Iron Metabolism in Thalassemia and Sickle Cell Disease
Raffaella Mariani, Paola Trombini, Matteo Pozzi and Alberto Piperno
Department of Clinical Medicine and Prevention, University of Milano-Bicocca and Centre for Diagnosis and Treatment of Hemochromatosis, S. Gerardo Hospital, Monza, Italy
Correspondence
to: Prof Alberto Piperno, Department of Clinical Medicine and
Prevention, University of Milano-Bicocca, Centre for Diagnosis and
Treatment of Hemochromatosis, S.Gerardo Hospital, Via Pergolesi 33,
20052, Monza, Tel: +39 039 2339555, Fax: +39 039 322274, Email: alberto.piperno@unimib.it
Published: October 27, 2009
Received: October 18, 2009
Accepted: October 25, 2009
Medit J Hemat Infect Dis 2009, 1(1): e2009006 DOI 10.4084/MJHID.2009.006
This article is available from: http://www.mjhid.org/article/view/5011
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
There
are two main mechanisms by which iron overload develops in
thalassemias: increased iron absorption due to ineffective
erythropoiesis and blood transfusions. In nontransfused patients with
severe thalassemia, abnormal dietary iron absorption increases body
iron burden between 2 and 5 g per year. If regular transfusions are
required, this doubles the rate of iron accumulation leading to earlier
massive iron overload and iron-related damage. Iron metabolism largely
differs between thalassemias and sickle cell disease, but chronic
transfusion therapy partially normalize many of the disparities between
the diseases, making iron overload an important issue to be considered
in the management of patients with sickle cell disease too. The present
review summarizes the actual knowledge on the regulatory pathways of
iron homeostasis. In particular, the data presented indicate the
inextricably link between erythropoiesis and iron metabolism and the
key role of hepcidin in coordinating iron procurement according to
erythropoietic requirement. The role of erythropoietin, hypoxia,
erythroid-dependent soluble factors and iron in regulating hepcidin
transcription are discussed as well as differences and similarities in
iron homeostasis between thalassemia syndromes and sickle cell disease.
Introduction
In
humans total body iron stores is maintained normally within the range
of 200-1500 mg (in men, the normal concentration of iron in the storage
pool is 13 mg/Kg and in women 5 mg/kg) by adequate adjustment of
intestinal iron absorption, since no excretory mechanisms exist1. Iron
overload is a common complication of thalassemia syndromes which could
lead per se to the development of organ damage and increased
mortality2. There are two main mechanisms by which iron overload
develops in thalassemias: increased iron absorption due to ineffective
erythropoiesis and blood transfusions. Due to the different
physiopathology of anemia in thalassemia and sickle cell disease (SCD),
and the different indications in the use of transfusions in thalassemia
major, intermedia, and SCD, there are significant differences in the
physiopathology of iron overload and iron-related complications in
these disorders.
Iron metabolism:
Body iron pathways. Because there is no known regulated mechanism for iron excretion, and the amount of iron entering the body each day represents less than 0.1% of the total body iron endowment, most circulating iron must be derived from the recycling of iron already within the system3 (Figure 1). From a quantitative point of view, the most important pathway of iron metabolism is the unidirectional recycling of iron from senescent red blood cells to the erythroid bone marrow through macrophages1,4. Renewed interest has been developed on the role of macrophages within the erythroblastic islands, the specialized niches in which erythroid precursors proliferate, differentiate, and enucleate in the bone marrow 5.
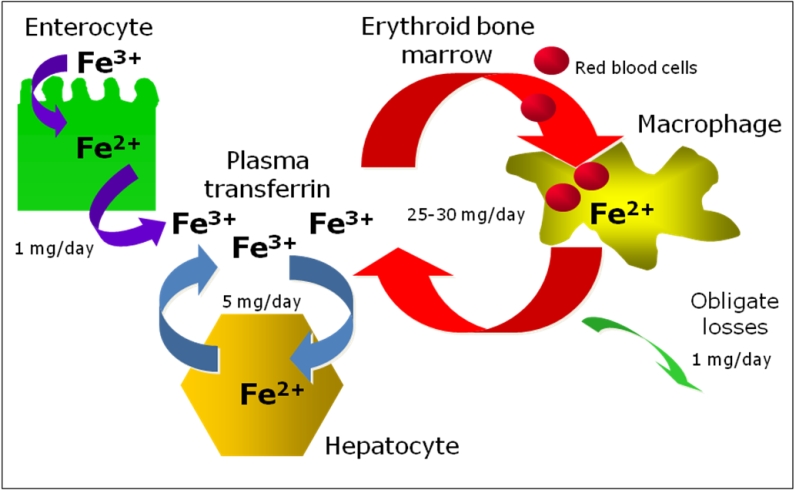
These hematopoietic subcompartments are composed of erythroblasts surrounding a central macrophage. It was suggested that the macrophage functions as a “nurse” cell, providing iron to developing erythroblasts for heme synthesis6. An attractive hypothesis proposed for central macrophages is that ferritin, synthesized by macrophages, secreted via exocytosis, and subsequently taken up by erythroblasts could be a source of iron for heme synthesis7. It is not known whether central macrophages might release iron also by the ferroportin-dependent exporting pathway and further studies are needed to clarify all this matter.
The second pathway is the cycling of iron from hepatocytes to the blood and viceversa, according to body needs and the third is iron absorption through duodenal and upper jejunum that balances the 1-2 mg daily iron loss occurring through cellular exfoliation (see below: iron absorption and iron storage paragraphs).
Iron transport and utilization: The systemic transporter of iron in the body is transferring, an abundant, high affinity iron-binding protein. Under normal circumstances, TF carries nearly all serum iron collected from duodenal absorptive epithelium, macrophages and hepatocytes, and dampens its reactivity. Very small amounts of iron may be loosely associated with albumin or small molecules. In normal human subjects, iron occupies approximately 30% of the iron-binding sites on plasma TF1. The saturation of TF by iron shows diurnal fluctuation exhibiting a morning peak and an evening nadir8. It is likely to be higher in the portal circulation, where recently absorbed iron from the intestine enters the circulation and passes through the liver. This first-pass effect may explain the periportal iron accumulation in the hepatic lobule observed in iron overload disorders associated with inappropriately increased intestinal iron absorption9,10. Conversely, TF saturation is likely lower than average in plasma leaving the erythroid bone marrow, where most of the iron present is extracted for use by erythroid precursor cells. The erythroid bone marrow is the largest consumer of iron. Normally, two-thirds of the body iron endowment is found in developing erythroid precursors and mature red blood cells. Erythroid precursors express cell surface transferrin receptors (TfR1) that take up iron from holo-TF by receptor-mediated endocytosis11. Targeted disruption of the murine TfR1 gene causes embryonic lethality, attributable to severe anemia12. Spontaneous mutations disrupting the TF gene demonstrate the importance of TF in animals 13 and humans 14. Lack of TF results in severe iron deficient mycrocytic anemia. However, iron deficiency occurs only in hematopoietic cells whereas other tissues develop massive iron overload. This underscores the importance of the TF-TfR1 endocytic cycle in erythropoiesis11, but further demonstrates that non-hematopoietic cells have alternative mechanisms to assimilate iron. It also highlights changes in overall iron homeostasis that result from iron deficient erythropoiesis. Hepcidin synthesis is markedly depressed and intestinal iron absorption is increased in congenital hypo-transferrinemia, apparently to try to compensate for the lack of iron available to erythroid precursors13,15,16. This is compelling evidence for a signaling mechanism that allows the erythroid bone marrow to communicate its iron needs to the intestine’s absorptive epithelium (see below).
Iron absorption: Iron in food is present as ferric iron or as heme. Heme is a biologically important iron containing compound and a key source of dietary iron but the mechanism by which the enterocyte takes up heme and catabolises it to utilise the iron is still poorly understood. Currently, there are two prevailing hypotheses explaining the mechanisms of this process; first, heme is taken up by receptor mediated endocytosis; secondly, the recent discovery of a heme transporter (PCFT/HCP1) that may have the capability of transferring heme from the small intestinal lumen directly into the cytoplasm17. One important criticism of the receptor mediated endocytosis hypothesis is that it assumes iron released from heme is transported out of the internalised vesicles in order to join the labile iron pool. Currently, no such transport process has been identified. However, it is possible that divalent metal transporter 1 (DMT1) may fulfil this role in a manner analogous to its role in the transferrin receptor cycle. In recent years, two mammalian heme transporters have been discovered, namely PCFT/HCP118,19 and FLVCR20 possibly acting at the apical and basolateral site of the small intestinal enterocyte, respectively. At this early stage, however, the physiological relevance of these transporters to intestinal heme iron absorption is unclear. PCFT/HCP1 has been independently characterised as a folate/proton symporter and appears to play a key role in intestinal folate absorption. Interestingly, the folate transport capabilities of PCFT/HCP1 are at least an order of magnitude higher than that observed for heme, suggesting that folate may be the more physiologically relevant target of this transport protein. FLVCR is a heme exporter relevant for erythropoietic activity, that acts as an overflow valve for excess manufactured heme that would otherwise result in cellular toxicity. Intestinal cell line CaCo-2 does express the protein and it might be hypothesised it can exert similar function in intestinal cells too21.
Much more knowledge has been developed on non-haem iron absorption4,17. Non-haem iron requires to be converted in ferrous iron by the apical ferric reductase duodenal cytochrome B although the physiological significance of this pathway is the subject of continued debate. Following the reduction, iron crosses into the cytoplasm via an apical iron transporter, DMT122. The physiological relevance of DMT1 in iron absorption, is confirmed in the Belgrade (b) rat and mk mouse which both exhibit a microcytic, hypochromic iron deficiency anaemia due to a G185R mutation to DMT1, resulting in a dramatic decrease in DMT1 function23.
Cells regulating body iron homeostasis: Besides enterocytes other cell lines, namely macrophages, hepatocytes in adults and placental cells during fetal life, have core functions within iron metabolism, that is to acquire iron from different sources (senescent erythrocytes and holotransferrin) and to deliver it to the rest of the body according to its needs (Figure 2). To do this important function these cells have a specialised mechanism for exporting iron to plasma, that is the iron exporter ferroportin24. Ferroportin needs copper-ferroxidases to release iron to plasma transferrin, namely hephaestin in duodenal cells25 and ceruloplasmin in hepatocytes, and macrophages4. When defective, these proteins induce cellular iron retention in specific cell types as shown in hephaestin deficient mice26 and in humans with aceruloplasminemia27. Ferroportin acts under the control of hepcidin and this interaction can explain the systemic regulation of iron metabolism28. Interestingly, it has been shown that hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Janus kinase (Jak2)29. Hepcidin binding to ferroportin results in the phosphorylation of ferroportin, a step necessary for its internalization by clathrin-coated pits and the kinase responsible for the phosphorylation is Jak2. This finding provides a mechanistic explanation for the dominant inheritance of hepcidin resistant ferroportin disease30 and suggests that Jak2 might represent an important link at the interface between erythropoiesis and iron metabolism.
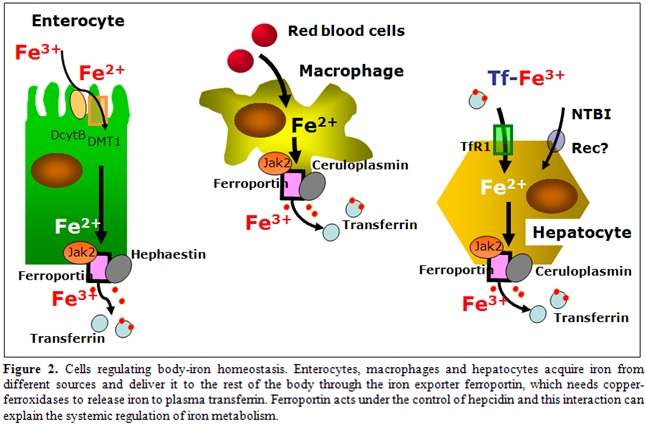
Iron storage: Following phagocytosis of old and damaged erythrocytes, tissue macrophages, particularly in the spleen, lyse cells and catabolyse hemoglobin presumably by heme oxygenase, to liberate iron. Some iron remains stored in macrophages, although some is exported to plasma TF. Ferroportin is critical for macrophage iron export and can be regulated to change the ratio between stored and released iron24. Hepatocytes represent the main depot for iron storage in normal conditions and in non-transfusional iron overload. Although the TF cycle may be involved in hepatocyte iron acquisition to some extent, non–transferrin-bound iron (NTBI) uptake pathways become particularly important when serum iron levels exceed TF binding capacity31,32. The identity of the hepatocyte NTBI uptake system is not known, but DMT1 is unlikely to be involved, because hepatocytes can accumulate iron in the absence of DMT133. Candidates for the NTBI transporter include Zip14, a member of the SLC39A zinc transporter family, which can mediate the uptake of zinc and NTBI in hepatocytes34. Hepatocytes have a large capacity to store excess iron. Most storage iron is probably in the form of ferritin, which can be mobilized when needed elsewhere in the body. Eventually, however, massive iron overload results in hepatotoxicity; hepatitis leads to fibrosis and cirrhosis. The liver is probably exposed to more NTBI than are other tissues because of the first-pass effect of the portal circulation. However, other tissues have NTBI uptake activities and load iron when NTBI is present in the plasma. The heart and endocrine tissues are particularly susceptible; cardiomyopathy and endocrinopathies are the predominant nonhepatic complications of iron overload. L-type calcium channels35 can mediate the uptake of NTBI in the myocardium, whereas other candidates, such as transient receptor potential canonical protein TRPC636, need further confirm.
Regulation of iron homeostasis.
Hepcidin: the master regulator. The liver peptide hepcidin regulates intestinal iron absorption and iron release from storage cells by binding ferroportin causing its internalization and degradation, thus exerting a general inhibitory effect on iron release in the body28,37,38. Animal models clarified the role of hepcidin as hepcidin knock-out mice developed massive iron overload39 and hepcidin over-expression induced iron deficiency 40. In physiological conditions, hepcidin production is tightly regulated in response to signals released from other organs, prevalently from bone marrow (erythroid regulator) and the iron stores (store regulator). Hepcidin levels increase in iron overload in order to limit iron absorption and are reduced up to undetectable levels in iron deficient erythropoiesis, either dependent from decreased iron supply or increased erythroid iron requirement, to allow iron acquisition16,37.
Studies on genetic disorders of iron metabolism and of corresponding animal models have identified the hemochromatosis proteins (HFE, TFR2 and HJV) as the iron-dependent regulators of hepcidin expression. Patients affected by hemochromatosis have a defective synthesis of hepcidin that is absent in JH, reduced in type 3 HH due to TFR2 mutation or inadequate to the amount of iron overload in classical HH38. Such difference is related to the role of each protein in hepcidin regulation. HFE, TFR2 and HJV are all positive regulators and represent only a part of the complex regulation of hepcidin transcription, as summarised in Figure 3, where mediators and signalling pathways of iron- inflammatory- and erythroid- dependent regulation of hepcidin are reported. Recent studies suggest a role for HFE as a component of an iron-sensing complex that involves interactions with diferric transferrin, TFR1 and TfR2 at the plasma membrane of hepatocytes 41,42.
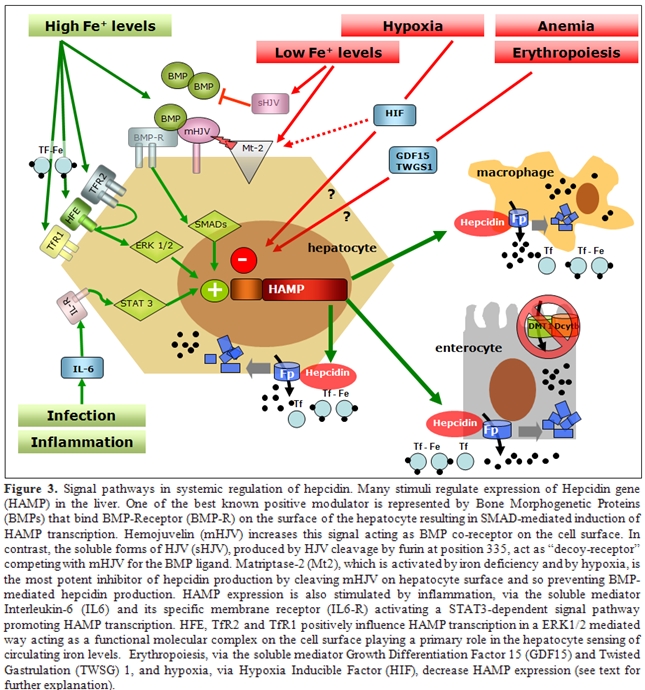
Defective HFE or TFR2 prevents formation of a functional iron sensor and signal transduction effector complex leading to dysregulated hepcidin expression as observed in hereditary hemochromatosis. Indeed, double heterozygotes for HFE and TFR2 mutations develop hemochromatosis 43, whereas this does not occur in carriers of single allelic mutations in both HFE and HJV44,45, suggesting they behave to different pathways of hepcidin regulation. The main activator of hepcidin in iron overload is HJV, the protein mutated in type 2a juvenile hemochromatosis: HJV-deficient patients and mice have undetectable levels of hepcidin46,47. HJV is a GPI linked protein that activate hepcidin as a co-receptor for Bone morphogenetic proteins (BMPs), a family of cytokines that signal through SMAD proteins as a second messenger48. Several BMPs may activate hepcidin in vitro but the relevant BMP in vivo is BMP6, since knock out mice for this BMP have no developmental defect but develop severe iron overload49. That the BMPs pathway is involved in iron homeostasis through hepcidin regulation is further demonstrated by the severe iron overload and very low hepcidin expression reported in Smad4 liver conditional knock out50. Several other signals regulate hepcidin expression. Infection and inflammation markedly increase hepcidin synthesis through the IL-6/IL-6 receptor and STAT3 pathway, a mechanism largely implicated in the pathogenesis of anemia of chronic diseases51,52. Hepcidin inhibition occurs in iron deficiency, hypoxia and erythropoiesis expansion in order to increase iron export to plasma. Several inhibitors of hepcidin have been proposed: HIF-1a that is stabilised in hypoxia/iron deficiency, reduces hepcidin transcription by binding a HIF responsive element of hepcidin promoter53, the soluble variant of HJV downregulates hepcidin in vitro by competing with mHJV for the BMP ligand54, and matriptase 2 is a serin protease recently identified as a strong hepcidin inhibitor by cleaving membrane-HJV55. Accordingly, mutations in the gene coding matriptase 2 cause the rare form of iron refractory iron deficiency anemia in mice and humans56.
Erythroid-dependent regulation of hepcidin: In vivo studies demonstrated that the effect is predominantly due to the activation of erythropoiesis and to secondary changes in plasma and tissue iron16. This was demonstrated by Pak et al57 who showed that erythropoietin suppresses hepcidin synthesis, but that this effect was lost by pharmacologic inhibition of erythropoiesis. Similarly, Vokurka et al.58 showed a dramatic increase in hepcidin expression in mice when erythropoiesis was inhibited by irradiation or posttransfusion polycythemia. The nature of the erythropoietic regulator of hepcidin is now debated, but may include one or more proteins secreted by developing erythrocytes. When HepG2 cells were treated with sera from -thalassemia patients, in which erythropoietic drive is greatly elevated, or with sera from HFE-hemochromatosis or control subjects, only -thalassemia sera decreased hepcidin mRNA59. Another study treating HepG2 cells with sera from patients with iron-deficiency anemia or -thalassemia showed similar results60. Regulation of hepcidin by erythropoietic activity is of particular importance in iron-loading anemias such as -thalassemias and diserytropoietic anemias. Studies in animal models of thalassemia indicate that in conditions of extreme ineffective erythropoiesis, there is relatively little peripheral destruction of red cells and iron accumulates more rapidly in the liver than in the spleen, consistent with the interpretation that iron loading results primarily from increased intestinal absorption59,61. Thus, very high erythropoietic activity and increased iron requirement from the bone marrow generates a signal that can override hepcidin regulation by iron, leading to increased iron absorption, but the marrow signal that modulates hepcidin expression is debated (Figure 4).
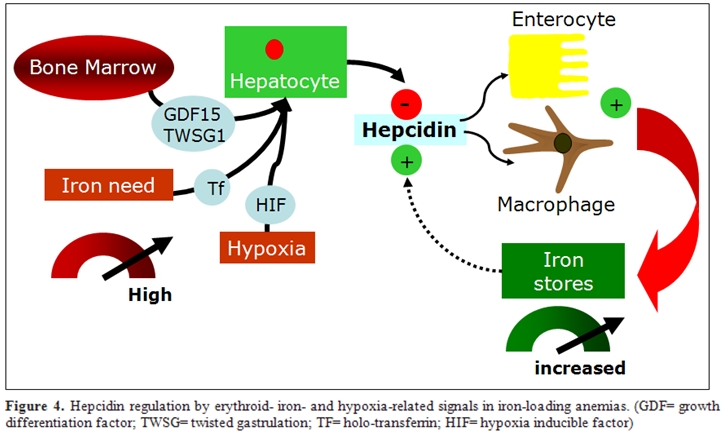
One proposed hepcidin inhibitor in these conditions is GDF15, a cytokine member of the TGF- superfamily which is strongly expressed in hemoglobinized erythroblasts at the final stages of human erythropoiesis. GDF15 is hyperexpressed in thalassemia62, CDA type 163 and, although at a lower level, in PKD and some myelodisplastic syndromes and it might mediate the bone marrow signal to the liver by partially suppressing hepcidin synthesis, as shown in hepatocyte cell colture16,62. Very recently, the same Authors, identified twisted gastrulation 1 (TWSG1) as a novel erythroid regulator of hepcidin expression in murine and human cells64. TWSG1 suppressed hepcidin in human hepatocytes through a BMP-dependent mechanism. It is proposed that TWSG1 and GDF15 act together to inappropriately inhibit hepcidin expression in thalassemia (Figure 4). Due to the expansion of erythropoiesis, TWSG1 expressed during the early stages of erythropoiesis acts indirectly by inhibiting BMP-mediated expression of hepcidin64. In thalassemia, over-expression of TWSG1 would inhibit the host’s ability to sense and respond to iron loading. It is also hypothesised that the increased expression of TWSG1 may also impact erythropoiesis in thalassemia by inhibiting the BMP4 dependent expansion of stress erythroblasts, which in turn would exacerbate the anemia.
The role of hypoxi: Several lines of evidence indicate that iron and oxygen homeostasis are tightly connected. Anemia generates tissue hypoxia and activates the cellular mediator of biological response to hypoxia: Hypoxia inducible transcription factors: HIFs. This induce a signalling cascade involving hundreds of genes. Seminal studies established an association between oxygen and iron regulation by showing that hypoxia results in higher iron absorption in mice and rats. Accordingly, both hypoxia and anemia induce the synthesis of erythropoietin (EPO) and are the two main signals that increase iron absorption independently of iron stores1,65. In addition, hypoxia was found to increase the expression of transferrin, transferrin receptor, ceruloplasmin and hemoxygenase-1 which are hypoxia-inducible HIF-1 target genes, thus enabling iron transport to erythroid tissue and cellular uptake, and iron recycling and release from stores.
Hepcidin is suppressed in human cultured hepatoma cells exposed to hypoxia66 and HIF-1 has been implicated in hypoxia-mediated hepcidin regulation53,66,67. HIF-1 binds to the hepcidin promoter in vitro and decreases is transactivation and stabilization of HIF in hepatocytes down-regulates hepcidin and increases ferroportin expression in mice. Thus, HIF-1 acts as a regulator of iron homeostasis, but recent studies also suggest a potential role of HIF-1 in iron sensing in the liver. In fact, iron deficiency increases HIF-1 levels in mice liver. In addition, one recent study demonstrates a role for HIF as a transcription factor that regulates the expression of divalent metal transporter (DMT)-1 and apical ferric reductase duodenal cytochrome b (Dcyt-b) genes, which are regulators of intestinal iron absorption68. Thus, HIF may act both as an iron sensor and iron regulator, and be an essential link between iron and oxygen homeostasis in vivo that through coordinate gene regulation mobilizes iron to support erythrocyte production. Other experimental studies further support the iron-oxygen connection, that involves iron deficient and hypoxia-dependent production of soluble-HJV69, GDF15 and Matriptase-2 in hepatocytes.
Iron metabolism in thalassemia and sickle cell disease:
Patients affected by the most severe forms of thalassemia require chronic blood transfusions to sustain life and chelation therapy to prevent iron overload. Those affected by -thalassemia intermedia do not require chronic blood transfusions but eventually develop elevated body iron loads due to ineffective erythropoiesis and hypoxia dependent hepcidin downregulation that, in turn, induces increased gastrointestinal iron absorption16,61,70. Iron absorption studies in patients affected by beta thalassemia intermedia show that the rate of iron loading from the gastrointestinal tract is approximately three to four times greater than normal71. In nontransfused patients with severe thalassemia, abnormal dietary iron absorption results in an increased body iron burden between 2 and 5 g per year depending on the severity of erythroid expansion72. If regular transfusions are required, as in -thalassemia major patients, this doubles the rate of iron accumulation. In addition to the transfusion-related iron overload, increased iron absorption also plays a role in -thalassemia major, in which its importance is inversely related to Hb levels61,73. As loading continues, the capacity of transferrin, the main transport protein of iron, to bind and detoxify this essential metal may be exceeded. The resulting nontransferrin-bound iron (NTBI) fraction within plasma may promote the generation of reactive oxygen species (ROS), propagators of oxygen-related damage74. Iron overload is responsible for the most damaging effects of the thalassemias, making iron chelation a focal point of the management of these diseases. In addition, as iron accumulates in the organs, dysfunction of the liver, endocrine glands, and heart become the main factors in limiting the survival of patients with -thalassemia2.
Iron metabolism in SCD largely differs as compared to thalassemia. SCD is an inherited disorder of hemoglobin synthesis characterized by life-long hemolytic anemia, increased erythropoiesis and a chronic inflammatory state with endothelial activation and enhanced red cell and leukocyte adhesion75,76. There is no evidence of iron overload in non-transfused SCD patients, and iron deficiency may be even develop, possibly related to intravascular hemolysis (which constitutes about a third of the hemolysis in SCD), and the resulting excessive urinary losses of iron77. The variable clinical spectrum of SCD is the consequence of multiple events and genetic susceptibility that goes beyond the occurrence of a single amino acid substitution in the beta globin chain of hemoglobin. SCD is as much a disease of endothelial dysfunction as it is a hemoglobinopathy that triggers erythrocyte polymerization. Hemolytic rate is associated with a growing list of clinical complications of SCD that fall into two partially overlapping subphenotypes: a viscosity–vaso-occlusion phenotype versus one of hemolysis-endothelial dysfunction. The viscosity-vaso-occlusion subphenotype is associated with a lower hemolytic rate, marked by a higher hemoglobin level, and low plasma hemoglobin, lactate dehydrogenase, bilirubin and arginase levels. Patients with these features have a higher incidence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis. In contrast, patients with the hemolysis-endothelial dysfunction subphenotype exhibit markers of high hemolytic rate, including low hemoglobin level, high plasma hemoglobin, LDH, bilirubin, and arginase, culminating in low nitric oxide bioavailability and high prevalence of pulmonary hypertension, leg ulceration, priapism, and stroke. Erythropoiesis is variably increased, but is not defective and nontransfused SCD patients do not spontaneously develop systemic iron overload, however, microvascular occlusion, erythrocyte sequestration and splenic infarction cause splenic iron overload78. In contrast, in nontransfused thalassemic patients the spleen is enlarged but not iron-loaded because of the lack of transfusions and because low hepcidin levels favor iron mobilization from reticuloendothelial stores78,79. Chronic transfusion therapy partially normalize many of the disparities between the diseases. In SCD patients, chronic transfusion therapy lowers the percent sickle hemoglobin to below 30%, dramatically decreasing intravascular hemolysis, splenic infarction, and the classic SCD vascular phenotypes associated with hemolysis nitric oxide dysregulation75,80. Patients with -thalassemia and chronically transfused patients with SCD develop severe iron overload with iron deposition in liver, heart, spleen, and endocrine organs2,81. Although having similar transfusional burdens and somatic iron stores, patients with chronically transfused SCD appear to be at lower risk for endocrinopathy, cardiac dysfunction, iron-mediated oxidative stress, and extrahepatic iron deposition2. Risk for hepatic fibrosis during chronic transfusion therapy for SCD occurs at a HIC level 2.7-fold higher than historically seen in thalassemia 81. Transferrin saturation and circulating nontransferrin bound iron levels are lower in transfused patients with SCD relative to patients with TM having comparable somatic iron stores, suggesting disease-specific iron handling2,74. Some early reports link NTBI to iron-related cardiac disease and may explain the prevalence of severe heart disease in thalassaemia with their higher levels of NTBI compared to almost no cases in transfused SCD2. It has been postulated that the greater systemic inflammation observed in transfused patients with SCD may limit reticuloendothelial iron export and iron absorption through hepcidin or other iron mediators, but this hypothesis has never been proven2,74. Recent data indicate that, despite transfused SCD patients exhibiting heavy total body iron burden, they are monitored on a less frequent basis for iron-related organ damage compared to thalassemia patients. The reasons for these discrepancies are multifactorial and also likely include the perceived importance of the procedure by either the practitioner or the patient. Patients with SCD have far more acute and unpredictable clinical crises compared to patients with thalassemia; therefore, iron risk assessment is not perceived as having high priority in clinical care management. What is clear from these preliminary baseline data is that the disparity in the care of SCD patients who receive transfusions requires further study and that guidelines for the assessment of this select group of patients should be supported by existing evidence and expert opinion, until the full effect of iron overload in this population is better understood.
Hepcidin levels in thalassemia and SCD patients: Thalassemia intermedia and major are the most studied human models of hepcidin modulation by ineffective erythropoiesis alone and the combined and opposite effect of both ineffective erythropoiesis and transfusion dependent iron overload, respectively. Regular transfusions, in fact, induce massive iron loading but also inhibit erythropoietic drive. Accordingly, hepcidin production is higher in thalassemia major than in thalassemia intermedia although still inappropriate to the massive transfusional iron loading that partially counteracts the erythropoietic-dependent hepcidin downregulation70,82,83. Transfusions strongly influence hepcidin production as shown by the significant post-transfusion increase of urinary hepcidin levels in thalassemia patients, probably related to transfusion dependent suppression of erythropoiesis84. This result is also supported by Jenkins et al85 who found 5- to 8-fold increase in hepcidin mRNA in liver specimens obtained 1–3 days after red cell transfusion in patients with thalassemia major and SCD. This indicates that hemoglobin levels at the time of biopsy are a significant variable in determining liver hepcidin mRNA values.
Data on hepcidin levels in SCD are very limited and no data are presently available on GDF15 and TWSG1 expression. Kearney et al found significantly lower levels of urinary hepcidin in nine SCD patients when compared with normal controls84. In their analysis, hepcidin levels were negatively correlated with markers of erythroid proliferation, thus supporting the theory that hepcidin production is suppressed in scenarios of chronic haemolytic anemia and increased erythropoiesis. This finding, however, contrasts with the common knowledge that non-transfused patients with SCD do not develop iron overload. Although chronic hemolysis is one of the main features of SCD, the pathophysiology of SCD is not entirely red-cell related however, as vaso-occlusive crises and resultant tissue damage have been shown to result in chronic inflammation74,76, a condition that might influence hepcidin production51,86. In addition, transfusional iron overload might further increase hepcidin production. Thus, hepcidin levels in patients with SCD might be under the influence of different and contrasting stimuli, possibly leading to marked variability. Very recently Kroot et al 87 studied hepcidin and several serum parameters of erythropoietic, inflammatory and iron status in 16 patients with SCD. They found various serum parameters to vary widely within this population. In particular, they observed very high serum ferritin levels not simply related to the transfusion history and in the presence of normal transferrin saturation. This finding confirms that serum ferritin in SCD might not be suitable in the evaluation of hepatocyte iron loading and suggest an iron distribution pattern of the anemia of chronic disease, with relatively more iron in the RE system. The median serum and urine hepcidin-25 levels were similar for patients and controls. More precisely, serum hepcidin-25 levels were below the lower limit of detection in 5 SCD patients while in the rest they were within the normal range. Interestingly and not so easy to explain, the five patients with the lowest hepcidin levels did not show marked alterations of serum iron indices (transferrin saturation and serum ferritin ranged between 23-72%, and 40-213 g/L, respectively). Results confirmed that also in SCD patients, erythropoiesis down-regulates hepcidin-25. In fact, when only sTfR was increased, serum hepcidin-25 levels were in the lower normal range or even not detectable. In cases where, together with a substantially increased sTfR, inflammation and/or high iron stores were also present, serum hepcidin-25 levels were in the normal range confirming the induction of hepcidin by inflammation and elevated iron stores in SCD patients.
Iron metabolism:
Body iron pathways. Because there is no known regulated mechanism for iron excretion, and the amount of iron entering the body each day represents less than 0.1% of the total body iron endowment, most circulating iron must be derived from the recycling of iron already within the system3 (Figure 1). From a quantitative point of view, the most important pathway of iron metabolism is the unidirectional recycling of iron from senescent red blood cells to the erythroid bone marrow through macrophages1,4. Renewed interest has been developed on the role of macrophages within the erythroblastic islands, the specialized niches in which erythroid precursors proliferate, differentiate, and enucleate in the bone marrow 5.

These hematopoietic subcompartments are composed of erythroblasts surrounding a central macrophage. It was suggested that the macrophage functions as a “nurse” cell, providing iron to developing erythroblasts for heme synthesis6. An attractive hypothesis proposed for central macrophages is that ferritin, synthesized by macrophages, secreted via exocytosis, and subsequently taken up by erythroblasts could be a source of iron for heme synthesis7. It is not known whether central macrophages might release iron also by the ferroportin-dependent exporting pathway and further studies are needed to clarify all this matter.
The second pathway is the cycling of iron from hepatocytes to the blood and viceversa, according to body needs and the third is iron absorption through duodenal and upper jejunum that balances the 1-2 mg daily iron loss occurring through cellular exfoliation (see below: iron absorption and iron storage paragraphs).
Iron transport and utilization: The systemic transporter of iron in the body is transferring, an abundant, high affinity iron-binding protein. Under normal circumstances, TF carries nearly all serum iron collected from duodenal absorptive epithelium, macrophages and hepatocytes, and dampens its reactivity. Very small amounts of iron may be loosely associated with albumin or small molecules. In normal human subjects, iron occupies approximately 30% of the iron-binding sites on plasma TF1. The saturation of TF by iron shows diurnal fluctuation exhibiting a morning peak and an evening nadir8. It is likely to be higher in the portal circulation, where recently absorbed iron from the intestine enters the circulation and passes through the liver. This first-pass effect may explain the periportal iron accumulation in the hepatic lobule observed in iron overload disorders associated with inappropriately increased intestinal iron absorption9,10. Conversely, TF saturation is likely lower than average in plasma leaving the erythroid bone marrow, where most of the iron present is extracted for use by erythroid precursor cells. The erythroid bone marrow is the largest consumer of iron. Normally, two-thirds of the body iron endowment is found in developing erythroid precursors and mature red blood cells. Erythroid precursors express cell surface transferrin receptors (TfR1) that take up iron from holo-TF by receptor-mediated endocytosis11. Targeted disruption of the murine TfR1 gene causes embryonic lethality, attributable to severe anemia12. Spontaneous mutations disrupting the TF gene demonstrate the importance of TF in animals 13 and humans 14. Lack of TF results in severe iron deficient mycrocytic anemia. However, iron deficiency occurs only in hematopoietic cells whereas other tissues develop massive iron overload. This underscores the importance of the TF-TfR1 endocytic cycle in erythropoiesis11, but further demonstrates that non-hematopoietic cells have alternative mechanisms to assimilate iron. It also highlights changes in overall iron homeostasis that result from iron deficient erythropoiesis. Hepcidin synthesis is markedly depressed and intestinal iron absorption is increased in congenital hypo-transferrinemia, apparently to try to compensate for the lack of iron available to erythroid precursors13,15,16. This is compelling evidence for a signaling mechanism that allows the erythroid bone marrow to communicate its iron needs to the intestine’s absorptive epithelium (see below).
Iron absorption: Iron in food is present as ferric iron or as heme. Heme is a biologically important iron containing compound and a key source of dietary iron but the mechanism by which the enterocyte takes up heme and catabolises it to utilise the iron is still poorly understood. Currently, there are two prevailing hypotheses explaining the mechanisms of this process; first, heme is taken up by receptor mediated endocytosis; secondly, the recent discovery of a heme transporter (PCFT/HCP1) that may have the capability of transferring heme from the small intestinal lumen directly into the cytoplasm17. One important criticism of the receptor mediated endocytosis hypothesis is that it assumes iron released from heme is transported out of the internalised vesicles in order to join the labile iron pool. Currently, no such transport process has been identified. However, it is possible that divalent metal transporter 1 (DMT1) may fulfil this role in a manner analogous to its role in the transferrin receptor cycle. In recent years, two mammalian heme transporters have been discovered, namely PCFT/HCP118,19 and FLVCR20 possibly acting at the apical and basolateral site of the small intestinal enterocyte, respectively. At this early stage, however, the physiological relevance of these transporters to intestinal heme iron absorption is unclear. PCFT/HCP1 has been independently characterised as a folate/proton symporter and appears to play a key role in intestinal folate absorption. Interestingly, the folate transport capabilities of PCFT/HCP1 are at least an order of magnitude higher than that observed for heme, suggesting that folate may be the more physiologically relevant target of this transport protein. FLVCR is a heme exporter relevant for erythropoietic activity, that acts as an overflow valve for excess manufactured heme that would otherwise result in cellular toxicity. Intestinal cell line CaCo-2 does express the protein and it might be hypothesised it can exert similar function in intestinal cells too21.
Much more knowledge has been developed on non-haem iron absorption4,17. Non-haem iron requires to be converted in ferrous iron by the apical ferric reductase duodenal cytochrome B although the physiological significance of this pathway is the subject of continued debate. Following the reduction, iron crosses into the cytoplasm via an apical iron transporter, DMT122. The physiological relevance of DMT1 in iron absorption, is confirmed in the Belgrade (b) rat and mk mouse which both exhibit a microcytic, hypochromic iron deficiency anaemia due to a G185R mutation to DMT1, resulting in a dramatic decrease in DMT1 function23.
Cells regulating body iron homeostasis: Besides enterocytes other cell lines, namely macrophages, hepatocytes in adults and placental cells during fetal life, have core functions within iron metabolism, that is to acquire iron from different sources (senescent erythrocytes and holotransferrin) and to deliver it to the rest of the body according to its needs (Figure 2). To do this important function these cells have a specialised mechanism for exporting iron to plasma, that is the iron exporter ferroportin24. Ferroportin needs copper-ferroxidases to release iron to plasma transferrin, namely hephaestin in duodenal cells25 and ceruloplasmin in hepatocytes, and macrophages4. When defective, these proteins induce cellular iron retention in specific cell types as shown in hephaestin deficient mice26 and in humans with aceruloplasminemia27. Ferroportin acts under the control of hepcidin and this interaction can explain the systemic regulation of iron metabolism28. Interestingly, it has been shown that hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Janus kinase (Jak2)29. Hepcidin binding to ferroportin results in the phosphorylation of ferroportin, a step necessary for its internalization by clathrin-coated pits and the kinase responsible for the phosphorylation is Jak2. This finding provides a mechanistic explanation for the dominant inheritance of hepcidin resistant ferroportin disease30 and suggests that Jak2 might represent an important link at the interface between erythropoiesis and iron metabolism.

Iron storage: Following phagocytosis of old and damaged erythrocytes, tissue macrophages, particularly in the spleen, lyse cells and catabolyse hemoglobin presumably by heme oxygenase, to liberate iron. Some iron remains stored in macrophages, although some is exported to plasma TF. Ferroportin is critical for macrophage iron export and can be regulated to change the ratio between stored and released iron24. Hepatocytes represent the main depot for iron storage in normal conditions and in non-transfusional iron overload. Although the TF cycle may be involved in hepatocyte iron acquisition to some extent, non–transferrin-bound iron (NTBI) uptake pathways become particularly important when serum iron levels exceed TF binding capacity31,32. The identity of the hepatocyte NTBI uptake system is not known, but DMT1 is unlikely to be involved, because hepatocytes can accumulate iron in the absence of DMT133. Candidates for the NTBI transporter include Zip14, a member of the SLC39A zinc transporter family, which can mediate the uptake of zinc and NTBI in hepatocytes34. Hepatocytes have a large capacity to store excess iron. Most storage iron is probably in the form of ferritin, which can be mobilized when needed elsewhere in the body. Eventually, however, massive iron overload results in hepatotoxicity; hepatitis leads to fibrosis and cirrhosis. The liver is probably exposed to more NTBI than are other tissues because of the first-pass effect of the portal circulation. However, other tissues have NTBI uptake activities and load iron when NTBI is present in the plasma. The heart and endocrine tissues are particularly susceptible; cardiomyopathy and endocrinopathies are the predominant nonhepatic complications of iron overload. L-type calcium channels35 can mediate the uptake of NTBI in the myocardium, whereas other candidates, such as transient receptor potential canonical protein TRPC636, need further confirm.
Regulation of iron homeostasis.
Hepcidin: the master regulator. The liver peptide hepcidin regulates intestinal iron absorption and iron release from storage cells by binding ferroportin causing its internalization and degradation, thus exerting a general inhibitory effect on iron release in the body28,37,38. Animal models clarified the role of hepcidin as hepcidin knock-out mice developed massive iron overload39 and hepcidin over-expression induced iron deficiency 40. In physiological conditions, hepcidin production is tightly regulated in response to signals released from other organs, prevalently from bone marrow (erythroid regulator) and the iron stores (store regulator). Hepcidin levels increase in iron overload in order to limit iron absorption and are reduced up to undetectable levels in iron deficient erythropoiesis, either dependent from decreased iron supply or increased erythroid iron requirement, to allow iron acquisition16,37.
Studies on genetic disorders of iron metabolism and of corresponding animal models have identified the hemochromatosis proteins (HFE, TFR2 and HJV) as the iron-dependent regulators of hepcidin expression. Patients affected by hemochromatosis have a defective synthesis of hepcidin that is absent in JH, reduced in type 3 HH due to TFR2 mutation or inadequate to the amount of iron overload in classical HH38. Such difference is related to the role of each protein in hepcidin regulation. HFE, TFR2 and HJV are all positive regulators and represent only a part of the complex regulation of hepcidin transcription, as summarised in Figure 3, where mediators and signalling pathways of iron- inflammatory- and erythroid- dependent regulation of hepcidin are reported. Recent studies suggest a role for HFE as a component of an iron-sensing complex that involves interactions with diferric transferrin, TFR1 and TfR2 at the plasma membrane of hepatocytes 41,42.

Defective HFE or TFR2 prevents formation of a functional iron sensor and signal transduction effector complex leading to dysregulated hepcidin expression as observed in hereditary hemochromatosis. Indeed, double heterozygotes for HFE and TFR2 mutations develop hemochromatosis 43, whereas this does not occur in carriers of single allelic mutations in both HFE and HJV44,45, suggesting they behave to different pathways of hepcidin regulation. The main activator of hepcidin in iron overload is HJV, the protein mutated in type 2a juvenile hemochromatosis: HJV-deficient patients and mice have undetectable levels of hepcidin46,47. HJV is a GPI linked protein that activate hepcidin as a co-receptor for Bone morphogenetic proteins (BMPs), a family of cytokines that signal through SMAD proteins as a second messenger48. Several BMPs may activate hepcidin in vitro but the relevant BMP in vivo is BMP6, since knock out mice for this BMP have no developmental defect but develop severe iron overload49. That the BMPs pathway is involved in iron homeostasis through hepcidin regulation is further demonstrated by the severe iron overload and very low hepcidin expression reported in Smad4 liver conditional knock out50. Several other signals regulate hepcidin expression. Infection and inflammation markedly increase hepcidin synthesis through the IL-6/IL-6 receptor and STAT3 pathway, a mechanism largely implicated in the pathogenesis of anemia of chronic diseases51,52. Hepcidin inhibition occurs in iron deficiency, hypoxia and erythropoiesis expansion in order to increase iron export to plasma. Several inhibitors of hepcidin have been proposed: HIF-1a that is stabilised in hypoxia/iron deficiency, reduces hepcidin transcription by binding a HIF responsive element of hepcidin promoter53, the soluble variant of HJV downregulates hepcidin in vitro by competing with mHJV for the BMP ligand54, and matriptase 2 is a serin protease recently identified as a strong hepcidin inhibitor by cleaving membrane-HJV55. Accordingly, mutations in the gene coding matriptase 2 cause the rare form of iron refractory iron deficiency anemia in mice and humans56.
Erythroid-dependent regulation of hepcidin: In vivo studies demonstrated that the effect is predominantly due to the activation of erythropoiesis and to secondary changes in plasma and tissue iron16. This was demonstrated by Pak et al57 who showed that erythropoietin suppresses hepcidin synthesis, but that this effect was lost by pharmacologic inhibition of erythropoiesis. Similarly, Vokurka et al.58 showed a dramatic increase in hepcidin expression in mice when erythropoiesis was inhibited by irradiation or posttransfusion polycythemia. The nature of the erythropoietic regulator of hepcidin is now debated, but may include one or more proteins secreted by developing erythrocytes. When HepG2 cells were treated with sera from -thalassemia patients, in which erythropoietic drive is greatly elevated, or with sera from HFE-hemochromatosis or control subjects, only -thalassemia sera decreased hepcidin mRNA59. Another study treating HepG2 cells with sera from patients with iron-deficiency anemia or -thalassemia showed similar results60. Regulation of hepcidin by erythropoietic activity is of particular importance in iron-loading anemias such as -thalassemias and diserytropoietic anemias. Studies in animal models of thalassemia indicate that in conditions of extreme ineffective erythropoiesis, there is relatively little peripheral destruction of red cells and iron accumulates more rapidly in the liver than in the spleen, consistent with the interpretation that iron loading results primarily from increased intestinal absorption59,61. Thus, very high erythropoietic activity and increased iron requirement from the bone marrow generates a signal that can override hepcidin regulation by iron, leading to increased iron absorption, but the marrow signal that modulates hepcidin expression is debated (Figure 4).

One proposed hepcidin inhibitor in these conditions is GDF15, a cytokine member of the TGF- superfamily which is strongly expressed in hemoglobinized erythroblasts at the final stages of human erythropoiesis. GDF15 is hyperexpressed in thalassemia62, CDA type 163 and, although at a lower level, in PKD and some myelodisplastic syndromes and it might mediate the bone marrow signal to the liver by partially suppressing hepcidin synthesis, as shown in hepatocyte cell colture16,62. Very recently, the same Authors, identified twisted gastrulation 1 (TWSG1) as a novel erythroid regulator of hepcidin expression in murine and human cells64. TWSG1 suppressed hepcidin in human hepatocytes through a BMP-dependent mechanism. It is proposed that TWSG1 and GDF15 act together to inappropriately inhibit hepcidin expression in thalassemia (Figure 4). Due to the expansion of erythropoiesis, TWSG1 expressed during the early stages of erythropoiesis acts indirectly by inhibiting BMP-mediated expression of hepcidin64. In thalassemia, over-expression of TWSG1 would inhibit the host’s ability to sense and respond to iron loading. It is also hypothesised that the increased expression of TWSG1 may also impact erythropoiesis in thalassemia by inhibiting the BMP4 dependent expansion of stress erythroblasts, which in turn would exacerbate the anemia.
The role of hypoxi: Several lines of evidence indicate that iron and oxygen homeostasis are tightly connected. Anemia generates tissue hypoxia and activates the cellular mediator of biological response to hypoxia: Hypoxia inducible transcription factors: HIFs. This induce a signalling cascade involving hundreds of genes. Seminal studies established an association between oxygen and iron regulation by showing that hypoxia results in higher iron absorption in mice and rats. Accordingly, both hypoxia and anemia induce the synthesis of erythropoietin (EPO) and are the two main signals that increase iron absorption independently of iron stores1,65. In addition, hypoxia was found to increase the expression of transferrin, transferrin receptor, ceruloplasmin and hemoxygenase-1 which are hypoxia-inducible HIF-1 target genes, thus enabling iron transport to erythroid tissue and cellular uptake, and iron recycling and release from stores.
Hepcidin is suppressed in human cultured hepatoma cells exposed to hypoxia66 and HIF-1 has been implicated in hypoxia-mediated hepcidin regulation53,66,67. HIF-1 binds to the hepcidin promoter in vitro and decreases is transactivation and stabilization of HIF in hepatocytes down-regulates hepcidin and increases ferroportin expression in mice. Thus, HIF-1 acts as a regulator of iron homeostasis, but recent studies also suggest a potential role of HIF-1 in iron sensing in the liver. In fact, iron deficiency increases HIF-1 levels in mice liver. In addition, one recent study demonstrates a role for HIF as a transcription factor that regulates the expression of divalent metal transporter (DMT)-1 and apical ferric reductase duodenal cytochrome b (Dcyt-b) genes, which are regulators of intestinal iron absorption68. Thus, HIF may act both as an iron sensor and iron regulator, and be an essential link between iron and oxygen homeostasis in vivo that through coordinate gene regulation mobilizes iron to support erythrocyte production. Other experimental studies further support the iron-oxygen connection, that involves iron deficient and hypoxia-dependent production of soluble-HJV69, GDF15 and Matriptase-2 in hepatocytes.
Iron metabolism in thalassemia and sickle cell disease:
Patients affected by the most severe forms of thalassemia require chronic blood transfusions to sustain life and chelation therapy to prevent iron overload. Those affected by -thalassemia intermedia do not require chronic blood transfusions but eventually develop elevated body iron loads due to ineffective erythropoiesis and hypoxia dependent hepcidin downregulation that, in turn, induces increased gastrointestinal iron absorption16,61,70. Iron absorption studies in patients affected by beta thalassemia intermedia show that the rate of iron loading from the gastrointestinal tract is approximately three to four times greater than normal71. In nontransfused patients with severe thalassemia, abnormal dietary iron absorption results in an increased body iron burden between 2 and 5 g per year depending on the severity of erythroid expansion72. If regular transfusions are required, as in -thalassemia major patients, this doubles the rate of iron accumulation. In addition to the transfusion-related iron overload, increased iron absorption also plays a role in -thalassemia major, in which its importance is inversely related to Hb levels61,73. As loading continues, the capacity of transferrin, the main transport protein of iron, to bind and detoxify this essential metal may be exceeded. The resulting nontransferrin-bound iron (NTBI) fraction within plasma may promote the generation of reactive oxygen species (ROS), propagators of oxygen-related damage74. Iron overload is responsible for the most damaging effects of the thalassemias, making iron chelation a focal point of the management of these diseases. In addition, as iron accumulates in the organs, dysfunction of the liver, endocrine glands, and heart become the main factors in limiting the survival of patients with -thalassemia2.
Iron metabolism in SCD largely differs as compared to thalassemia. SCD is an inherited disorder of hemoglobin synthesis characterized by life-long hemolytic anemia, increased erythropoiesis and a chronic inflammatory state with endothelial activation and enhanced red cell and leukocyte adhesion75,76. There is no evidence of iron overload in non-transfused SCD patients, and iron deficiency may be even develop, possibly related to intravascular hemolysis (which constitutes about a third of the hemolysis in SCD), and the resulting excessive urinary losses of iron77. The variable clinical spectrum of SCD is the consequence of multiple events and genetic susceptibility that goes beyond the occurrence of a single amino acid substitution in the beta globin chain of hemoglobin. SCD is as much a disease of endothelial dysfunction as it is a hemoglobinopathy that triggers erythrocyte polymerization. Hemolytic rate is associated with a growing list of clinical complications of SCD that fall into two partially overlapping subphenotypes: a viscosity–vaso-occlusion phenotype versus one of hemolysis-endothelial dysfunction. The viscosity-vaso-occlusion subphenotype is associated with a lower hemolytic rate, marked by a higher hemoglobin level, and low plasma hemoglobin, lactate dehydrogenase, bilirubin and arginase levels. Patients with these features have a higher incidence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis. In contrast, patients with the hemolysis-endothelial dysfunction subphenotype exhibit markers of high hemolytic rate, including low hemoglobin level, high plasma hemoglobin, LDH, bilirubin, and arginase, culminating in low nitric oxide bioavailability and high prevalence of pulmonary hypertension, leg ulceration, priapism, and stroke. Erythropoiesis is variably increased, but is not defective and nontransfused SCD patients do not spontaneously develop systemic iron overload, however, microvascular occlusion, erythrocyte sequestration and splenic infarction cause splenic iron overload78. In contrast, in nontransfused thalassemic patients the spleen is enlarged but not iron-loaded because of the lack of transfusions and because low hepcidin levels favor iron mobilization from reticuloendothelial stores78,79. Chronic transfusion therapy partially normalize many of the disparities between the diseases. In SCD patients, chronic transfusion therapy lowers the percent sickle hemoglobin to below 30%, dramatically decreasing intravascular hemolysis, splenic infarction, and the classic SCD vascular phenotypes associated with hemolysis nitric oxide dysregulation75,80. Patients with -thalassemia and chronically transfused patients with SCD develop severe iron overload with iron deposition in liver, heart, spleen, and endocrine organs2,81. Although having similar transfusional burdens and somatic iron stores, patients with chronically transfused SCD appear to be at lower risk for endocrinopathy, cardiac dysfunction, iron-mediated oxidative stress, and extrahepatic iron deposition2. Risk for hepatic fibrosis during chronic transfusion therapy for SCD occurs at a HIC level 2.7-fold higher than historically seen in thalassemia 81. Transferrin saturation and circulating nontransferrin bound iron levels are lower in transfused patients with SCD relative to patients with TM having comparable somatic iron stores, suggesting disease-specific iron handling2,74. Some early reports link NTBI to iron-related cardiac disease and may explain the prevalence of severe heart disease in thalassaemia with their higher levels of NTBI compared to almost no cases in transfused SCD2. It has been postulated that the greater systemic inflammation observed in transfused patients with SCD may limit reticuloendothelial iron export and iron absorption through hepcidin or other iron mediators, but this hypothesis has never been proven2,74. Recent data indicate that, despite transfused SCD patients exhibiting heavy total body iron burden, they are monitored on a less frequent basis for iron-related organ damage compared to thalassemia patients. The reasons for these discrepancies are multifactorial and also likely include the perceived importance of the procedure by either the practitioner or the patient. Patients with SCD have far more acute and unpredictable clinical crises compared to patients with thalassemia; therefore, iron risk assessment is not perceived as having high priority in clinical care management. What is clear from these preliminary baseline data is that the disparity in the care of SCD patients who receive transfusions requires further study and that guidelines for the assessment of this select group of patients should be supported by existing evidence and expert opinion, until the full effect of iron overload in this population is better understood.
Hepcidin levels in thalassemia and SCD patients: Thalassemia intermedia and major are the most studied human models of hepcidin modulation by ineffective erythropoiesis alone and the combined and opposite effect of both ineffective erythropoiesis and transfusion dependent iron overload, respectively. Regular transfusions, in fact, induce massive iron loading but also inhibit erythropoietic drive. Accordingly, hepcidin production is higher in thalassemia major than in thalassemia intermedia although still inappropriate to the massive transfusional iron loading that partially counteracts the erythropoietic-dependent hepcidin downregulation70,82,83. Transfusions strongly influence hepcidin production as shown by the significant post-transfusion increase of urinary hepcidin levels in thalassemia patients, probably related to transfusion dependent suppression of erythropoiesis84. This result is also supported by Jenkins et al85 who found 5- to 8-fold increase in hepcidin mRNA in liver specimens obtained 1–3 days after red cell transfusion in patients with thalassemia major and SCD. This indicates that hemoglobin levels at the time of biopsy are a significant variable in determining liver hepcidin mRNA values.
Data on hepcidin levels in SCD are very limited and no data are presently available on GDF15 and TWSG1 expression. Kearney et al found significantly lower levels of urinary hepcidin in nine SCD patients when compared with normal controls84. In their analysis, hepcidin levels were negatively correlated with markers of erythroid proliferation, thus supporting the theory that hepcidin production is suppressed in scenarios of chronic haemolytic anemia and increased erythropoiesis. This finding, however, contrasts with the common knowledge that non-transfused patients with SCD do not develop iron overload. Although chronic hemolysis is one of the main features of SCD, the pathophysiology of SCD is not entirely red-cell related however, as vaso-occlusive crises and resultant tissue damage have been shown to result in chronic inflammation74,76, a condition that might influence hepcidin production51,86. In addition, transfusional iron overload might further increase hepcidin production. Thus, hepcidin levels in patients with SCD might be under the influence of different and contrasting stimuli, possibly leading to marked variability. Very recently Kroot et al 87 studied hepcidin and several serum parameters of erythropoietic, inflammatory and iron status in 16 patients with SCD. They found various serum parameters to vary widely within this population. In particular, they observed very high serum ferritin levels not simply related to the transfusion history and in the presence of normal transferrin saturation. This finding confirms that serum ferritin in SCD might not be suitable in the evaluation of hepatocyte iron loading and suggest an iron distribution pattern of the anemia of chronic disease, with relatively more iron in the RE system. The median serum and urine hepcidin-25 levels were similar for patients and controls. More precisely, serum hepcidin-25 levels were below the lower limit of detection in 5 SCD patients while in the rest they were within the normal range. Interestingly and not so easy to explain, the five patients with the lowest hepcidin levels did not show marked alterations of serum iron indices (transferrin saturation and serum ferritin ranged between 23-72%, and 40-213 g/L, respectively). Results confirmed that also in SCD patients, erythropoiesis down-regulates hepcidin-25. In fact, when only sTfR was increased, serum hepcidin-25 levels were in the lower normal range or even not detectable. In cases where, together with a substantially increased sTfR, inflammation and/or high iron stores were also present, serum hepcidin-25 levels were in the normal range confirming the induction of hepcidin by inflammation and elevated iron stores in SCD patients.
Conclusion
Erythropoiesis
and iron metabolism are inextricably linked. Hepcidin has a key role in
coordinating iron procurement according to erythropoietic
requirement. The recent tremendous advancement in the iron field have
shed light on the various signals regulating hepcidin production and
showed that erythroid-dependent regulation is absolutely prevailing.
There are however, some issues to be clarified: a. if mediators other
than GDF15 and TWSG1 exist and which are their interactions and
mechanisms of hepcidin regulation; b. why, despite anemia and increased
iron demand, and the evidence of reduced hepcidin synthesis, patients
with haemolytic anemias do not develop the same amount of iron overload
observed in the iron-loading anemias where ineffective erythropoiesis
prevails on hemolysis. New links between erythropoiesis and iron
metabolism have recently suggested: the link between
Epo/EpoR/Jak2/Stat5 signalling and TfR1 mRNA transcription 88 and the
role of Jak2 in the phosphorylation and recycling of ferroportin 29. In
addition, based on preliminary studies in animal models of thalassemia,
it has been suggested that use of Jak2 and analogous of hepcidin
peptide might have a role in limiting ineffective erythropoiesis and
increased iron absorption 73. Greater understanding on hepcidin
regulation and function, and on the other molecules involved in the
intricate association between iron and erythropoiesis, will shed light
on new scenarios and will help develop more effective therapeutic
options for disorders of iron homeostasis and erythropoiesis.
References
- Finch C. Regulators of iron balance in
humans. Blood. 1994;84:1697-1702.

- Fung EB, Harmatz P, Milet M, et al.
Morbidity and mortality in chronically transfused subjects with
thalassemia and sickle cell disease: A report from the multi-center
study of iron overload. Am J Hematol. 2007;82:255-265.

- De Domenico I, McVey Ward D, Kaplan J.
Regulation of iron acquisition and storage: consequences for
iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72-81.

- Andrews NC, Schmidt PJ. Iron homeostasis.
Annu Rev Physiol. 2007;69:69-85.

- Chasis JA, Mohandas N. Erythroblastic
islands: niches for erythropoiesis. Blood. 2008;112:470-478.

- Bessis MC, Breton-Gorius J. Iron
metabolism in the bone marrow as seen by electron microscopy: a
critical review. Blood. 1962;19:635-663.

- Leimberg MJ, Prus E, Konijn AM, Fibach
E. Macrophages function as a ferritin iron source for cultured human
erythroid precursors. J Cell Biochem. 2008;103:1211-1218.

- Uchida T, Akitsuki T, Kimura H, Tanaka
T, Matsuda S, Kariyone S. Relationship among plasma iron, plasma iron
turnover, and reticuloendothelial iron release. Blood. 1983;61:799-802.

- Piperno A. Classification and diagnosis of
iron overload. Haematologica. 1998;83:447-455.

- Deugnier YM, Loreal O, Turlin B, et
al. Liver pathology in genetic hemochromatosis: a review of 135
homozygous cases and their bioclinical correlations. Gastroenterology.
1992;102:2050-2059.

- Hentze MW, Muckenthaler MU, Andrews
NC. Balancing acts: molecular control of mammalian iron metabolism.
Cell. 2004;117:285-297.

- Levy JE, Jin O, Fujiwara Y, Kuo F,
Andrews NC. Transferrin receptor is necessary for development of
erythrocytes and the nervous system. Nat Genet. 1999;21:396-399.

- Bernstein SE. Hereditary
hypotransferrinemia with hemosiderosis, a murine disorder resembling
human atransferrinemia. J Lab Clin Med. 1987;110:690-705.

- Goldwurm S, Casati C, Venturi N, et
al. Biochemical and genetic defects underlying human congenital
hypotransferrinemia. Hematol J. 2000;1:390-398.

- Trombini P, Coliva T, Nemeth E, et
al. Effects of plasma transfusion on hepcidin production in human
congenital hypotransferrinemia. Haematologica. 2007;92:1407-1410.

- Nemeth E. Iron regulation and
erythropoiesis. Curr Opin Hematol. 2008;15:169-175.

- West AR, Oates PS. Mechanisms of heme
iron absorption: current questions and controversies. World J
Gastroenterol. 2008;14:4101-4110.

- Inoue K, Nakai Y, Ueda S, et al.
Functional characterization of PCFT/HCP1 as the molecular entity of the
carrier-mediated intestinal folate transport system in the rat model.
Am J Physiol Gastrointest Liver Physiol. 2008;294:G660-668.

- Shayeghi M, Latunde-Dada GO, Oakhill
JS, et al. Identification of an intestinal heme transporter. Cell.
2005;122:789-801.

- Quigley JG, Yang Z, Worthington MT,
et al. Identification of a human heme exporter that is essential for
erythropoiesis. Cell. 2004;118:757-766.

- Uc A, Stokes JB, Britigan BE. Heme
transport exhibits polarity in Caco-2 cells: evidence for an active and
membrane protein-mediated process. Am J Physiol Gastrointest Liver
Physiol. 2004;287:G1150-1157.

- Andrews NC. The iron transporter DMT1. Int
J Biochem Cell Biol. 1999;31:991-994.

- Fleming MD, Romano MA, Su MA, Garrick
LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade
(b) rat: evidence of a role for Nramp2 in endosomal iron transport.
Proc Natl Acad Sci U S A. 1998;95:1148-1153.

- Ganz T. Cellular iron: ferroportin is the
only way out. Cell Metab. 2005;1:155-157.

- Frazer DM, Vulpe CD, McKie AT, et al.
Cloning and gastrointestinal expression of rat hephaestin: relationship
to other iron transport proteins. Am J Physiol Gastrointest Liver
Physiol. 2001;281:G931-939.

- Vulpe CD, Kuo YM, Murphy TL, et al.
Hephaestin, a ceruloplasmin homologue implicated in intestinal iron
transport, is defective in the sla mouse. Nat Genet. 1999;21:195-199.

- Mariani R, Arosio C, Pelucchi S, et
al. Iron chelation therapy in aceruloplasminaemia: study of a patient
with a novel missense mutation. Gut. 2004;53:756-758.

- Nemeth E, Tuttle MS, Powelson J, et
al. Hepcidin regulates cellular iron efflux by binding to ferroportin
and inducing its internalization. Science. 2004;306:2090-2093.

- De Domenico I, Lo E, Ward DM, Kaplan
J. Hepcidin-induced internalization of ferroportin requires binding and
cooperative interaction with Jak2. Proc Natl Acad Sci U S A.
2009;106:3800-3805.

- De Domenico I, Ward DM, Musci G,
Kaplan J. Iron overload due to mutations in ferroportin. Haematologica.
2006;91:92-95.

- Barisani D, Berg CL, Wessling-Resnick
M, Gollan JL. Evidence for a low Km transporter for
non-transferrin-bound iron in isolated rat hepatocytes. Am J Physiol.
1995;269:G570-576.

- Anderson GJ. Non-transferrin-bound iron
and cellular toxicity. J Gastroenterol Hepatol. 1999;14:105-108.

- Gunshin H, Fujiwara Y, Custodio AO,
Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal
iron absorption and erythropoiesis but dispensable in placenta and
liver. J Clin Invest. 2005;115:1258-1266.

- Liuzzi JP, Aydemir F, Nam H, Knutson
MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron
uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612-13617.

- Oudit GY, Sun H, Trivieri MG, et al.
L-type Ca2+ channels provide a major pathway for iron entry into
cardiomyocytes in iron-overload cardiomyopathy. Nat Med.
2003;9:1187-1194.

- Mwanjewe J, Grover AK. Role of
transient receptor potential canonical 6 (TRPC6) in
non-transferrin-bound iron uptake in neuronal phenotype PC12 cells.
Biochem J. 2004;378:975-982.

- Andrews NC. Forging a field: the golden
age of iron biology. Blood. 2008;112:219-230.

- Piperno A, Mariani R, Trombini P,
Girelli D. Hepcidin modulation in human diseases: from research to
clinic. World J Gastroenterol. 2009;15:538-551.

- Nicolas G, Bennoun M, Devaux I, et
al. Lack of hepcidin gene expression and severe tissue iron overload in
upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci
U S A. 2001;98:8780-8785.

- Nicolas G, Bennoun M, Porteu A, et
al. Severe iron deficiency anemia in transgenic mice expressing liver
hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596-4601.

- Pantopoulos K. Function of the
hemochromatosis protein HFE: Lessons from animal models. World J
Gastroenterol. 2008;14:6893-6901.

- Gao J, Chen J, Kramer M, Tsukamoto H,
Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis
protein HFE with transferrin receptor 2 is required for
transferrin-induced hepcidin expression. Cell Metab. 2009;9:217-227.

- Pietrangelo A, Caleffi A, Henrion J,
et al. Juvenile hemochromatosis associated with pathogenic mutations of
adult hemochromatosis genes. Gastroenterology. 2005;128:470-479.

- van Dijk BA, Kemna EH, Tjalsma H, et
al. Effect of the new HJV-L165X mutation on penetrance of HFE. Blood.
2007;109:5525-5526.

- Wallace DF, Dixon JL, Ramm GA,
Anderson GJ, Powell LW, Subramaniam N. Hemojuvelin (HJV)-associated
hemochromatosis: analysis of HJV and HFE mutations and iron overload in
three families. Haematologica. 2005;90:254-255.

- Papanikolaou G, Tzilianos M,
Christakis JI, et al. Hepcidin in iron overload disorders. Blood.
2005;105:4103-4105.

- Niederkofler V, Salie R, Arber S.
Hemojuvelin is essential for dietary iron sensing, and its mutation
leads to severe iron overload. J Clin Invest. 2005;115:2180-2186.

- Babitt JL, Huang FW, Xia Y, Sidis Y,
Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling
in vivo regulates systemic iron balance. J Clin Invest.
2007;117:1933-1939.

- Camaschella C. BMP6 orchestrates iron
metabolism. Nat Genet. 2009;41:386-388.

- Wang RH, Li C, Xu X, et al. A role of
SMAD4 in iron metabolism through the positive regulation of hepcidin
expression. Cell Metab. 2005;2:399-409.

- Nemeth E, Rivera S, Gabayan V, et al.
IL-6 mediates hypoferremia of inflammation by inducing the synthesis of
the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-1276.

- Verga Falzacappa MV, Vujic Spasic M,
Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic
hepcidin expression and its inflammatory stimulation. Blood.
2007;109:353-358.

- Peyssonnaux C, Zinkernagel AS,
Schuepbach RA, et al. Regulation of iron homeostasis by the
hypoxia-inducible transcription factors (HIFs). J Clin Invest.
2007;117:1926-1932.

- Lin L, Goldberg YP, Ganz T.
Competitive regulation of hepcidin mRNA by soluble and cell-associated
hemojuvelin. Blood. 2005;106:2884-2889.

- Silvestri L, Pagani A, Nai A, De
Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2
(TMPRSS6) inhibits hepcidin activation by cleaving membrane
hemojuvelin. Cell Metab. 2008;8:502-511.

- Melis MA, Cau M, Congiu R, et al. A
mutation in the TMPRSS6 gene, encoding a transmembrane serine protease
that suppresses hepcidin production, in familial iron deficiency anemia
refractory to oral iron. Haematologica. 2008.

- Pak M, Lopez MA, Gabayan V, Ganz T,
Rivera S. Suppression of hepcidin during anemia requires erythropoietic
activity. Blood. 2006;108:3730-3735.

- Vokurka M, Krijt J, Sulc K, Necas E.
Hepcidin mRNA levels in mouse liver respond to inhibition of
erythropoiesis. Physiol Res. 2006;55:667-674.

- Weizer-Stern O, Adamsky K, Amariglio
N, et al. Downregulation of hepcidin and haemojuvelin expression in the
hepatocyte cell-line HepG2 induced by thalassaemic sera. Br J Haematol.
2006;135:129-138.

- Kemna EH, Kartikasari AE, van Tits
LJ, Pickkers P, Tjalsma H, Swinkels DW. Regulation of hepcidin:
insights from biochemical analyses on human serum samples. Blood Cells
Mol Dis. 2008;40:339-346.

- Gardenghi S, Marongiu MF, Ramos P, et
al. Ineffective erythropoiesis in beta-thalassemia is characterized by
increased iron absorption mediated by down-regulation of hepcidin and
up-regulation of ferroportin. Blood. 2007;109:5027-5035.

- Tanno T, Bhanu NV, Oneal PA, et al.
High levels of GDF15 in thalassemia suppress expression of the iron
regulatory protein hepcidin. Nat Med. 2007;13:1096-1101.

- Tamary H, Shalev H, Perez-Avraham G,
et al. Elevated growth differentiation factor 15 expression in patients
with congenital dyserythropoietic anemia type I. Blood.
2008;112:5241-5244.

- Tanno T, Porayette P, Sripichai O, et
al. Identification of TWSG1 as a second novel erythroid regulator of
hepcidin expression in murine and human cells. Blood. 2009;114:181-186.

- Smith TG, Robbins PA, Ratcliffe PJ.
The human side of hypoxia-inducible factor. Br J Haematol.
2008;141:325-334.

- Braliou GG, Verga Falzacappa MV,
Chachami G, Casanovas G, Muckenthaler MU, Simos G.
2-Oxoglutarate-dependent oxygenases control hepcidin gene expression. J
Hepatol. 2008;48:801-810.

- Choi SO, Cho YS, Kim HL, Park JW. ROS
mediate the hypoxic repression of the hepcidin gene by inhibiting
C/EBPalpha and STAT-3. Biochem Biophys Res Commun. 2007;356:312-317.

- Shah YM, Matsubara T, Ito S, Yim SH,
Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are
essential for iron absorption following iron deficiency. Cell Metab.
2009;9:152-164.

- Silvestri L, Pagani A, Camaschella C.
Furin-mediated release of soluble hemojuvelin: a new link between
hypoxia and iron homeostasis. Blood. 2008;111:924-931.

- Origa R, Galanello R, Ganz T, et al.
Liver iron concentrations and urinary hepcidin in beta-thalassemia.
Haematologica. 2007;92:583-588.

- Pippard MJ, Callender ST, Warner GT,
Weatherall DJ. Iron absorption and loading in beta-thalassaemia
intermedia. Lancet. 1979;2:819-821.

- Hershko C, Rachmilewitz EA. Mechanism
of desferrioxamine-induced iron excretion in thalassaemia. Br J
Haematol. 1979;42:125-132.

- Rivella S. Ineffective erythropoiesis and
thalassemias. Curr Opin Hematol. 2009;16:187-194.

- Walter PB, Fung EB, Killilea DW, et
al. Oxidative stress and inflammation in iron-overloaded patients with
beta-thalassaemia or sickle cell disease. Br J Haematol.
2006;135:254-263.

- Morris CR. Mechanisms of vasculopathy
in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ
Program. 2008:177-185.

- Stuart MJ, Nagel RL. Sickle-cell disease.
Lancet. 2004;364:1343-1360.

- Koduri PR. Iron in sickle cell disease: a
review why less is better. Am J Hematol. 2003;73:59-63.

- Brewer CJ, Coates TD, Wood JC. Spleen
R2 and R2* in iron-overloaded patients with sickle cell disease and
thalassemia major. J Magn Reson Imaging. 2009;29:357-364.

- Nemeth E, Ganz T. Hepcidin and
iron-loading anemias. Haematologica. 2006;91:727-732.

- Wahl S, Quirolo KC. Current issues in
blood transfusion for sickle cell disease. Curr Opin Pediatr.
2009;21:15-21.

- Brown K, Subramony C, May W, et al.
Hepatic iron overload in children with sickle cell anemia on chronic
transfusion therapy. J Pediatr Hematol Oncol. 2009;31:309-312.

- Kattamis A, Papassotiriou I,
Palaiologou D, et al. The effects of erythropoetic activity and iron
burden on hepcidin expression in patients with thalassemia major.
Haematologica. 2006;91:809-812.

- Camberlein E, Zanninelli G, Detivaud
L, et al. Anemia in beta-thalassemia patients targets hepatic hepcidin
transcript levels independently of iron metabolism genes controlling
hepcidin expression. Haematologica. 2008;93:111-115.

- Kearney SL, Nemeth E, Neufeld EJ, et
al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood
Cancer. 2007;48:57-63.

- Jenkins ZA, Hagar W, Bowlus CL, et
al. Iron homeostasis during transfusional iron overload in
beta-thalassemia and sickle cell disease: changes in iron regulatory
protein, hepcidin, and ferritin expression. Pediatr Hematol Oncol.
2007;24:237-243.

- Nemeth E, Valore EV, Territo M,
Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of
anemia of inflammation, is a type II acute-phase protein. Blood.
2003;101:2461-2463.

- Kroot JJ, Laarakkers CM, Kemna EH,
Biemond BJ, Swinkels DW. Regulation of serum hepcidin levels in sickle
cell disease. Haematologica. 2009;94:885-887.

- Kerenyi MA, Grebien F, Gehart H, et
al. Stat5 regulates cellular iron uptake of erythroid cells via IRP-2
and TfR-1. Blood. 2008;112:3878-3888.
