [21]. These along with activation of platelets, endothelial cells and monocytes along with dysfunction of the coagulation system have been associated with thromboembolic phenomenon, especially in splenectomised TI patients [21]. Moreover, haemolysis carries a role in the dysregulation of nitric oxide (NO) homeostasis which is correlated with PHT and probably thrombotic phenomena [22] (Figure 1).
Three main factors are responsible for the clinical manifestations of TI: ineffective erythropoiesis, chronic haemolytic anaemia and iron overload. The severity of clinical sequelae primarily depends on the underlying molecular defects. Alpha-chains are highly unstable and precipitate within erythroid precursors in the bone marrow, causing membrane damage and cell death – this is ineffective erythropoiesis [17]. Hypertrophy of erythroid marrow in medullary and extramedullary sites, a consequence of severe ineffective erythropoiesis, results in characteristic deformities of the skull and face and may also cause cortical thinning and pathological fractures of long bones [16,18]. The degree of ineffective erythropoiesis is the primary determinant of the development of anaemia, while peripheral haemolysis of mature RBCs and an overall reduction in Hb synthesis are secondary. However, haemolysis per se has been linked to the development of a hypercoagulable state and pulmonary hypertension (PHT) in this patient population [19,20]. Damaged circulating RBCs expose negatively charged phosphatidyl-serine residues through the ‘Flip-Flop’ phenomenon [21]. These along with activation of platelets, endothelial cells and monocytes along with dysfunction of the coagulation system have been associated with thromboembolic phenomenon, especially in splenectomised TI patients [21]. Moreover, haemolysis carries a role in the dysregulation of nitric oxide (NO) homeostasis which is correlated with PHT and probably thrombotic phenomena [22] (Figure 1).

In contrast to patients with
TM, in whom iron loading occurs mainly as
a result of transfusion therapy, patients with TI accumulate iron
primarily due to increased intestinal iron absorption [23].
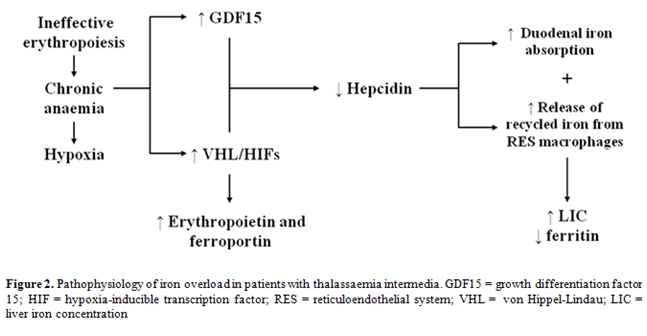
The chronic anaemia and ineffective erythropoiesis that are
characteristic of TI are associated with reduced expression of
hepcidin, a hepatic peptide that plays a central role in iron
homeostasis [23]. Recent studies indicate that
growth and differentiation factor 15 (GDF15), secreted by erythroid
precursors, is significantly increased in patients with TM, inhibiting
hepcidin production [24]. In addition, other studies
indicate that hypoxia-inducible transcription factors (HIFs) control
iron homeostasis by coordinating the downregulation of hepcidin and the
upregulation of erythropoietin and ferroportin. Under normal
conditions, HIFs play a useful role by mobilizing iron and supporting
erythrocyte production in response to anaemia/hypoxia [25-27].
However, the same mechanism may contribute to the harmful accumulation
of iron in response to the chronic anaemia of TI. The combination of
ineffective erythropoiesis (leading to increased GDF15) and chronic
anaemia/hypoxia (altering the expression of HIF) results in hepcidin
suppression, increased iron absorption and increased release of
recycled iron from the reticuloendothelial (RE) system. This results in
depletion of macrophage iron, and relatively low levels of serum
ferritin. The situation in TI is similar to that seen in patients with
hereditary hemochromatosis syndromes, which is characterized by
impaired hepcidin production (Figure 2).

By contrast, in transfused TM
patients iron is preferentially
distributed to the RE system, stimulating ferritin synthesis and its
release to the circulation and leading to high serum ferritin levels [28].
Blood transfusions modify hepcidin production by altering all three
factors responsible for the clinical manifestations of TI described
above namely: (i) by correcting anaemia, transfusion therapy will
suppress ineffective erythropoiesis and the associated increase in
GDF15; (ii) by correcting anaemia, blood transfusions will prevent the
hypoxia responsible for increased HIF production and hypoxia-associated
suppression of hepcidin; and (iii) increased body iron and transferrin
saturation will stimulate hepcidin production. The result will be a
relative increase in hepcidin production in polytransfused TM patients
countering the hepcidin-inhibitory effects of anaemia and ineffective
erythropoiesis. These considerations may explain the differences in
iron homeostasis characterizing TM compared with untransfused TI
patients. Thus, although the rate of iron loading may be slower than
that in patients with TM, TI patients will inevitably suffer from iron
overload which in turn can cause a number of serious complications
including cardiac failure and endocrine abnormalities such as diabetes
mellitus and hypogonadism.
Clinical Sequelae
As a consequence of the aforementioned pathophysiology, several complications have been identified in patients with TI. Some of these complications have been termed unique to TI as compared to TM, especially in the splenectomised, transfusion naďve patient (Table 3).
Clinical Sequelae
As a consequence of the aforementioned pathophysiology, several complications have been identified in patients with TI. Some of these complications have been termed unique to TI as compared to TM, especially in the splenectomised, transfusion naďve patient (Table 3).

Cholelithiasis
Gallstones are much more common in TI than in TM because of ineffective
erythropoiesis and peripheral haemolysis. Recently, unrelated genetic
factors such as uridine 5’-diphospho-alpha-D-glucose (UDPG) deficiency
(Gilbert’s syndrome) have been reported to increase gallstone formation
in patients with thalassaemia [29]. For this reason,
the gallbladder should be inspected during splenectomy and a
cholecystectomy performed if necessary, particularly if the patient is
experiencing symptomatic gallstones. This should be undertaken to
prevent cholecystitis, which can have serious consequences in the
splenectomised patient.
Extramedullary haematopoiesis. Extra-medullary haematopoiesis (EMH) is a compensatory mechanism where bone marrow activity increases in an attempt to overcome the chronic anaemia of TI, leading to the formation of erythropoietic tissue masses that primarily affect the spleen, liver and lymph nodes. These masses can be detected by magnetic resonance imaging (MRI). They may cause neurological problems such as spinal cord compression and paraplegia, and intrathoracic masses [30,31]. Extramedullary haemato-poiesis can be managed by radiotherapy (since haematopoietic tissue is highly radiosensitive) [32], transfusion therapy or hydroxyurea [30,33,34].
Leg ulcers. Leg ulcers are more common in older than in younger patients with TI. It is unclear why ulcers develop in some patients who are maintained at relatively low Hb levels and have the same amount of foetal Hb (HbF) as others in whom ulcers do not develop. The skin at the extremities of elderly TI patients can be thin due to reduced tissue oxygenation, and this makes the subcutaneous tissue fragile and increases the risk of lesions from minimal trauma. Once an ulcer has started to develop it is very painful and difficult to cure, although regular blood transfusions may provide some relief in persistent cases. Simple measures may be beneficial, such as keeping the patient’s legs and feet raised above the level of the heart for 1–2 hours during the day or sleeping with the end of the bed raised. Zinc supplementation [35] and pentoxifylline, which alters the rheological properties of the RBCs [36], can help accelerate the healing of ulcers. Hydroxyurea also has some benefit, either alone or in combination with erythropoietin [37]. In addition, the use of an oxygen chamber can provide moderate relief since tissue hypoxia may be an underlying cause of the ulceration [38].
Thromboembolic events. Taher et al. [39] analyzed data from 8860 thalassaemia patients (6670 TM and 2190 TI). In this study thromboembolic events (TEE) occurred 4.38 times more frequently in TI than TM patients, with more venous events occurring in TI and more arterial events occurring in TM. In another series of TI patients, 24 patients (29%) developed either deep vein thrombosis (DVT), pulmonary embolism, or portal vein thrombosis during a 10-year follow up [40]. In a recent survey involving nine Italian pediatric thalassaemia centres, TEE was observed in 4% of 683 patients with TM and in 9.6% of 52 patients with TI41. Studies collectively revealed that the incidence of TEE is higher in splenectomised patients [39,40]. The incidence of overt stroke in TI patients is low [8]; however, a study done to assess the rate of asymptomatic brain damage in patients with benign haemoglobinopathies reported that 37.5% of patients with TI showed silent brain ischemia [42].
Pulmonary hypertension and congestive heart failure. Pulmonary hypertension (PHT) is prevalent in patients with TI (59.1%) [20], and is thought to be the primary cause of congestive heart failure in this patient population. A retrospective analysis showed that splenectomised females with significant anaemia, thrombocytosis and elevated ferritin levels, were at greatest risk for developing PHT [43]. Preliminary results have demonstrated that PHT is reversible by blood transfusion and treatment with aspirin and warfarin. Several echocardiographic studies have confirmed that cardiac ejection fraction is rarely affected in TI [44]. Nevertheless, patients with TI often have an increased cardiac output, and left ventricular wall dimensions proportional to the dilutional volume overload secondary to chronic anaemia [45].
As anaemia and iron overload are uncommon in well-transfused and chelated TM patients, they are likely to be at the heart of the pathophysiology of PHT. A recent study demonstrated a significant correlation between iron overloading in the liver and pulmonary artery systolic pressure independent of left ventricular filling pressures [46]. Regular transfusion and iron chelation therapy is therefore indicated in TI patients who are well-stratified according to the early detection of PHT indices. Sildenafil has also been successfully used to treat PHT [47], although data from large patient numbers are lacking in TI.
Pregnancy and related complications. Women with TI may have spontaneous successful pregnancies although complications during pregnancy may occur [48]. The chronic anaemia of TI can cause an increase in spontaneous abortions, pre-term labour and intrauterine growth retardation, while endocrine complications due to haemo-siderosis are common [49]. The course and outcome of 19 pregnancies was assessed in 16 women with thalassaemia, including four with TI [50]. All pregnancies were uneventful and elective Caesarean section was performed in each case. The mean birth weight of the babies was 3000 g and all were normal except for one case of omphalocele. The largest study to date assessed 44 TI women who had 83 pregnancies, all spontaneous, 30 from Lebanon and 53 from Italy [51]. These pregnancies resulted in 20.5% abortions, 77.1% live-births and 2 intrauterine foetal deaths at 26 and 36 weeks’ gestation. The mean gestational age at delivery was 36.5 weeks and mean birth weight was 2551 g. In pregnancies progressing > 20 weeks’ gestation, pre-term delivery and intrauterine growth restriction (IUGR) were noted in 31.8% and 24.2% of cases, respectively. In those complicated by IUGR, Caesarean delivery (CS) rate was 87.5%. Two women (Italy) developed severe alloimmune haemolytic anaemia. One progressed to cardiac failure at 35 weeks’ gestation and had CS. The other underwent CS for IUGR and non-reassuring foetal heart monitoring and was scheduled for a splenectomy postpartum. Worsening alloimmune anaemia also developed in 2 women from Lebanon who required splenectomy within eight weeks postpartum. Transfusion was required in 35/44 women during pregnancy (79.5%), with 27.3% requiring transfusion during pregnancy for the first time. The lowest mean Hb level was 6.7±2.0 vs. 8.3±1.2 g/dL in Lebanon and Italy respectively. The average ferritin level before pregnancy was 885.2 ± 658.9 vs.1232.8 ± 902.9 µg/L after pregnancy. In total, CS was performed in 48 pregnancies (72.7%), the indications being elective (41.7%), repeat (31.2%) and obstetrical (27.1%). Pregnancy outcome was similar between Lebanon and Italy with the exception of a significantly higher rate of live births in Italy [51].
Folic acid deficiency is common in TI and occurs due to poor absorption, low dietary intake, or, most significantly, an increased demand for folic acid from active bone marrow. This is a particular concern in pregnancy since deficiency can cause neural tube defects, such as spina bifida, in the growing foetus. During pregnancy, women with TI should therefore be given oral folic acid supplementation (around 1 mg/day), and should be carefully monitored in order to assess the need for transfusion therapy and to avoid haemodynamic compromises. The major fear of initiating transfusions during pregnancy is the development of alloantibodies. These can aggravate anaemia and progress into severe haemolytic anaemia refractory to transfusions and thus increase the complication rate. CS may be required in TI patients due to the associated cephalopelvic disproportion secondary to skeletal deformity and short stature, especially in non-transfused women. Splenectomy is usually performed in TI women for decreased levels of haemoglobin, hyperactivity of the spleen, leukopenia and symptomatic thrombocytopenia [5,6].
Extramedullary haematopoiesis. Extra-medullary haematopoiesis (EMH) is a compensatory mechanism where bone marrow activity increases in an attempt to overcome the chronic anaemia of TI, leading to the formation of erythropoietic tissue masses that primarily affect the spleen, liver and lymph nodes. These masses can be detected by magnetic resonance imaging (MRI). They may cause neurological problems such as spinal cord compression and paraplegia, and intrathoracic masses [30,31]. Extramedullary haemato-poiesis can be managed by radiotherapy (since haematopoietic tissue is highly radiosensitive) [32], transfusion therapy or hydroxyurea [30,33,34].
Leg ulcers. Leg ulcers are more common in older than in younger patients with TI. It is unclear why ulcers develop in some patients who are maintained at relatively low Hb levels and have the same amount of foetal Hb (HbF) as others in whom ulcers do not develop. The skin at the extremities of elderly TI patients can be thin due to reduced tissue oxygenation, and this makes the subcutaneous tissue fragile and increases the risk of lesions from minimal trauma. Once an ulcer has started to develop it is very painful and difficult to cure, although regular blood transfusions may provide some relief in persistent cases. Simple measures may be beneficial, such as keeping the patient’s legs and feet raised above the level of the heart for 1–2 hours during the day or sleeping with the end of the bed raised. Zinc supplementation [35] and pentoxifylline, which alters the rheological properties of the RBCs [36], can help accelerate the healing of ulcers. Hydroxyurea also has some benefit, either alone or in combination with erythropoietin [37]. In addition, the use of an oxygen chamber can provide moderate relief since tissue hypoxia may be an underlying cause of the ulceration [38].
Thromboembolic events. Taher et al. [39] analyzed data from 8860 thalassaemia patients (6670 TM and 2190 TI). In this study thromboembolic events (TEE) occurred 4.38 times more frequently in TI than TM patients, with more venous events occurring in TI and more arterial events occurring in TM. In another series of TI patients, 24 patients (29%) developed either deep vein thrombosis (DVT), pulmonary embolism, or portal vein thrombosis during a 10-year follow up [40]. In a recent survey involving nine Italian pediatric thalassaemia centres, TEE was observed in 4% of 683 patients with TM and in 9.6% of 52 patients with TI41. Studies collectively revealed that the incidence of TEE is higher in splenectomised patients [39,40]. The incidence of overt stroke in TI patients is low [8]; however, a study done to assess the rate of asymptomatic brain damage in patients with benign haemoglobinopathies reported that 37.5% of patients with TI showed silent brain ischemia [42].
Pulmonary hypertension and congestive heart failure. Pulmonary hypertension (PHT) is prevalent in patients with TI (59.1%) [20], and is thought to be the primary cause of congestive heart failure in this patient population. A retrospective analysis showed that splenectomised females with significant anaemia, thrombocytosis and elevated ferritin levels, were at greatest risk for developing PHT [43]. Preliminary results have demonstrated that PHT is reversible by blood transfusion and treatment with aspirin and warfarin. Several echocardiographic studies have confirmed that cardiac ejection fraction is rarely affected in TI [44]. Nevertheless, patients with TI often have an increased cardiac output, and left ventricular wall dimensions proportional to the dilutional volume overload secondary to chronic anaemia [45].
As anaemia and iron overload are uncommon in well-transfused and chelated TM patients, they are likely to be at the heart of the pathophysiology of PHT. A recent study demonstrated a significant correlation between iron overloading in the liver and pulmonary artery systolic pressure independent of left ventricular filling pressures [46]. Regular transfusion and iron chelation therapy is therefore indicated in TI patients who are well-stratified according to the early detection of PHT indices. Sildenafil has also been successfully used to treat PHT [47], although data from large patient numbers are lacking in TI.
Pregnancy and related complications. Women with TI may have spontaneous successful pregnancies although complications during pregnancy may occur [48]. The chronic anaemia of TI can cause an increase in spontaneous abortions, pre-term labour and intrauterine growth retardation, while endocrine complications due to haemo-siderosis are common [49]. The course and outcome of 19 pregnancies was assessed in 16 women with thalassaemia, including four with TI [50]. All pregnancies were uneventful and elective Caesarean section was performed in each case. The mean birth weight of the babies was 3000 g and all were normal except for one case of omphalocele. The largest study to date assessed 44 TI women who had 83 pregnancies, all spontaneous, 30 from Lebanon and 53 from Italy [51]. These pregnancies resulted in 20.5% abortions, 77.1% live-births and 2 intrauterine foetal deaths at 26 and 36 weeks’ gestation. The mean gestational age at delivery was 36.5 weeks and mean birth weight was 2551 g. In pregnancies progressing > 20 weeks’ gestation, pre-term delivery and intrauterine growth restriction (IUGR) were noted in 31.8% and 24.2% of cases, respectively. In those complicated by IUGR, Caesarean delivery (CS) rate was 87.5%. Two women (Italy) developed severe alloimmune haemolytic anaemia. One progressed to cardiac failure at 35 weeks’ gestation and had CS. The other underwent CS for IUGR and non-reassuring foetal heart monitoring and was scheduled for a splenectomy postpartum. Worsening alloimmune anaemia also developed in 2 women from Lebanon who required splenectomy within eight weeks postpartum. Transfusion was required in 35/44 women during pregnancy (79.5%), with 27.3% requiring transfusion during pregnancy for the first time. The lowest mean Hb level was 6.7±2.0 vs. 8.3±1.2 g/dL in Lebanon and Italy respectively. The average ferritin level before pregnancy was 885.2 ± 658.9 vs.1232.8 ± 902.9 µg/L after pregnancy. In total, CS was performed in 48 pregnancies (72.7%), the indications being elective (41.7%), repeat (31.2%) and obstetrical (27.1%). Pregnancy outcome was similar between Lebanon and Italy with the exception of a significantly higher rate of live births in Italy [51].
Folic acid deficiency is common in TI and occurs due to poor absorption, low dietary intake, or, most significantly, an increased demand for folic acid from active bone marrow. This is a particular concern in pregnancy since deficiency can cause neural tube defects, such as spina bifida, in the growing foetus. During pregnancy, women with TI should therefore be given oral folic acid supplementation (around 1 mg/day), and should be carefully monitored in order to assess the need for transfusion therapy and to avoid haemodynamic compromises. The major fear of initiating transfusions during pregnancy is the development of alloantibodies. These can aggravate anaemia and progress into severe haemolytic anaemia refractory to transfusions and thus increase the complication rate. CS may be required in TI patients due to the associated cephalopelvic disproportion secondary to skeletal deformity and short stature, especially in non-transfused women. Splenectomy is usually performed in TI women for decreased levels of haemoglobin, hyperactivity of the spleen, leukopenia and symptomatic thrombocytopenia [5,6].
Management
There are a number of options currently available for managing patients with TI, including splenectomy, transfusion therapy, iron chelation therapy, modulation of HbF production and haematopoietic stem cell transplantation.
Splenectomy. As per expert opinion, the current indications for splenectomy in TI include growth retardation or poor health, leukopenia, thrombocytopenia, increased transfusion demand, or symptomatic splenomegaly [6]. Clinical observations, however, have suggested that splenectomy in TI can contribute to an increased susceptibility to TEE and PHT [39,40,43]. This calls for a review of splenectomy as a procedure of choice, especially with its potential role in increasing TI-related complications and the inherent risk of infection associated with the procedure even for individuals without haematological disorders [52].
Transfusion and iron chelation therapy. In patients with TM, a remarkable improvement in life expectancy and prevention of morbidity has been achieved in recent decades [53]. This is mainly attributed to improved methods of blood transfusion, better understanding of iron toxicity, and evolution in iron chelation therapy [53]. On the other hand, TI has been regarded as a clinical entity with limited complicationsso as the general approach was to avoid early blood transfusions and the associated need for chelation therapy. However, increasing evidence is delineating the benefit of transfusion therapy in decreasing the incidence of complications as PHT, TEE, and EMH, to name a few [30,39,54]. Thus although the common practice was to initiate transfusion when complications ensue, it may be worthwhile start transfusion therapy earlier as a preventive approach which will also help alleviate the increased risk of alloimmunisation with delayed initiation of transfusion [55]. Although earlier introduction of blood transfusions will increase the rate of iron accumulation, effective methods of iron chelation are now available [56,57], and the benefits of transfusion therapy would greatly outweigh the cost and inconvenience of iron chelation therapy.
The initiation of iron chelation therapy in patients with TI depends not only on the amount of excess iron, but also on the rate of iron accumulation, the duration of exposure to excess iron and various other factors in individual patients. A direct assessment of LIC is recommended, either by biopsy or by a non-invasive method such as R2 MRI. Where LIC measurement is not possible, threshold serum ferritin values of 400-500 ng/mL (which are lower than those generally accepted in patients with TM) could be considered as an indicator for initiation of iron chelation therapy. Chelation therapy should generally be initiated if LIC exceeds 7 mg/g dry weight of liver tissue, however lower levels of LIC for initiation of chelation therapy must be considered particularly now with the availability of oral iron chelators [6].
Modulation of foetal haemoglobin production. Increasing the synthesis of HbF can help alleviate anaemia and therefore improve the clinical status of patients with TI [58]. Production of HbF is reactivated during recovery from marrow suppression after treatment with cytotoxic drugs, therefore it is postulated that these agents may alter the pattern of erythropoiesis and increase the expression of gamma-chain genes. Several cytotoxic agents with this effect have been identified, including cytosine arabinoside and hydroxyurea [59-61]. Recently published results from Iran, evaluating six years of hydroxyurea therapy in transfusion dependent patients with TI, are encouraging. A significant decrease in the need for blood transfusions was observed in many patients; the need was completely obviated in some patients [62]. Erythropoietin has also been shown to increase HbF levels in some patients with TI [58]. Preliminary trials with intravenous and oral butyric acid derivatives have shown increases in foetal and total Hb levels in patients with TI [63-66], and the acceptable safety profile of these agents makes them promising therapeutic targets. It is unclear how butyrates stimulate gamma-globin production or why some patients respond to treatment while others do not.
However, the overall trial results with HbF-stimulating agents are somewhat disappointing. Studies using combined treatments have shown greater promise than the individual agents alone [67]. Further clinical evaluation is required to clarify the value of this approach, especially in view of the reduced oxygen delivery capacity of HbF, as this might favour the implementation of a target Hb level higher than 10 g/dL in response to increased need (e.g. PHT, coronary heart disease and chronic obstructive pulmonary disease (COPD)) and an increased ratio of HbF/HbA.
Haematopoietic stem cell transplantation. Haematopoietic stem cell transplantation (HSCT), where the marrow of an affected patient is replaced from the stem cells of an uaffected donor, is an established treatment for beta-thalassaemia. Although successful HSCT can offer a cure, it can be unsuccessful (e.g. if the thalassaemia returns), may lead to complications (e.g. graft-versus-host disease, growth impairment, neurological complications), and can even result in death [68-71]; the risk for a failed transplantation depends primarily on the health and age of the patient. The decision as to which patients are eligible for transplantation is complex and is related to both the quality of life and expected survival-time of the transplanted patient, when compared with supportive care only. This is particularly relevant in patients with TI, especially in those who are only mildly affected. Due to the risks involved, transplantation is considered appropriate only for patients with a human leukocyte antigen (HLA)-matched donor, which comprises only 30–40% of all beta-thalassaemia patients, at most [72]. As HLA type is genetically determined, there is a 25% chance that any two siblings will be a match.
There are a number of options currently available for managing patients with TI, including splenectomy, transfusion therapy, iron chelation therapy, modulation of HbF production and haematopoietic stem cell transplantation.
Splenectomy. As per expert opinion, the current indications for splenectomy in TI include growth retardation or poor health, leukopenia, thrombocytopenia, increased transfusion demand, or symptomatic splenomegaly [6]. Clinical observations, however, have suggested that splenectomy in TI can contribute to an increased susceptibility to TEE and PHT [39,40,43]. This calls for a review of splenectomy as a procedure of choice, especially with its potential role in increasing TI-related complications and the inherent risk of infection associated with the procedure even for individuals without haematological disorders [52].
Transfusion and iron chelation therapy. In patients with TM, a remarkable improvement in life expectancy and prevention of morbidity has been achieved in recent decades [53]. This is mainly attributed to improved methods of blood transfusion, better understanding of iron toxicity, and evolution in iron chelation therapy [53]. On the other hand, TI has been regarded as a clinical entity with limited complicationsso as the general approach was to avoid early blood transfusions and the associated need for chelation therapy. However, increasing evidence is delineating the benefit of transfusion therapy in decreasing the incidence of complications as PHT, TEE, and EMH, to name a few [30,39,54]. Thus although the common practice was to initiate transfusion when complications ensue, it may be worthwhile start transfusion therapy earlier as a preventive approach which will also help alleviate the increased risk of alloimmunisation with delayed initiation of transfusion [55]. Although earlier introduction of blood transfusions will increase the rate of iron accumulation, effective methods of iron chelation are now available [56,57], and the benefits of transfusion therapy would greatly outweigh the cost and inconvenience of iron chelation therapy.
The initiation of iron chelation therapy in patients with TI depends not only on the amount of excess iron, but also on the rate of iron accumulation, the duration of exposure to excess iron and various other factors in individual patients. A direct assessment of LIC is recommended, either by biopsy or by a non-invasive method such as R2 MRI. Where LIC measurement is not possible, threshold serum ferritin values of 400-500 ng/mL (which are lower than those generally accepted in patients with TM) could be considered as an indicator for initiation of iron chelation therapy. Chelation therapy should generally be initiated if LIC exceeds 7 mg/g dry weight of liver tissue, however lower levels of LIC for initiation of chelation therapy must be considered particularly now with the availability of oral iron chelators [6].
Modulation of foetal haemoglobin production. Increasing the synthesis of HbF can help alleviate anaemia and therefore improve the clinical status of patients with TI [58]. Production of HbF is reactivated during recovery from marrow suppression after treatment with cytotoxic drugs, therefore it is postulated that these agents may alter the pattern of erythropoiesis and increase the expression of gamma-chain genes. Several cytotoxic agents with this effect have been identified, including cytosine arabinoside and hydroxyurea [59-61]. Recently published results from Iran, evaluating six years of hydroxyurea therapy in transfusion dependent patients with TI, are encouraging. A significant decrease in the need for blood transfusions was observed in many patients; the need was completely obviated in some patients [62]. Erythropoietin has also been shown to increase HbF levels in some patients with TI [58]. Preliminary trials with intravenous and oral butyric acid derivatives have shown increases in foetal and total Hb levels in patients with TI [63-66], and the acceptable safety profile of these agents makes them promising therapeutic targets. It is unclear how butyrates stimulate gamma-globin production or why some patients respond to treatment while others do not.
However, the overall trial results with HbF-stimulating agents are somewhat disappointing. Studies using combined treatments have shown greater promise than the individual agents alone [67]. Further clinical evaluation is required to clarify the value of this approach, especially in view of the reduced oxygen delivery capacity of HbF, as this might favour the implementation of a target Hb level higher than 10 g/dL in response to increased need (e.g. PHT, coronary heart disease and chronic obstructive pulmonary disease (COPD)) and an increased ratio of HbF/HbA.
Haematopoietic stem cell transplantation. Haematopoietic stem cell transplantation (HSCT), where the marrow of an affected patient is replaced from the stem cells of an uaffected donor, is an established treatment for beta-thalassaemia. Although successful HSCT can offer a cure, it can be unsuccessful (e.g. if the thalassaemia returns), may lead to complications (e.g. graft-versus-host disease, growth impairment, neurological complications), and can even result in death [68-71]; the risk for a failed transplantation depends primarily on the health and age of the patient. The decision as to which patients are eligible for transplantation is complex and is related to both the quality of life and expected survival-time of the transplanted patient, when compared with supportive care only. This is particularly relevant in patients with TI, especially in those who are only mildly affected. Due to the risks involved, transplantation is considered appropriate only for patients with a human leukocyte antigen (HLA)-matched donor, which comprises only 30–40% of all beta-thalassaemia patients, at most [72]. As HLA type is genetically determined, there is a 25% chance that any two siblings will be a match.
Conclusion
Our
understanding of the molecular and pathophysiological mechanisms
underlying the disease process in patients with TI has substantially
increased over the past decade. Today, a large body of evidence
documents the incidence and consequences of the different clinical
complications associated with the disease. Although there are still
currently no clear guidelines for the optimal management plan of this
disease entity, the emerging body of evidence looks promising.
Declaration Of Interest.
The authors report no conflicts of interest. The authors alone are
responsible for the content and writing of the paper. This study did
not receive external funding.
References
- Weatherall DJ. Thalassaemia: the long road
from bedside to genome. Nat Rev Genet 2004; 5:625-631.

- Sturgeon P, Itano HA, Bergren WR. Genetic
and biochemical studies of intermediate types of Cooley's anaemia. Br J
Haematol 1955l; 1:264-277.

- Galanello R, Cao A. Relationship between
genotype and phenotype. Thalassemia intermedia. Ann NY Acad Sci 1998;
850:325-333.

- Weatherall D. The molecular basis for
phenotypic variability of the common thalassaemias. Mol Med Today 1995;
1:15-20.

- Taher A, Ismaeel H, Cappellini MD.
Thalassaemia Intermedia: Revisited. Blood Cells Mol Dis 2006; 37:12-20

- Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the Clinical Management of Thalassaemia. 2nd Revised Edition. Thalassaemia International Federation 2008.
- Camaschella C, Mazza U, Roetto, Gottardi E,
Parziale A, Travi M, Fattore S, Bacchiega D, Fiorelli G, Cappellini MD.
A et al. Genetic interactions in thalassemia intermedia: analysis of
beta-mutations, alpha-genotype, gamma-promoters, and beta-LCR
hypersensitive sites 2 and 4 in Italian patients. Am J Hematol 1995;
48:82-87.

- Camaschella C, Kattamis AC, Petroni D,
Roetto A, Sivera P, Sbaiz L, Cohen A, Ohene-Frempong K, Trifillis P,
Surrey S, Fortina P. Different hematological phenotypes caused by the
interaction of triplicated alpha-globin genes and heterozygous
beta-thalassemia. Am J Hematol 1997; 55:83-88.

- Sampietro M, Cazzola M, Cappellini MD,
Fiorelli G. The triplicated alpha-gene locus and heterozygous beta
thalassaemia: a case of thalassaemia intermedia. Br J Haematol 1983;
55:709-710.

- Beris P, Solenthaler M, Deutsch S,
Darbellay R, Tobler A, Bochaton-pialat ML, Gabbiani G Severe inclusion
body beta-thalassaemia with haemolysis in a patient double heterozygous
for beta(0)-thalassaemia and quadruplicated apla-globin gene
arrangement of the anti-4.2 type. Br J Haematol 1999; 105:1074-80

- Taher A, Shammaa D, Bazarbachi A, Itani D,
Zaatari G, Greige L, Otrock ZK, Mahfouz RA. Absence of JAK2 V617F
mutation in thalassemia intermedia patients. Mol Biol Rep
2009;36:1555-1557.

- Weatherall DJ. Thalassemia intermedia:
cellular and molecular aspects. J Hematol 2001; 86:186-188.

- Ho PJ, Hall GW, Luo LY, Weatherall DJ,
Thein SL. Beta-thalassaemia intermedia: is it possible consistently to
predict phenotype from genotype? Br J Haematol 1998; 100:70-78.

- Rund D, Oron-Karni P, Filon D, Goldfarb A,
Rachmilewitz E, Oppenheim A. Genetic analysis of beta-thalassemia
intermedia in Israel: diversity of mechanisms and unpredictability of
phenotype. Am J Hematol 1997; 54:16-22.

- Phadke SR, Agarwal S. Phenotype score to
grade the severity of thalassemia intermedia. Indian J Pediatr 2003;
70:477-481.

- Camaschella C, Cappellini MD. Thalassemia
intermedia. Haematologica 1995; 80:58-68.

- Olivieri NF. The beta-thalassemias. N Engl
J Med 1999; 341:99-109.

- Cappellini MD, Cerino M, Marelli S et al. Thalassemia intermedia: clinical aspects and management. Haematologica 2001; 86:194-196.
- Taher A, Abou-Mourad Y, Abchee A, Zallouaa
P, Shamseddine A. Pulmonary thromboembolism in beta-thalassemia
intermedia: are we aware of this complication? Hemoglobin 2002;
26:107-112.

- Aessopos A, Farmakis D, Karagiorga M,
Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J,
Loukopoulos D. Cardiac involvement in thalassemia intermedia: a
multicenter study. Blood 2001; 97: 3411-3416.

- Taher AT, Otrock ZK, Uthman I, Cappellini
MD. Thalassemia and hypercoagulability. Blood Rev 2008; 22:283-292.

- Ataga KI, Cappellini MD, Rachmilewitz EA.
Beta-thalassaemia and sickle cell anaemia as paradigms of
hypercoagulability. Br J Haematol 2007; 139:3-13.

- Origa R, Galanello R, Ganz T, Giagu N,
Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary
hepcidin in b-thalassemia. Haematologica 2007; 92:583-588.

- Tanno T, Bhanu NV, Oneal PA, Goh SH,
Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE,
Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of
GDF15 in thalassemia suppress expression of the iron regulatory protein
hepcidin. Nat Med 2007; 13:1096-1101.

- Valore EV, Ganz T. Posttranslational
processing of hepcidin in human hepatocytes is mediated by the
prohormone convertase furin. Blood Cells Mol Dis 2008; 40:132-138.

- Silvestri L, Pagani A, Camaschella C.
Furin-mediated release of soluble hemojuvelin: a new link between
hypoxia and iron homeostasis. Blood 2008; 111:924-931.

- Peyssonnaux C, Zinkernagel AS, Schuepbach
RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of
iron homeostasis by the hypoxia-inducible transcription factors (HIFs).
J Clin Invest 2007; 117:1926-1932.

- Pakbaz Z, Fischer R, Fung E, Nielsen P,
Harmatz P, Vichinsky E. Serum ferritin underestimates liver iron
concentration in transfusion independent thalassemia patients as
compared to regularly transfused thalassemia and sickle cell patients.
Pediatr Blood Cancer 2007; 49:329-332.

- Borgna-Pignatti C, Rigon F, Merlo L,
Chakrok R, Micciolo R, Perseu L, Galanello R. Thalassemia minor, the
Gilbert mutation, and the risk of gallstones. Haematologica 2003;
88:1106-1109.

- Chehal A, Aoun E, Koussa S, Skoury H,
Koussa S, Taher A. Hypertransfusion: a successful method of treatment
in thalassemia intermedia patients with spinal cord compression
secondary to extramedullary hematopoiesis. Spine 2003; 28:E245-249.

- Castelli R, Graziadei G, Karimi M,
Cappellini MD. Intrathoracic masses due to extramedullary
hematopoiesis. Am J Med Sci 2004; 328:299-303.

- Smith PR, Manjoney DL, Teitcher JB, Choi
KN, Braverman AS. Massive hemothorax due to intrathoracic
extramedullary hematopoiesis in a patient with thalassemia intermedia.
Chest 1988; 94:658-660.

- Saxon BR, Rees D, Olivieri NF. Regression
of extramedullary haemopoiesis and augmentation of fetal haemoglobin
concentration during hydroxyurea therapy in beta thalassaemia. Br J
Haematol 1998; 101:416-419.

- Cario H, Wegener M, Debatin KM. Treatment
with hydroxyurea in thalassemia intermedia with paravertebral
pseudotumors of extramedullary hematopoiesis. Ann Hematol 2002;
81:478-482.

- Gupta VL, Choubey BS. RBC survival, zinc
deficiency, and efficacy of zinc therapy in sickle cell disease. Birth
Defects Orig Artic Ser 1987; 23:477-483.

- Dettelbach HR, Aviado DM. Clinical
pharmacology of pentoxifylline with special reference to its
hemorrheologic effect for the treatment of intermittent claudication. J
Clin Pharmacol 1985; 25:8-26.

- al Momen AK. Recombinant human
erythropoietin induced rapid healing of a chronic leg ulcer in a
patient with sickle cell disease. Acta Haematol 1991; 86:46-48.

- Gimmon Z, Wexler MR, Rachmilewitz EA.
Juvenile leg ulceration in beta-thalassemia major and intermedia. Plast
Reconstr Surg 1982; 69:320-325.

- Taher A, Isma’eel H, Mehio G, Bignamini D,
Kattamis A, Rachmilewitz EA. Cappellini MD. Prevalence of
thromboembolic events among 8,860 patients with thalassaemia major and
intermedia in the Mediterranean area and Iran. Thromb Haemost 2006;
96:488-491.

- Cappellini MD, Robbiolo L, Bottasso BM,
Coppola R, Fiorelli AP, Mannucci AP. Venous thromboembolism and
hypercoagulability in splenectomized patients with thalassaemia
intermedia. Br J Haematol 2000; 111:467-473.

- Borgna Pignatti C, Carnelli V, Caruso V,
Dore F, De Mattia D, Di Palma A, Di Gregorio F, Romeo MA, Longhi R,
Mangiagli A, Melevendi C, Pizzarelli G, Musumeci S. Thromboembolic
events in beta thalassemia major: an Italian multicenter study. Acta
Haematol 1998; 99:76-79.

- Manfre L, Giarratano E, Maggio A, Banco A,
Vaccaro G, Lagalla R. MR imaging of the brain: findings in asymptomatic
patients with thalassemia intermedia and sickle cell-thalassemia
disease. AJR Am J Roentgenol 1999; 173:1477-1480.

- Atichartakarn V, Likittanasombat K,
Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P,
Aryurachai K. Pulmonary arterial hypertension in previously
splenectomized patients with beta-thalassemic disorders. Int J Hematol
2003; 78:139-145.

- Aessopos A, Farmakis D, Deftereos S,
Tsironi M, Tassiopoulos S, Moyssakis I, Karagiorga M. Thalassemia heart
disease: a comparative evaluation of thalassemia major and thalassemia
intermedia. Chest 2005; 127:1523- 1530.

- Gharzuddine WS, Kazma HK, Nuwayhid IA,
Bitar FF, Koussa SF, Moukarbel GV, Taher AT. Doppler characterization
of left ventricular diastolic function in beta-thalassaemia major.
Evidence for an early stage of impaired relaxation. Eur J Echocardiogr
2002; 3:47-51.

- Isma’eel H, Chafic AH, Rassi FE, Inati A,
Koussa S, Daher R, Gharzuddin W, Alam S, Taher A. Relation between
iron-overload indices, cardiac echo-Doppler, and biochemical markers in
thalassemia intermedia. Am J Cardiol 2008; 102: 363-367.

- Derchi G, Forni GL, Formisano F,
Cappellini MD, Galanello R, D'Ascola G, Bina P, Magnano C, Lamagna M.
Efficacy and safety of sildenafil in the treatment of severe pulmonary
hypertension in patients with hemoglobinopathies. Haematologica 2005;
90:452-458.

- Savona-Ventura C, Bonello F.
Beta-thalassemia syndromes and pregnancy. Obstet Gynecol Surv 1994;
49:129-137.

- Skordis N, Christou S, Koliou M, Pavlides
N, Angastiniotis M. Fertility in female patients with thalassemia. J
Pediatr Endocrinol Metab 1998; 11:35-943.

- Karagiorga-Lagana M. Fertility in
thalassemia: the Greek experience. J Pediatr Endocrinol Metab 1998;
11:945-951.

- Nassar A, Naja M, Cesaretti C, Eprassi B,
Cappellini MD, Taher A. Pregnancy outcome in patients with
beta-thalassemia intermedia at two tertiary care centers, in Beirut and
Milan. Haematologica 2008;93:1586-1587.

- Cadili A, de Gara C. Complications of
splenectomy. Am J Med 2008; 121:371-375.

- Borgna-Pignatti C, Rugolotto S, De Stefano
P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini
MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients
with thalassemia major treated with transfusion and deferoxamine.
Haematologica 2004; 89:1187-1193.

- Aessopos A, Kati M, Meletis J. Thalassemia
intermedia today: should patients regularly receive transfusions?
Transfusion 2007; 47:792-800.

- Eder AF, Chambers LA. Noninfectious
complications of blood transfusion. Arch Pathol Lab Med 2007;
13:708-718.

- Cossu P, Toccafondi C, Vardeu F, Sanna G,
Frau F, Lobrano R, Cornacchia G, Nucaro A, Bertolino F, Loi A, De
Virgiliis S, Cao A. Iron overload and desferrioxamine chelation therapy
in beta-thalassemia intermedia. Eur J Pediatr 1981; 137:267-271.

- Pootrakul P, Sirankapracha P, Sankote J,
Kachintorn U, Maungsub W, Sriphen K, Thakernpol K, Atisuk K, Fucharoen
S, Chantraluksri U, Shalev O, Hoffbrand AV. Clinical trial of
deferiprone iron chelation therapy in beta-thalassaemia/haemoglobin E
patients in Thailand. Br J Haematol 2003; 122:305-310.

- Olivieri NF. Reactivation of fetal
hemoglobin in patients with beta-thalassemia. Semin Hematol 1996;
33:24-42.

- Arruda VR, Lima CS, Saad ST, Costa FF.
Successful use of hydroxyurea in beta-thalassemia major. N Engl J Med
1997;336: 964.

- Bradai M, Abad MT, Pissard S, Lamraoui F,
Skopinski L, de Montalembert M. Hydroxyurea can eliminate transfusion
requirements in children with severe beta-thalassemia. Blood 2003;
102:1529-1530.

- Dixit A, Chatterjee TC, Mishra P, Choudhry
DR, Mahapatra M, Tyagi S, Kabra M, Saxena R, Choudhry VP. Hydroxyurea
in thalassemia intermedia-a promising therapy. Ann Hematol 2005;
84:441-446.

- Karimi M, Darzi H, Yavarian M. Hematologic
and clinical responses of thalassemia intermedia patients to
hydroxyurea during 6 years of therapy in Iran. J Pediatr Hematol Oncol
2005; 27:380-385.

- Perrine SP, Ginder GD, Faller DV, Dover
GH, Ikuta T, Witkowska HE, Cai SP, Vichinsky EP, Olivieri NF. A
short-term trial of butyrate to stimulate fetalglobin- gene expression
in the beta-globin disorders. N Engl J Med 1993; 328:81-86.

- Cappellini MD, Graziadei G, Ciceri L,
Comino A, Bianchi P, Porcella A, Fiorelli G. Oral isobutyramide therapy
in patients with thalassemia intermedia: results of a phase II open
study. Blood Cells Mol Dis 2000; 26:105-111.

- Sher GD, Ginder GD, Little J, Yang S,
Dover GJ, Olivieri NF. Extended therapy with intravenous arginine
butyrate in patients with beta-hemoglobinopathies. N Engl J Med 1995;
332:1606-1610.

- Collins AF, Pearson HA, Giardina P,
McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy
in homozygous beta thalassemia: a clinical trial. Blood 1995; 85:43-49.

- Olivieri NF, Rees DC, Ginder GD, Thein SL,
Brittenham GM, Waye JS, Weatherall DJ. Treatment of thalassaemia major
with phenylbutyrate and hydroxyurea. Lancet 1997; 350:491-492.

- Piga A, Longo F, Voi V, Facello S, Miniero
R, Dresow B. Late effects of bone marrow transplantation for
thalassemia. Ann N Y Acad Sci 1998; 850:294-299.

- Apperley JF. Bone marrow transplant for
the haemoglobinopathies: past, present and future. Baillieres Clin
Haematol 1993; 6:299-325.

- Uckan D, Cetin M, Yigitkanli I, Tezcan I,
Tuncer M, Karasimav D, Oguz KK, Topçu M. Life-threatening neurological
complications after bone marrow transplantation in children. Bone
Marrow Transplant 2005; 35:71-76.

- Khojasteh NH, Zakernia M, Ramzi M,
Haghshenas M. Bone marrow transplantation for hematological
disorders-Shiraz experience. Indian J Pediatr 2002; 69: 31-32.

- Rund D, Rachmilewitz E. Advances in the
pathophysiology and treatment of thalassemia. Crit Rev Oncol Hematol
1995; 20:237-254
