Infections in Thalassemia and Hemoglobinopathies: Focus on Therapy-Related Complications
Bianca Maria Ricerca, Arturo Di Girolamo* and Deborah Rund°
Hematology Department, Catholic University, Rome (Italy), *Infectious Diseases Department, G. d’Annunzio University, Chieti-Pescara (Italy), ° Hebrew University-Hadassah Medical Center, Ein Kerem, Jerusalem, Israel IL 91120
Correspondence
to: Bianca Maria Ricerca, Servizio di Ematologia, Policlinico A.
Gemelli, Largo A Gemelli 8. 00168 Rome (Italy), Tel: +39
0630154968, e-mail: bmricerca@rm.unicatt.it
Published: December 28 , 2009
Received: December 6, 2009
Accepted: December 26, 2009
Medit J Hemat Infect Dis 2009, 1(1):e2009028 DOI 10.4084/MJHID.2009.028
This article is available from: http://www.mjhid.org/article/view/5229
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
The
clinical approach to thalassemia and hemoglobinopathies, specifically
Sickle Cell Disease (SCD), based on transfusions, iron chelation and
bone marrow transplantation has ameliorated their prognosis.
Nevertheless, infections still may cause serious complications in these
patients. The susceptibility to infections in thalassemia and SCD
arises both from a large spectrum of immunological abnormalities and
from exposure to specific infectious agents. Four fundamental issues
will be focused upon as central causes of immune dysfunction: the
diseases themselves; iron overload, transfusion therapy and the role of
the spleen. Thalassemia and SCD differ in their pathogenesis and
clinical course. It will be outlined how these differences affect
immune dysfunction, the risk of infections and the types of most
frequent infections in each disease. Moreover, since transfusions are a
fundamental tool for treating these patients, their safety is paramount
in reducing the risks of infections. In recent years, careful
surveillance worldwide and improvements in laboratory tests reduced
greatly transfusion transmitted infections, but the problem is not
completely resolved. Finally, selected topics will be discussed
regarding Parvovirus B19 and transfusion transmitted infections as well
as the prevention of infectious risk postsplenectomy or in presence of
functional asplenia.
Introduction: Infections
are a frequent complication of thalassemias and hemo-globinopathies and
they can be fatal. The morbility and mortality rate for infections vary
throughout the world depending on differences in the epidemiology of
each infection and on the socio-economic level of each country and also
vary depending on the preventive and therapeutic strategies adopted. In
an Italian multicenter study [1], infections were the
second cause of
death after heart failure in thalassemia. Similar results were reported
in Greece [2] and in Taiwan [3],
while in E-beta thalassemia patients in
Thailand, infections are the primary cause of morbidity and mortality [4].
In this review we will
compare and contrast the different mechanisms
which predispose to infectious complications in thalassemia and in
hemoglobinopathies, specifically SCD. We will distinguish between
those aspects deriving from the disease itself and those which are
essentially therapy related. Thereafter, we will examine only selected
issues from the large amount of data on the clinical management of
infectious diseases, trying to determine if there are infections to
which these patients are naturally susceptible and others that are
primarily due to treatment. Finally, the last point on which we will
focus is how much some clinical aspects of these diseases (for
example iron overload (IOL), and splenic absence (or
hypofunction) influence the outcome of certain infection such as
Acquired Immunodeficiency Syndrome (AIDS), hepatitis C virus
(HCV) or bacterial infections.
Etiology Of Risks Of Infections In
Thalassemia And Hemoglobinopathies:
The susceptibility to infections in thalassemia and SCD arises both
from a large spectrum of immunological abnormalities and from the
exposure to infectious agents.
To simplify the
complex scenario of immune system perturbations, four
fundamental issues can be addressed: the disease itself, i.e. all those
changes inherent to the pathological process which can interfere with
the immune systems; IOL, transfusion therapy and the role of the spleen.
Transfusion and
chelation therapies represent true progress in the
management of these diseases. In fact, they dramatically ameliorated
the prognosis of thalassemia and SCD, as epidemiological data clearly
demonstrate[1,2,9].
Nevertheless, the benefits offered by allogenic blood
transfusions (ABTs) come together with the disadvantages of the high
transfusion burden in terms of direct exposure to infectious risks and,
indirectly, transfusion related immunomodulation (TRIM) and IOL.
Moreover, other therapeutic options (splenectomy, central venous
catheters, bone marrow transplantation) or nutritional deficiency (zinc
deficiency) contribute to the infectious risks.
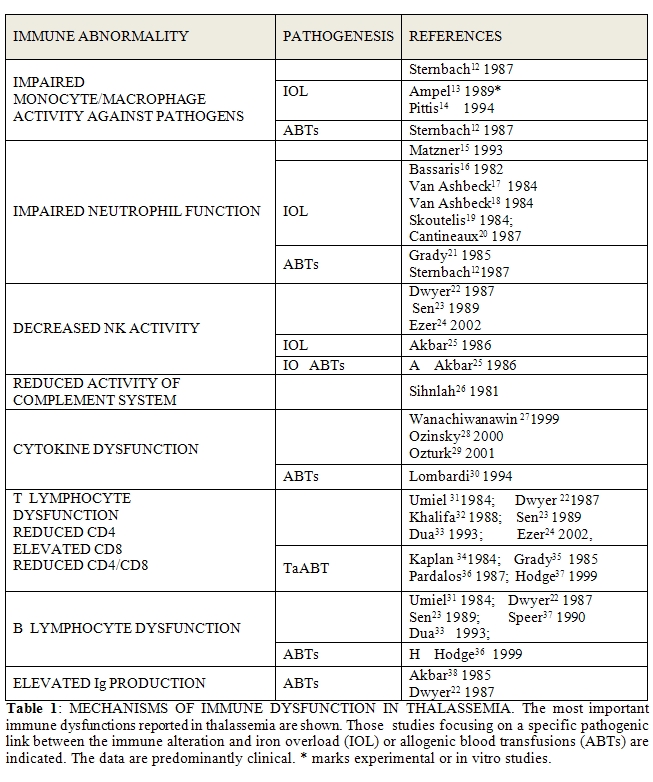
Immunological Abnormalities In Thalassemia
And SCD:
Recently, the immunological abnormalities observed in thalassemic
patients were reviewed and listed in two publications [10,11].
The immune
alterations concern both the innate and the adaptive immune systems.
The CD4/CD8 ratio is lower than normal, neutrophil and macrophage
phagocytosis, neutrophil chemotaxis, natural killer (NK) function are
compromised; C3 and C4 are reduced. High immuglobulins (Ig)
were reported and B lymphocytes were found to be increased, activated
with impaired differentiation. Table 1
summarizes the most important evidence in the literature (experimental
or clinical), indicating, where noted, the relationship between the
immune alteration and the ABTs or the IOL. There are few
inconsistencies among the various reports.
The role of the
disease itself in inducing immune abnormalities can be
explained by pathophysiological mechanisms of the disease, as is
reported in the literature.
The pathogenesis of
thalassemia is based on ineffective erythropoiesis,
hemolysis, and a tendency to increased iron absorption, inherent in the
disease itself. For the first two reasons, the monocyte/macrophage
compartment undergoes gross hyperplasia and is hyperactive in
phagocytizing all defective erythroid precursors and
erythrocytes [39,40,41]. This increased phagocytic
activity very likely
reduces the capacity of the phagocytic system to defend against
pathogenic microorganisms. For the same reason, the pattern recognition
receptors (PRR) are overwhelmed [28]. Moreover, in a
study
conducted in a mouse model of β-thalassemia, susceptibility to
infection by L. Monocytogenes and of S. Typhimurium was demonstrated as
a result of low phagocytotic activity [13]. The
authors suggest that, in
this model, the relationship of this alteration to IOL not caused
by transfusions but results from the disease itself. Finally, in
clinical practice, it has been observed that severe anemia, itself, is
a risk factor for bacterial infections in thalassemia, predominantly
pneumonia [4,43]. The current
criteria for transfusion therapy recommend
the maintenance of Hb level above 9 g/dl but in some countries with
lower socio-economic levels, this optimal regimen is not assured. In
these cases, anemia itself represents another risk factor for
infections.
As far as SCD disease
is concerned, its pathogenesis is quite different
from thalassemia. Ineffective erythropoiesis does not play a central
role as in thalassemia. HbS polymerization is the trigger, able to
initiate the catastrophic chain of events responsible for chronic
hemolytic anemia and for vaso-occlusive (VOC) crises. The latter may
cause organ damage in all parts of the body and it accounts for the
enormous clinical complexity of this disease. Much evidence is
consistent with the existence of a chronic inflammatory state in SCD,
exacerbated during the VOC episodes [44,45 ]with
participation of cells
(neutrophils, macrophages platelets), cytokines and adhesion molecules.
Many signs of high oxidative stress and decreased anti-oxidant defense
are present [46]. Moreover, high interleukin-6
(IL-6) levels were observed
in SCD47,[48] in addition to interleukin-4 and
interleukin-10 [48,49]. This
cytokine elevation suppresses humoral and cell-mediated immune
function, increasing infectious risks [49,50]. High
values of soluble IL-2
receptors (sIL-2R), observed in a large number of SCD patients, were
interpreted as the effect of continuous IL-6 stimulation [51].
Regarding the cellular
aspects of the immune system, monocytes are
continously activated, as is demonstrated by the upregulation and the
atypical expression of CD152. Neutrophil dysfunction was considered a
very important functional defect involved in the high susceptibility to
infections53. For example, neutrophils from SCD patients show high
expression of CD18, a molecule correlated with adhesive properties, and
they respond, in vitro, to IL-8 with enhanced sensitivity [54].
This
feature renders neutrophils important participants in the initiation of
vaso-occlusion (VOCs) but they are thus less available for
defense tasks.
In fact, VOC crises
are responsible for further immune
abnormalities which are present to a lesser degree or absent in the
steady state of the disease [55]. For example,
phagocytic activity rises
during VOCs [56]. Neutrophil chemotaxis is
normal or clearly
reduced in the steady state of the disease but increases during VOC
crises [57]. This hyperactivity of the
monocyte/macrophage and neutrophil
compartments is not committed to defending against pathogens but it
contributes to VOCs. Moreover, it is a source of oxidative stress which
impairs the immune response (see below).
As a further sign of
inflammatory activation, the alternate (pathway of
complement (AP50) is reduced for consumption in SCD patients and
has a significant inverse correlation with the number of crises,
while circulating immune complexes are elevated and they directly
correlate with the number of complications of the disease [58].
The last factor to
consider is that in SCD, VOCs themselves can
predispose, locally, to the onset of infectious complications.
Respiratory infections, frequently following the acute chest syndromes
(ACSs), or osteomyelitis are examples of this mechanism [59].
Another difference
between thalassemic and SCD patients concerns
splenic function: SCD patients undergo functional asplenia due to
recurrent episodes of vaso-occlusion in this organ. Thus, the
immunodeficiency observed in thalassemia after splenectomy is often
naturally present even early in the life in SCD[60].
This state
particularly favors infections by encapsulated bacteria [61].
Finally, we mention
that some immune alterations similar to those
mentioned for thalassemia were also found in SCD: CD4 lymphocyte
reduction and CD4/CD8 ratio reduction [55, 62-64]; natural killer
lymphocyte reduced activity [64]; high serum
immunoglobulin [65], and
elevated B lymphocytes [55]. On the other hand, the
published data are
less uniform and there are also some studies reporting the normality of
these immunological features [66,67].
Risks Related To Iron Overload:
Hereditary hemocromatosis patients represent an ideal model to
understand the effects of IOL on immunity. Indeed, many studies have
demonstrated that immunological function is largely and negatively
influenced by iron excess [68]. Many of the
alterations observed in
hereditary hemochromatosis were confirmed also in thalassemic patients (Table 1).
To comment on the
numerous data, we will outline only some specific
aspects: for example the dual and opposing roles of the phagocytic
system (monocyte/macrophages and neutrophils). IOL damage derives from
a disequilibrium between iron oxidation (through the Fenton reaction)
and the effectiveness and availability of those systems able to
counteract oxidative stress. In this sense, in addition to the
antioxidant systems, ferritin and the monocyte/macrophage compartment
also participate in clearing up toxic iron. Indeed, lysosomes in these
cells are able to endocytose both free iron and ferritin and this
contributes toward protection from iron [68] (Figure 1).
Additional oxidative stress can destabilize the secondary lysosomes of
the macrophage, and their protective role is lost. Moreover,
phagocytosis of microorganisms, of dyserythropoietic precursors and of
senescent or damaged red blood cells (intravascularly and/or
extravascularly) causes oxidative stress [69] which
compounds that
deriving from IOL. Finally, IOL impairs phagocytosis [70]
and its negative
effect on neutrophil function has been clearly demonstrated [70,71].
Phagocytic function is the center of a vicious cycle, acting as a
double edged sword: protective against oxidative stress while also
generating oxidative stress on the one hand, and on the other hand,
having its own function impaired by the same oxidative stress (Figure 1).
Finally, the scanty
detoxifying properties of lymphocyte are the reason
for their numerous functional alterations related to IOL.
In addition, regarding
IOL, SCD seems to be a different disease. Indeed
non transfused SCD patients may present with iron deficiency (due to
intravavascular hemolysis)[72] and even in
transfused patients, the
organ damage due to iron overload is less severe [73].
Perhaps this
difference derives from the significant contribution of inflammation to
the pathogenesis of the disease, as recent studies evaluating the role
of hepcidin in these diseases have led us to hypothesize [74].
A recent
multicenter prospective study [75] seems to support
the influence of ABTs
and IOL on the prevalence of infections requiring hospitalization, and,
in general, on the rate of hospitalization, in SCD patients.
Nevertheless, the data analysis shows a very complex scenario and the
results suggest that this topic needs further studies to be clarified.
Indeed, the transfused SCD are overall adult patients with more severe
and advanced disease and, as the authors conclude, the differences
observed may be, but not necessarily, attributable to ABTs and to IOL.
We conclude by
mentioning that in patients who underwent hematopoietic
stem cell transplantation, IOL severity is related to high infectious
risk and it negatively influences the outcome of infections in this
patient group[76].
Risks Related To Allogenic Blood
Transfusions (ABTs):
The data regarding transfusion transmitted infection (TTIs) risks in
patients with thalassemia and hemoglobinopathies does not differ from
the evidence in the literature regarding multitransfused patients
(MTPs) in general. Hepatitis C virus (HCV), Hepatitis B virus (HBV),
Human Immunodeficiency virus (HIV) and Syphilis are the most common
infection agents transmitted via transfusions and routine screening is
performed for these agents throughout the entire world. Other agents
are routinely screened for, in different countries, according to
epidemiologic alerts but also commensurate with economic resources. In
the USA, for example, screening for Human T-cell Lymphotropic virus
(HTLV), West Nile virus (WNV), Trypanosoma cruzi and Cytomegalovirus
(CMV) is also routinely performed on blood units and screening is
performed for bacteria in platelet units [77]. Many
other infectious
agents are transfusion transmissible. The data in the literature
demonstrated that some of these agents do not cause any clinical
disease (GBV-C/HGV, SEN-V, TTV, HHV-8) while others represent a
transfusional risk according to epidemiologic evidence. Thus, the risk
of these agents can vary in different parts of the world. As summarized
by Vanvakas et al [77] additional infectious agents
which can be
transmitted by transfusion include: Parvovirus B19, Dengue fever virus
(DFV), Babesia microti, Plasmodia species, Leishmania, Brucella and
Creutzfeldt-Jakob disease (vCJD) prions.
The prevention of HBV,
HCV and HIV transfusion transmission represented
a challenge for transfusion medicine. Two weapons play a fundamental
role in the war against these viral agents. The primary preventive
measure is the selection of appropriate eligibility criteria for blood
donors; the second line of prevention includes testing the units to be
transfused by various laboratory methods. Both tools have been and are
always in continuous evolution. Health surveillance throughout the
world, including rapid information about disease epidemiology and
travel patterns of people, as well as the economic and political
choices of each country and technological progress, have all
contributed in the past and continue contributing to assure transfusion
safety. Since the discovery of HBsAg in 1963, diagnostic accuracy has
improved progressively. The introduction of Nuclear Amplification Tests
(NAT) represented a milestone. A suitable example is transfusion
transmitted HCV and HIV. Recently, the centralized data of the American
Red Cross blood donor population were reviewed [78]
and the prevalence
rates of disease marker positivity and the residual risk attributable
to the window period were evaluated. A continuous statistically
significant decrease (p<0.001) of prevalence rates for infectious
disease markers among first-time donors was observed in the period
between 1995 and 2001. Examining the data, the effect of the
introduction of NAT testing is clear: the estimated risk of collecting
blood during the infectious window period for HCV was 1:276,000 and
1:1,935,000 respectively with only antibody determination compared to
NAT, respectively. Similarly, the risk for HIV was 1:1,468,000 and
1:2,135,000. The important role of the introduction of NAT is
indirectly confirmed by the evidence that a less impressive reduction
rate was recorded for HBV for which no relevant diagnostic improvements
were achieved (1:205,000). Furthermore, another interesting approach to
TTI evaluation is the application of mathematical models to calculate
the residual risk of infection. The results obtained in the USA [79] for
HCV, HBV and HIV, are similar to those reported by Dodd et al. In
England [80] and in Canada [81]
the residual risk is substantially lower,
in comparison to the USA, for HCV (1: 30 million and 1:13 million
respectively) while for HIV only in Canada the residual risk is lower
(1:7-8 million). Many clinical reports can be quoted to demonstrate the
effect of the more advanced diagnostic tools adopted in transfusion
field. For example, in Italy, a recent epidemiologic study of 708
multitransfused children, showed that HCV hepatitis,
transmitted by transfusion, disappeared after 199282.
Furthermore, in another Italian study, performed retrospectively from
1990 until 2007, HCV-RNA negative thalassemic patients were
significantly younger than positive patients (p<0.001)[83].
A survey
of 399 patients with thalassemia and SCD in Turkey [84]
reported a
prevalence of 0.75%, 4.5% and 0 of positivity to HBsAg, HCV and
HIV antibodies respectively but the majority of this positivity (77.7%)
was found in patients transfused before the introduction of second
generation testing. The most recent data, although encouraging, suggest
some considerations: different levels of blood safety are achieved
among various countries. It derives that donor screening strategies can
be ameliorated. Finally the problem of HCV and also HBV (we will expand
on this below) is far from a complete resolution.
As far as the
influence of ABTs on immune system is concerned, over 30
years ago, it was noted that patients who had received many ABTs prior
to renal transplantation showed a better rate of allograft survival.
This was the onset of a long and heated debate focused on understanding
the immunomodulation induced by ABTs [85-87]. The
debate initially began
from the data of approximately 40 studies which indicated that surgical
patients receiving perioperative ABTs have a higher risk of bacterial
infections, demonstrating the link between multiple transfusions and
infectious risk. Recently, Vamvakas and Blajchman [87]
reviewed extensive
evidence regarding this issue, summarizing the beneficial and
deleterious effects of ABTs. TRIM could contribute to all immunological
alterations listed above and it also reduces delayed-type
hypersensitivy and it induces antiidiotypic and anticlonotypic antibody
production. A central role in pathogenesis of TRIM is played by
allogenic mononuclear cells, both for their presence and for the
soluble substances they release during storage of blood components.
Moreover, the soluble HL-A class I peptides that circulate free in
allogenic plasma also contribute to the generation of TRIM. The
similarity between donor WBC HLA antigens and those of the recipient is
able to induce alloimmunization (if HLA-DR mismatch is high) or
tolerance and immunosuppression (if the mismatch is for only one HLA-DR
antigen). For these reasons, universal blood unit leukodepletion
in the prestorage phase should be an important measure to prevent TRIM.
Thalassemic patients represented an ideal setting to verify the
usefulness of ABT leukodepletion. Although leukodepletion reduces
non-hemolytic febrile reactions (NHFR)[88-90] and
anti-leukocyte
antibodies and anti-platelet production [91, 92] it
does not modify
substantially the immunologic alterations observed in thalassemic
patients [92] Probably, their pathogenesis is very
complex and TRIM
represents only one of the numerous factors interfering with immunity.
Risks Related To Splenectomy Or Functional
Asplenia:
At the present time, as an effect of the hypertransfusion regimen,
fewer thalassemic patients undergo splenectomy [93].
However, when
transfusional needs rise excessively, splenic enlargement, or
hypersplenism and/or compressive damage occurs, splenectomy is
indicated. We already outlined that SCD patients often present with
functional asplenia early in life.
The spleen is very
important for immunological surveillance. It is an
important reservoir of immunocompetent lymphocytes [94].
In asplenia or
functional hyposplenia, antibody production in response to new
antigens, mediated by CD4 function, is impaired [95].
Efficient
phagocytosis depends on splenic macrophages and on the production of
many substances (opsonins, properdin, tufsin) which are reduced in
asplenic organisms [96, 97]. Chemotaxis is
also impaired [98]. For all
these reasons, when the spleen is absent or poorly functioning, sepsis
can occur for any pathogen agent. However, encapsulated pathogens
(Streptococcus pneumoniae, Haemophilus influenza type B, Escherichia
coli, Neisseria menigitidis) are the most fearsome. Hansen et al [99]
reviewed the literature regarding overwhelming sepsis in subjects with
surgical or functional asplenia. They compared the number of events of
sepsis and fatal sepsis in recent reports to the same data obtained in
1973 [100]. In 1973, sepsis occurred in 119 of 2796
cases (4.3%) and fatal
sepsis occurred in 71 (2.5%). In the most recent series, sepsis
occurred in 270 of 7872 cases (3.5%) and was fatal in 169 (2.1%) The
percent reduction of sepsis from 1973 to most recent years was
estimated -18 for sepsis and -16 for fatal sepsis. In both series,
thalassemia patients have the highest frequency of sepsis and fatal
sepsis. No comparison was possible for SCD because data before 1973
were lacking. The preventive strategy based on penicillin prophylaxis
and vaccinations (see below) has been fundamental for this reduction of
sepsis and fatal sepsis.
Zinc deficiency: The
link between zinc deficiency and immunodeficiency
is well known [101]. Some reports, concerning SCD
patients focus on this
aspect and the beneficial role of zinc supplementation [102,103].
Selected Topics Regarding Clinical Aspects
Of Infections In Thalassemia And Hemoglobinopathies:
The amount of published data on the clinical aspects of infections in
thalassemia and hemoglobinopathies is enormous and it is difficult to
summarize it. In part, they are recently reviewed by Vento et al11. In
the following section, we will focus on some specific aspects or new
evidence arising from the literature, concluding by emphasizing the
importance of preventive measures in splenectomized patients.
Human Parvovirus B19:
Human parvovirus (HPV) B19 is a small, non enveloped, single stranded
DNA virus with a terminal hairpin [104]. During
replication, two proteins
(VP1 and VP2) are produced but also in the absence of replication it
can exert its toxic effects. After infection, a transient high titer
viremia lasts one week; the HPV DNA disappears during the production of
neutralizing antibodies (IgM for 6-8 weeks and afterwards, IgG). This
protective reaction can be absent in immunocompromised patients leading
to the persistence of viral DNA. The clinical course is characterized
by a flu-like syndrome (fever, chills, headache, gastrointestinal
discomfort, arthropathy and a typical slapped-cheek rash which, after
two days also involves the arms and legs), sometimes complicated by a
transient red cell aplasia (TRCA). In fact, HPV B19 it is also called
erythrovirus because it has a high and almost specific tropism for
erythroid progenitors inducing them to undergo apoptosis by the
activation of the caspase pathway. In subjects with high erythroid turn
over (such as those with congenital red cell defects) severe anemia
with low reticulocyte counts may develop, requiring transfusion or an
intensification of a previous transfusion regimen. Moreover, it is
presumed that the virus can stay in the bone marrow for lifelong
duration, although this point is not completely clarified and there is
evidence that persistently infected blood donors can transmit the
infection through transfusions [105], although the
main route of
transmission is always respiratory. For these reasons the course of HPV
B19 infection in thalassemia and hemo-globinopathies can be quite
different from that in a healthy subject.
A large
epidemiological study of 633 children with SCD (older than 12
months) has been reported [106]. They were examined
between November 1996
and December 2001. At the start of the study, 187 children (29.5%) had
already contracted the disease (HPV B19 IgG+ and IgM-); their mean age
was higher than that of serologically negative subjects (p<0.001)
and fewer underwent chronic therapies (regular ransfusion or
hydroxyurea-HU). The second cohort of patients (446; 70.4%) included
those completely negative (IgG and IgM-) and those with a recent
infection (IgG-, IgM+). The follow up of 372 children belonging to this
group revealed important information: the rate of seroconversion; the
features of seroconverted subjects, the prevalence of TRCA (severe or
mild) and the variables related to the clinical course.
One hundred-ten
children (29.5%) seroconverted during the follow up
(incidence rate 11.3 for 100 patient-years; 95% confidence interval
[CI] 8.2-14.4). It is very interesting that among them, fewer were
receiving transfusions (7 out of 49; 14.3%; incidence rate 5.9 for 100
patients years, 95% CI 1-15) than those treated with hydroxyurea (9 out
of 29; 31% ) or not transfused (global incidence rate for
non-transfused and HU groups: 11.9 per 100 patients years; 95% CI
7.6-16.2 p<0.06). Moreover, the only risk factor for seroconversion
was having a sibling with a recent HPV B19 infection. These data can be
important for what we will discuss later. SCD genotype, sex, age at the
first serological test did not affect seroconversion.
Sixty-eight TRCA were
observed during the study: 3 in the HPV B19 IgG
positive group (1.6%) and 65 in the other (59%). The univariate
analysis showed a strong association between acute HPV B19 infections
with fever and acute splenic sequestration (ASS), while the
multivariable analysis identified predisposing factors as ASS and
painful episodes. Although the same evidence was not clear for acute
chest syndrome (ACS), examining all children admitted with fever and
pain, ACS was more common in those with HPV B19 infections. The only
risk factor for TRCA was the high reticulocyte count before the
infection. This study is rich in information and outlines many aspects
of an infectious disease which has some peculiarities in SCD as
compared to other diseases with high erythropoietic turnover.
Nevertheless, an important debate is taking place in the literature as
to whether transfusions are an important source of HPV B19. This
hypothesis arises from the detection of HPV B19 DNA in asymptomatic
blood donors. In the previous report [106], treated
children (transfusion
or HU) seemed to have less seroconversion, perhaps because a lower
proliferation rate of the erythroid compartment. Other reports coming
from the transfusion medicine field [107-109]
support the evidence that
transmission of HPV B19 through transfusion always plays a secondary
role compared to respiratory transmission. As a result, there is
currently no consensus regarding the application of preventive measures
to blood donors, blood units or to patients.
Yersinia Enterocolitica: The
well known problematic of Yersinia enterocolitica sepsis in thalassemia
is another area in which some features of the disease combined with the
side effects of therapy increase the risk of infection. In fact
Yersinia infection is favored by IOL either related to the disease or
to transfusions and it can be triggered by deferoxamine therapy. [110, 111]
Transfusion Transmitted Infections (TTI)s:
In a manner analogous to the risks of infectious diseases, the course
and the outcome of the most common TTIs in thalassemia and
hemoglobinopathies are influenced by the pathogenic features of these
diseases in terms of immunodysfunction and by IOL.
HIV: Human
Immunodeficiency Virus (HIV) disease is a viral- related progressive
immune depression that leads to depletion of CD4+ lymphocytes, and
renders the individual at risk for many types of opportunistic
infections [112]. As previously stated, a low
CD4/CD8 ratio is one of the
most frequent abnormalities in patients with thalassemias and
hemoglobinopathies; thus, HIV disease is an example of negative
interactions and bidirectional combination of the hematological with
the infectious disease. Similarly, the substantial degree of
immunodysfunction related to IOL would influence the outcome of these
diseases. However, there are all too few studies dealing the clinical
aspects of HIV infection in thalassemia and hemoglobinopahies.
Some years ago a large
multicenter study was published [113] which
included 79 HIV positive thalassemia patients from various countries
(Brazil, Italy, Greece, Spain, France, United Kingdom, Cyprus), the
majority of whom were followed in Italy (71%) and Cyprus (16%). The
mean age was low enough (12 ± 6.6 years) to presume a prevalent
transfusion transmission of HIV infection. The progression to overt
AIDS after seroconversion was estimated 1.4% after three years and 9%
after five; no significant statistical association was found with age,
sex, acute infection, or splenectomy. Two years later, the same
investigator focused on the inverse relationship between the rate of
progression of HIV and the dose of deferoxamine used: the rate of
progression decreases as the mean daily dose of drug increases
(p<0.02)[114]. In a further publication [115] reporting the follow-up of
the same patients, a multivariate Cox proportional hazard analysis
demonstrated a direct relationship between disease progression and
ferritin values. These studies, published at the beginning of the
nineties, included some patients treated with zidovudine. In subsequent
years until the present time, a large spectrum of therapeutic options
are available for HIV infected patients: nucleoside analogues (NAs),
non nucleoside reverse transcriptase inhibitors (NNRTIs), protease
inhibitors (PIs), fusion inhibitors, CCR5 (receptor) inhibitors and
integrase inhibitors [116], which are used also in
patients with
thalassemia and hemoglobinopathies. Finally, we mention that the effect
in vitro of iron chelators (deferoxamine, deferiprone, deferasirox) on
HIV replication is an interesting area of experimental research [117, 118].
HCV: Hepatitis C Virus
still represents a fearsome disease, widespread worldwide: it is
estimated that one hundred million people are infected throughout the
world [119]. It can have a mild presentation, not
infrequently
asymptomatic, in its acute phase and in a high percentage of cases, the
initial infection goes unnoticed. However, the evolution rate to
chronic disease of HCV hepatitis is high (at least 80% of acute cases)
and the further evolution towards end-stage liver disease, cirrhosis,
and hepatocellular carcinoma (HCC) are not infrequent [120].
The influence of IOL
on the outcome of HCV infection was the subject of
debate both in nonthalassemic[121,122]
and thalassemic
patients.Di Marco et al [83] reported that, in thalassemics, the severity of
liver damage (i.e. the finding of fibrosis and histologic signs of
cirrhosis) is clearly related to persistent HCV infection (HCV RNA
positivity), predominantly for genotypes 1 and 4. In the same study,
the data on the influence of IOL on liver damage in HCV RNA positive
patients, although less impressive, are however suggestive. Many other
authors focused their attention on the relationship between IOL and the
outcome of HCV; although these studies may reflect some reporting bias,
the results consistently demonstrate the presence of this
negative link [123-128]. Much important evidence
was obtained in patients
who survived hemopoietic stem cell transplantation: serial liver
biopsies, performed to evaluate histology and hepatic iron content,
demonstrated that either HCV or IOL are independent risk factors for
the progression of liver fibrosis and they have an additive
effects [129].
Since the 1990's, the
management of HCV has been characterized by
remarkable improvements which initially began with the use of
α-Interferon 2a (α-IFN). The first clinical results obtained with α-IFN
were encouraging [130, 131]. α-IFN also showed long
term efficacy128: 36.5
months (range 25-49 months). Syriopoulou132 reported a complete
sustained response after 8 years of therapy in 45% of thalassemic
patients. In the first of these two studies, upon multivariate
analysis, the absence of cirrhosis, low iron content and infection with
non 1b C virus type were independently associated with a complete
sustained response. In the second study, younger patients, who were not
splenectomized, with a shorter duration of the infection, were more
likely to respond to therapy. α-IFN was used also in patients after
bone marrow transplantation: it did not adverse engraftment and was
demonstrated to be efficacious and safe [133].
Thereafter, treatment
options were enriched by the introduction
of pegylated IFN (PegIFN) and ribavirin. There is currently an
ongoing debate regarding the use of a combination of α-IFN (or
Peg-IFN) plus ribavirin in the treatment of HCV in thalassemia. This
option could be considered at least for patients infected by type 1b
virus which results in a more severe disease and it is resistant to
α-IFN as a single agent. On the other hand, it is well known that
ribavirin is able to induce hemolysis and so in thalassemic patients
the drug could increase the need for transfusions, thus worsening IOL.
Although this is a definite possibility, preliminary experiences [134-136]
with this combination are positive in terms of efficacy on HCV
infection. Inati et al [135] reported a complete
sustained response
in 62% of patients using both drugs in comparison to 30%
using IFN monotherapy (p=0.19). The patients required more transfusions
but no worsening of IOL was observed. After the discontinuation of
antiviral therapy, blood consumption returned to pre-therapy
level. Other authors [134, 136]
reported similar results.
The last point
concerns SCD patients; Teixera et al [137],
described the
histopathologic features of SCD patients with or without HCV. This work
has many limitations, as the authors state. Nevertheless, it gives
interesting information: liver damage in SCD was present in subjects
infected with HCV. In those not infected, the liver changes were mild
and, despite IOL, little fibrosis was present. These observations are
consistent with those made by Harmatz et al [138]
and they imply that SCD
differs from thalassemia in terms of the interaction between iron
overload and HCV in SCD.
HBV: The strategies
adopted in transfusion medicine as far as the widespread use of
vaccination against HBV has reduced the prevalence of this hepatitis
among multitransfused patients. Nevertheless, HBV hepatitis is still a
serious public health problem. The reasons for this phenomenon are
related to several factors. The routes of infection can be different
(transfusion as compared to sexual or perinatal); the patients can
be overt (HBsAg+) or occult (HBsAg – or anti HBc+/ HBsAg-)
carriers; and the virus can be reactivated in the setting of
immunosuppression. Finally, the protection offered by vaccination is
not absolute [139]. How can the risks be managed?
All transfused patients
(who were vaccinated) or those with HBsAg+, must be tested annually for
all HBV markers. The appearance of anti HBc positivity is a very
important event which mandates careful clinical evaluation
HBV may present as an
acute hepatitis with a wide range of
manifestations, from mild disease, sometimes asymptomatic, to a severe
one which, in some instances, can evolve to fulminant hepatic necrosis
which is not uncommonly fatal [140]. Apart from the
acute phase, between
2 to 10% of patients evolve to chronic liver disease, and thereafter,
end-stage liver disease, cirrhosis and hepatocellular carcinoma
(HCC) [141]. The first line treatment, available
for chronic HBV disease,
is α-IFN. This drug should be used for one year. During this period the
goal of therapy should be the complete clearance of HBV [142,
143].
Unfortunately, only 25%-40% of patients are noted to have a good
response and the use of other antiviral drugs (adefovir, tenofovir,
lamivudine, telbivudine, and entecavir) is often necessary [142].
Unfortunately, the major drawback of such therapies is that they are
not “curative”, i.e. these drugs can reduce the viral replication, but
they do not achieve complete viral clearance. Nonetheless, treatment is
considered effective when liver fibrosis does not progress to
cirrhosis [144].
Prevention Of Bacterial Infections In
Splenectomized Patients:
The risk of invasive bacterial infection in splenectomized patients is
well known. The data collected by Bisharat et al [145]
supports this
concept. They reviewed 28 studies amounting to 6,942 well-documented
patients, 209 of whom developed invasive infection (3%). The incidence
of infection was highest among patients with thalassemia major (8.2%),
and sickle-cell anaemia (7.3%). Furthermore, the highest mortality
rates were observed among patients with thalassaemia major (5.1%), and
sickle-cell anaemia (4.8%). Both incidence and mortality were
significantly higher in children than in adults. Streptococcus
pneumoniae was responsible for the majority of the infections (66%),
with a 55.3% mortality rate. It is followed for incidence by H.
influenzae type b, Escherichia coli, and Neiserria meningitides146.
Less common causative bacteria are Staphylococci, Streptococci,
Pseudomonas, and Salmonella species [147]. The
highest mortality rates
were attributed to gram negative bacteria (62%), and Neisseria
meningiditis (58.8%).
Thus the prevention
and treatment of bacterial infections in
splenectomized thalassemia and SCD patients is a life-saving
intervention. Adamkiewicz et al [148], reviewing
the records of 1,247
children born after 1983, reported a clear beneficial effect of
pneumococcal conjugate vaccine in the reduction of the incidence of
invasive pneumococcal disease.
Some issues are of
particular interest for clinical
practice: the optimal timing of vaccine administration, the
efficacy of various vaccination strategies, the duration of penicillin
prophylaxis, and the role of partial splenectomy. Splenectomized and
hyposplenic patients must receive routine vaccination, including both
live attenuated and killed vaccines [149], but they
should also be
immunized against Streptococcus pneumoniae, H. influenzae type b, and
Neisseria meningitides [147,150].
In the case of elective splenectomy,
vaccinations should be completed at least 2 weeks prior to the date of
surgery.
However, vaccination
does not completely protect against infection with
encapsulated bacteria [151] and prophylactic
antibiotics have a role as
well. In a prospective multicentre randomized study in pediatric SCD
patients aged <3yrs, penicillin prophylaxis reduced the incidence of
pneumococcal bacteremia by 84%. There are no prospective studies
in different clinical settings, but in a retrospective observation [152],
the incidence of post-splenectomy sepsis (PSS) infection and mortality
were reduced, by 47% and 88% respectively, after the introduction of
penicillin prophylaxis. The patients had undergone splenectomy for
different reasons, but the most relevant characteristic of the series
is that 70% of the patients were immunized (54% out of them only
against pnemococcus). Consequently, antibiotic prophylaxis is
recommended for all children <5 years of age, regardless of
immunization status, for all asplenic children <5yrs, for a duration
of at least for 2 years following splenectomy, since most series
demonstrate that 50% of PSS occurs within this period [153].
The debate
about the duration of prophylaxis is still open and the emergence of
penicillin-resistant pneumococci indicate that alternate therapy may be
warranted.
Notwithstanding the
risk of overtreatment, the potential catastrophic
clinical course of bacterial sepsis in the splenectomized individual
induces the physicians to start antibiotics at the first sign of
infection. Patients should carry a medical alert card to improve the
speed and appropriateness of treatment of postsplenectomy sepsis.
Subtotal splenectomy
may reduce the risk of postsplenectomy sepsis [154].
Nevertheless, there are not, at the moment, specific recommendations
for this procedure which has technical drawbacks in this population
including regrowth of the spleen and the need for reoperation [155].
Thus, also after a
subtotal splenectomy, the guidelines mentioned above for total
splenectomy should still be applied.
Conclusions:
Thalassemia
and SCD each have a different pathogenesis and this implies some
differences in the risks factors for infectious complications. The
strong inflammatory imprint and the frequent functional asplenia early
in life in SCD are the most important, although not the only,
differences between the two conditions. Moreover, although transfusions
and bone marrow transplantation are important modalities to treat or
cure both diseases, the additional problems arising from these
procedures or from their adverse effects (for example IOL), have
different implications. The knowledge of these differences is essential
to efficiently target future research in experimental and clinical
fields and also to define the best practical approach in the prevention
and in the treatment of infectious diseases in these complex patients.
Although much progress has been
made, infectious diseases still
represent a major challenge in the efforts for assuring these patients
enjoy a good quality of life and prolonged survival. The complexity of
infectious complications, involving different regions of the body
demonstrates that satisfactory cooperation among specialists in various
disciplines (hematology, microbiology, immunology, hepatology), both in
experimental and in clinical fields, is fundamental. Moreover, as a
consequence of routine use of transfusions in these patients,
transfusion medicine plays a central role. Ultimately, infectious
diseases in thalassemia and hemoglobinopathies represent an example for
which global surveillance, involving countries throughout the world,
coupled with an open exchange of information are essential for
achieving a high standard of patient care.
References
- Borgna-Pignatti C, Rugolotto S, De Stefano
P et al. Survival and complications in patients with thalassemia major
treated with transfusion and deferoxamine. Haematologica
2004;89:1187-1193.

- Ladis V, Chouliaras G Bedousi H et
al. Longitudinal study of survival and causes of death in
patients with thalassemia major in Greece. Ann N Y Acad Sci
2005;1054:445-50.

- Chern JP, Su S, Lin KH et al: Survival,
mortality, and complications in patients with beta-thalassemia major in
northern Taiwan. Pediatr Blood Cancer 2007;48:550-554

- Wanachiwanawin W. Infections in E-beta
thalassemia. Pediatr Hematol Oncol. 2000; 22(6):581-7.

- Manci EA, Culberson DE, Yang YM et al.
Causes of death in sickle cell disease: an autopsy study. Br J
Haematol. 2003;123(2): 359-365.

- Darbari DS, Kple-Faget P, Kwagyan Jet al.
Circumstances of death in adult sickle cell disease patients. Am J
Hematol. 2006; 81:858-63

- Van-Dunem JC, Alves JG, Bernardino L et al.
Factors associated with sickle cell disease mortality among
hospitalized Angolan children and adolescents. West Afr J Med.
2007;26(4):269-273.

- Perronne V, Roberts-Harewood M, Bachir D et
al. Patterns of mortality in sickle cell disease in adults in France
and England. Hematol J. 2002; 3(1):56-60.

- Quinn CT, Rogers ZR, Buchanan GR. Survival
of children with sickle cell disease. Blood. 2004;103(11):4023-4027

- Farmakis D, Giakoumis A, Polymeropoulo E
et al. Pathogenetic aspects of immune deficiency associated with β
thalassemia. Med Sci Monit 2003: 9: RA19-22

- Vento S, Cainelli F, Cesario F. Infections
in talassemia. Lancet Infect Dis 2006; 6:226-233

- Sternbach MS, Tsoukas C, Pasquin M et
al. Monocyte-Macrophage functions in asyntomatic and
supertransfused hemophiliacs and thalassemics. Clin Invest Med. 1987:
10:275-281.

- Ampel HM, van Wyck DM, Aguirre ML et al.
Resistance to infection in murine beta thalassaemia. Infect Immun 1989;
57: 1011–1017

- Pittis MG, Estevez ME and Diez RA.
Decreased phagolysosomal fusion of peripheral blood monocytes from
patients with thalassaemia major. Acta Haematol 1994;92: 66–70.

- Matzner Y, Goldlarb A, Abrahamov A et al.
Impaired neutrophil chemotaxis in patients with thalassemia major. Br J
Haematol 1993; 85:153- 158.

- Bassaris HP, Lianou PE, Skoutelis AT et
al. Defective adherance of polymorphonuclear leucocytes to nylon
induced by thalassemic serum. J Infect Dis
1982; 146:52–55.

- Van Ashbeck BS, Marx JJM, Struyvenberg A
et al.(A) Effect of iron (III) in the presence of various ligands on
the phagocytic and metabolic activity of human polymorphonuclear
leukocytes. J Immunol 1984;132: 851–856.

- Van Ashbeck BS, Marx JJM, Stryvenberg A.
et al. (B) Functional defects in phagocytic cells from patients with
iron overload. J Infect Dis. 1984; 8: 232–240.

- Skoutelis AT, Lianou E, Papavassilion T et
al. Defective phagocytic and bactericidal functions of
polymorphonuclear leucocytes in patients with beta-thalassaemia major.
J Infect 1984; 8: 118–122.

- Cantinieaux B, Hariga C, Ferster A, et
al. Neutrophil dysfunction in thalassaemia major: The role of
iron overload. Eur. J. Haematol. 1987; 39: 28–34.

- Grady RW, Akbar, AN., Giardina PJ et al.
Disproportionate lymphoid cell subsets in thalassemia major: the
relative contribution of transfusion and splenectomy. Br. J. Haematol.
1985; 59: 713-720.

- Dwyer J, Wood C, McNamara j et al.
Abnormalities in the immune system of children with beta-thalassemia
major. Clin Exp Immunol 1987; 68: 621-630

- Sen I, Goicoa Ma, Nualart PJ et al. immunological studies in talassemia major. Medicina (B Aires)1989, 49: 31-4
- Ezer U, Gulderen F, Culha VK et al.
Immunological status of thalassemia syndrome. Pediatr Hematol Oncol
2002;19:51-58

- Akbar AR, Fitzgerald-Bocarsly PA, De Sousa
M et al. Decreased natural killer activity in thalassemia major: a
possible consequence of iron overload. J Immunol 1986; 136:1635-1640.

- Sihnlah D, Yadav M. Elevated IgG and
decreased complement component C3 and factor B in β thalassemiamajor.
Acta pediatr scand 1981; 70: 547-560

- Wanachiwanawin W, Wiener E,
Siripaniaphinyo U et al. Serum levels of tumor Necrosis
factor a, interleukin-1 and interferon-g in b-thalassaemia/HbE and
their clinical significance. Interferon Cytokine Res 1999;
19:105–110.

- Ozinsky A, Underhill DM, Fontenot
J.D et al. The repertoire for pattern recognition of pathogens by
the innate immune system is defined by cooperation between toll-like
receptors. Proc Natl Acad Sci USA 2000; 97:
13766–13771.

- Ozturk O, Yaylim I, Aydin M, et al.
Increased plasma levels of interleukin-6 and interleukin-8 in beta
thalassaemia major, Haematologica 2001; 31: 237–244.

- Lombardi G, Matera R, Minervini MM et al.
Serum levels of cytokines and soluble antigens in polytransfused
patients with beta talassemia major relationship with immune status.
Hematologica 1994; 79:406-412

- Umiel T, Friedman E, Luria D et al:
Impaired immune regulationin children and adolescent with hemophilia
and thalassemia in Israel. Am J Pediatr Hematol Oncol 1984;6: 371-378

- Khalifa AS, Maged Z, Khalil R et al.
T-cell function in infants and children with beta-thalassemia. Acta
Haematol 1988; 79: 153-156

- Dua D, Choundury M, PrakashK.
Altered T and B Limphocytes in multitransfused patients of
thalassemia major . Indian pediatr 1993; 30: 893-896

- Kaplan J, Sarnaik S, Gitlin J et al.
Diminished helper/suppressor lymphocyte ratios and natural killer
activity in recipients of repeated blood transfusions. Blood.
1984;64:308-310

- Pardalos G, Kanakoudi-Tsakalidis
Iron-related disturbances ofF, Malaka-Zafiriu M et al. Iron-related
disturbances of cell-mediated immunity in multitransfused children with
thalassemia major. Clin Exp Immunol 1987; 68: 138-145

- Hodge G, Lloyd JV, Hodge s et al.
Functional lymphocytic immunophenothypes observed in thalasemia and
haemophilia patients receiving current blood product preparations. Br J
Haematol 1999; 105: 817-825

- Speer GP, Gahr M, Schuff- Werner P et al.
Immunologic evaluation of children with homozygous beta-thalassemia
treated with desferrioxamine. Acta Haematol 1990; 83: 75-81.

- Akbar AN, Giardina PJ, Hilgartner MW et
al. Immunological abnormalities in thalassemia major. A transfusion
related increase in cytoplasmic immunoglobulin positive cells. Clin
Experimental Immunol.1985;62:397-404.

- Wanachiwanawin W, Siripaniaphinyo U, Fucharoen S et al. Activation of monocytes for the immune clearance of red cellls in b0-thalassaemia/HbE. Br J Haematol 1993; 85: 363–369
- Wiener, E, Wanachiwanawin W,
Chinprasertsuk, S et al. Increased serum levels of macrophage colony
stimulating factor (M-CSF) in a- and b-thalassaemia syndromes. Eur J
Haematol 1996; 57:363–369

- Wiener E, Allen D, Siripaniaphinyo U et
al. Role of FcgRI (CD64) in erythrocyte elimination and its
upregulation in thalassaemia Br J Haematol 1999; 106:
923–930

- Ampel HM, van Wyck DM, Aguirre ML et al.
Resistance to infection in murine beta thalassaemia. Infect Immun 1989;
57: 1011–1017

- Model B, Berdoukas V. The clinical approach to Talassemia. London: Grune & Stratton. 1984: 140-150.
- Moore CM, Ehlayed M, Leiva LE et al. New
concepts in the immunology of sickle cell disease. Ann Allergy Asthma
Immunol 1996; 70: 385-400

- Chies JA, Nardi NB. Sickle cell disease: a
chronic inflammatory condition. Med Hypotheses 2001; 57:46-50

- Wood Kc, Granger DN Sickle cell disease:
role of reactive oxygen nitrogen metabolites. Clin Exp Pharmacol
Physiol.2007;34:926-932

- Taylor SC, Shacks SJ, Mitchell RA et al.
Serum interleukin-6 levels in steady state of sickle cell disease.
Interferon Cytokine Res 1995;15: 1061-1064

- Taylor SC, Shacks SJ, Qu Z et al. Type 2
cytokine serum levels in healthy sickle cell disease patients. J Natl
Med Assoc. 1997;89:753-757.

- Raghupathy R, Haider MZ, Azizich F et al.
Th1 and Th2 cytokine profiles in sickle cell disease. Acta Hematol
2000; 103:197-202.

- Taylor S, Shacks S, Qu Z. Effect of anti
Il-6 and anti IL-10 monoclonal antibodies on the suppression of the
normal T lymphocyte mitogenic responses by steady state sickle cell
disease sera. Immunol Invest 2001; 30: 209-219

- Taylor S, Shacks S, Qu Z. In vivo
production of type1 citokines in healty sickle cell disease patients, J
Natl Med Assoc. 1999; 91; 619-624

- Sloma I, Zilber MT, Charron D et al.
Upregulation and atypical expression of the CD1 molecules on monocytes
in sickle cell disease. Hum Immunol. 2004 Nov;65:1370-6.

- Humbert JR, Winsur EI, Githens JM et al
Neutrophil dysfunctions in sickle cell disease. Biomed Pharmacother
1990; 44:153-158.

- Lum AF, Wum T, Staunton D et al.
Inflammatory potential of neutrophils detected in sickle cell disease.
Am J Hematol 2004;76:126-133.

- Adedeji MO. Lymphocyte subpopulations in
homozygous sickle cell anemia. Acta Hematol 1985;74:10-13

- Mendoza E, Gutgsell N, Temple JD et al.
Nonocytic phagocitic activity in sickle cell disease. Acta haematol
1991;85: 199-201

- Lachant NA, Oseas RS. Vaso-occlusive
crisis-associated neutrophils dysfunction in patients with sickle-cell
disease. Am J Med Sci 1987;294:253-257.

- Anyaegbu CC, Okpala IE, Aken'ova AY et al.
Complement haemolytic activity, circulating immune complexes and the
morbidity of sickle cell anaemia. APMIS. 1999 Jul;107(7):699-702

- Agua P, Castello-Herbreteau B. Severe infections in children with sickle cell disease: clinical aspects and prevention. Arch Pediatr 2001; 8(S4): 732s-741s
- Gaston MH, Verter JI, Woods G, et al.
Prophylaxis with oral penicillin in children with sickle cell anemia. A
randomized trial. N Engl J Med 1986;314:1593–1599

- Overturf G, Powers D. Infections in sickle
cell anemia pathogenesis and control. Tex Rep Biol Med 1980; 40:
283-292.

- Glassman AB, Deas DV, Berlinsky FS et al.
Lymphocyte blast transformation and lymphocyte percentage in patients
with sickle cell disease. Ann Clin Lab Sci 1980; 10: 9-12.

- Ballester OF, Abdallah JM, Prasad AS.
Lymphocyte subpopulation abnormalities in sickle cell anemia: a
distinctive pattern from that of AIDS. Am J Hematol 1986, 21:23-27

- Rivero RA, Macaas C, Del Valle L et al.
Immunnologic changes in sickle cell anemia. Sangre 1991; 36:15-20.

- Wang W, Herrod H, Presbury g et al.
Lymphocyte phenotype and function in chronically transfused children
eith sickle cell disease. Am J Hematol 1985;20:31-3

- Hendriks J, De Ceulaer K, Williams E et
al. Mononuclear cells in sickle cell disease: subpopulations and in
vitro response to mitogens. J Clin Lab immunol 1984; 13. 129-32.

- Cetiner S, Akoazlu TF, kilina Y et al.
Immunological studies in sickle cell disease: comparison of homozygote
mild and severe variants. Clin Immunol Immunopathol 1990; 55: 492-497.

- Walker EM, Walker SM. Effects of iron
overload on immune system. Ann Clin Lab Sci 2000;30: 354-365.

- Fibach E, Rachmilewitz E. The role of
oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8:609-619

- Wiener E. Impaired phagocyte antibacterial
effector functionsin b-thalassemia: a likely factor in the increased
susceptibility to bacterial infections. Hematology, 2003; 8: 35–40

- Amer J, Fibach E. Chronic oxidative stress
reduces the respiratory burst response of neuthrophils from beta
thalassemia patients. Br J Haematol 2005;129:435-441

- Koduri PR. Iron in sickle cell disease: a
review why less is better. Am J Hematol 2003; 73:59-63.

- Vichinsky E, Butensky E, Fung E et al.
Comparison of organ dysfunction in transfused patients with SCD or beta
thalssemia. Am J Hematol. 2005;80:70-4.

- Kroot JJ, Laarakkers CM, Kemna EH et al.
Regulation of serum hepcidin levels in sickle cell disease.
Haematologica 2009;94:885-887

- Fung EB, Harmatz P, Milet M et al.
Morbidity and mortality in chronically transfused subjects with
thalassemia and sickle cell disease: a report from a multicenter study
on iron overload. Am J Hematol 2007;82:255-265.

- De Witthe T: the role of iron in patients
after bone marrow transplantation. Blood Rev 2008;22(S2):S22-S28

- Vanvakas E, Bajchman MA. Transfusion
related mortality: the ongoing risks of allogenic blood transfusion and
the available strategies for their prevention. Blood 2009;113:3406-3417

- Dodd RY, Notari IV, Stramer SL. Current
prevalence and incidence of infectious disease markers and estimated
window-period risk in the America Red Cross blood donor population.
Transfusion. 2002: 42:975-979

- Busch, MP, Glynn SA, Stramer SL et al. A
new strategy for estimating risks of transfusion transmitted viral
infections based on rates of detection of recently infected donors.
Transfusion 2005; 45: 254–264.

- Soldan K, Barbara JA, Ramsay ME et al.
Estimation of the risk of HBV, HCV and HIV infectious donations
entering the blood supply in England, 1993–2001. Vox Sanguinis
2003; 84: 274–286

- O’Brien SF, Yi QL, Foon W et al. Current
incidence and estimated residual risk of transfusion transmitted
infections in donations made for Canadian Blood Service. Transfusion
2007; 47, 316–325.

- Bortolotti F, Iorio R, Resti M et
al.Epidemiological profile of 806 Italian children with hepatitis C
virus infection over a 15-year period. J Hepatol. 2007;47:311-17.

- Di Marco V, Capra M, Gagliardotto F et al.
Liver disease in chelated transfusion-dependent thalassemic: the role
of iron overload and chronic epatitis C. Haematologica
2008;93:1243-1246.

- Ocak S, Kaya H, Cetin M, Gali E et al.
Seroprevalence of hepatitis B and hepatitis C in patients with
thalassemia and sickle cell anemia in a long-term follow-up. Arch Med
Res. 2006;37(7):895-898.

- Rouger P. Transfusion induced immunomodulation: myth or reality? Transf Clin Biol 2004; 11:115-116.
- Blumberg N. Deleterious effect of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion 2005; 45S:S33-39
- Vamvakas EC, Blajchman MA. Transfusion
related immunomodulation (TRIM): an update. Transfusion 2007;
21:327-348.

- James J, Matthews RN, Holdsworth R et al.
The role of filtration in the provision of leukocyte poor red cells to
multitransfused patients. Pathology. 1986;18:127-130.

- Tan KK, Lee WS, Liaw LC et al. A
prospective study on the use of leucocyte-filters in reducing blood
transfusion reactions in multi-transfused thalassemic children.
Singapore Med J. 1993; 34:109-111

- Cabibbo S, Fidone C, Antolino A et al.
Clinical effects of different types of red cell concentrates in
patients with thalassemia and sickle cell disease. Transfus Clin Biol.
2007;14:542-550.

- Sirchia G, Rebulla P, Mascaretti L et al.
Effectiveness of red blood cells filtered through cotton wool to
prevent antileukocyte antibody production in multitransfused patients.
Vox Sang. 1982;42:190-197

- Sirchia G, Rebulla P, Mascaretti Let al.
The clinical importance of leukocyte depletion in regular erythrocyte
transfusions. Vox Sang. 1986;51 (S 1):2-8.

- Rebulla P, Model B. Transfusion
requirement and effects in patients with thalassemia major.
Lancet 1991; 337: 277-280.

- Amlot PL, Hayes AE. Impaired human
antibody response to the thymus-independent antigen, DNP-ficoll, after
splenectomy. Lancet 1985;1:1008–1011.

- Wolf HM, Eibl MM, Georgi E, et al.
Long-term decrease of CD41 CD45 RA1 T cells and impaired primary immune
response after post-traumatic splenectomy. Br J Haematol 1999;107:55–68.

- Constantopoulos A, Najjar VA, Smith JW.
Tuftsin deficiency: a new syndrome with defective phagocytosis. J
Pediatr 1972;80:564–572.

- Hashimoto T, Mahour GH, Church JA, Lipsey
AI. Plasma fibronectin levels after splenectomy and splenic
autoimplantation in rats with and without dietary ascorbic acid
supplementation. J Pediatr Surg 1983;18:805–810.

- Simon M Jr, Djawari D, Hohenberger W.
Impairment of polymorphonuclear leukocyte and macrophage functions in
splenectomized patients. N Engl J Med 1985;1089–1092.

- Hansen K, Singer D. Aspenic-hyposplenic
overwhelming sepsis:postsplenectomy sepsis revisited. Ped Develop
Pathol 2001; 4:105-121

- Singer DB. Postsplenectomy sepsis.
Perspect Pediatr Pathol 1973;1:285–311

- Fraker PJ, King LE, Laakko T et al.
The dynamic link between the integrity of the immune system and zinc
status. J Nutr. 200;130(5S):1399S-406S.

- Prasad AS, Kaplan J, Brewer GJ et
al. Immunological effects of zinc deficiency in sickle cell
anemia (SCA). Prog Clin Biol Res. 1989;319:629-47

- Prasad AS, Beck FW, Kaplan J et al.
Effect of zinc supplementation on incidence of infections and hospital
admissions in sickle cell disease (SCD). Am J Hematol. 1999
Jul;61(3):194-202.

- Cotmore SF,Tattersal P. Characterization
and molecular cloning of human parvovirus genome. Science
1984;226:1161-1165

- Cassinotti P, Siegl G. Quantitative
evidence for persistence of human parvovirus B19 medicine.Summary of a
workshop. Transfusion.2001;41:130-135.DNA in an immunocompetent
individual. Eur J Clin Microbiol Infec Dis 2000;19:886-895

- Smith-Whitley K, Zhao H, Hodinka RL et
al. Epidemiology of human parvovirus B19 in children with sickle cell
disease. Blood 2004;103:422-427.

- Brown KE, Young NS, Alvin BM et al.
Parvovirus B19: implications for transfusion medicine. Summary of a
workshop.Transfusion. 2001 Jan;41(1):130-5.

- Kleinman S, Glynn SA, Lee T et al. A
linked donor-recipient study to evaluate parvovirus B19 transmissionby
blood component transfusion. Blood 2009;114:3677-3683.

- Lefrère JJ, Servant-Delmas A, Candotti D
et al. Persistent B19 in immunocompetent individuals: implications for
transfusion safety. Blood 2005;106:2890-2895

- Baumler AJ, Hantke K. Ferrioxamine uptake
in Yersinia enterocolitica: characterization of the receptor protein
FoxA. Mol Microbiol 1992; 6: 1309–1321.

- Autenrieth IB, Bohn E, Ewald JH et al.
Deferoxamine B but not deferoxamine G1 inhibits cytokine production in
murine bone marrow macrophages. J Infect Dis 1995; 172: 490–96.

- Nowak MA, Anderson RM, Boerlijst MC et
al. HIV-1 evolution and disease progression. Science. 1996;8:1008-11.

- Costagliola DG, Girot R, Rebulla P et al.
Incidence of AIDS in HIV1 infected talassemia patients. Br J Haematol
1992;81:109-112.

- Costagliola DG, deMontalembert M, Lefrere
JJ et al. Dose of desferrioxamine and evolution ofHIV-1 infection in
thalassaemic patients. Br J Haematol. 1994;87:849–52

- Salhi Y, Costagliola D, Rebulla P, et al.
Serum ferritin, desferrioxamine, and evolution of HIV-1infection in
thalassemic patients. J Acquired Immune Defic Syndr Hum Retrovirol.
1998;18:473–8

- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. November 3, 2008;1-39. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- Georgiou NA, van der Bruggen T, Oudshoorn
M et al. Inhibition of human immunodeficiency virus type 1 replication
in human mononuclear blood cells by the iron chelators deferoxamine,
deferiprone, and bleomycin. J Infect Dis. 2000;181:484-490.

- Debebe Z, Ammosova T, Jerebtsova M et
al. Iron chelators ICL670 and 311 inhibit HIV-1 transcription.
Virology. 2007;367:324-333.

- (no Author) Hepatitis C-global prevalence
(update).Wkly Epidemiol Rec 2000;75:18-19

- Burra P. Hepatitis C. Semin Liver Dis.
2009;29:53-65.

- Shedlofsky SI. Role of iron in the
natural history and clinical course of hepatitis C disease.
Hepatogastroenterology. 1998;45:349-55.

- Fujita N, Sugimoto R, Urawa N, et al.
Hepatic iron accumulation is associated with disease progression and
resistance to interferon/ribavirin combination therapy in chronic
epatitis C. J Gastroenterol Hepatol. 2007;22:1886–1893

- Li CK, Chik KW, Lam CWK et al. Liver
disease in transfusion dependent thalassaemia major. Arch Dis Child
2002;86:344-7.

- Ardalan FA, Osquei MR, Toosi MN et al.
Synergic effect of chronic hepatitis C infection and beta thalassemia
major with marked hepatic iron overload on liver fibrosis: a
retrospective cross-sectional study. BMC Gastroenterol 2004;4:17.

- Cunningham MJ, Macklin EA, Neufeld EJ et
al. Complications of beta-thalassemia major in North America.
Blood 2004;104:34-39.

- Prati D, Maggioni M, Milani S et
al. Clinical and histological characterization of liver disease
in patients with transfusion-dependent beta-thalassemia. A multicenter
study of 117 cases. Haematologica 2004;89:1179-86.

- Perifanis V, Tziomalos K, Tsatra I et al.
Prevalence and severity of liver disease in patients with
beta-thalassemia major. A single-institution fifteen-year experience.
Haematologica 2005;90:1136-1138.

- Di Marco V,Lo Iacono P, Almasio P et al.
Long-term efficacy of α-Interferon in β-thalassemics with chronic
hepatitis C. Blood 1997;90:2207-2212.

- Angelucci E, Muretto P, Nicolucci A et
al. Effects of iron overload and hepatitis C virus positivity in
determining progression of liver fibrosis in thalassemia following bone
marrow transplantation. Blood 2002;100:17-21.

- Di Marco V, Lo Iacono O, Capra M et al.
Alpha-interferon treatment of chronic C hepatitis of young
patients with homozygous β-thalassemia. Haematologica 1992;77:502-506

- Donohue SM, WonkeB, Hoffbrand AV et al.
Alpha interferon in the treatment of chronic Hepatitis C in fection in
Thalassemia major. Br J Haematol 1993;83:491-497

- Syriopoulou V, Daikos GL, manolaki N et
al. Sustained response to interferon α-2a in thalassemic patients with
chronic hepatitis C. A prospective 8-years follow-up study.
Haematologica 2005; 90:129-131

- Giardini C, Galimberti M, Lucarelli G et
al. α-Interferon treatment of chronic hepatitis C after bone marrow
transplantation for homozygous β-thalassemia. Bone Marrow
Transpl;.1997;20:767-772

- Li CK, Chan PKS, Ling S et al. Interferon
and ribavirin as frontline treatment for chronic hepatitis C infection
in thalassemia major. Br J Haematol 2002;117:755–758

- Inati A, Taher A, Ghorra S et al.
Efficacy and tolerability of peginterferon alpha2a with or without
ribavirin in thalassemia major patients with chronic hepatitis C
infection. Br J Haematol 2005; 130: 644-646.

- Harmatz P, Jonas MM, Kwiatkowski JL et
al. Safety and efficacy of pegylated interferon α-2a and ribavirin for
the treatment of hepatitis C in patients with thalassemia.
Haematologica 2008:93:1247-1251.

- Teixera AL, Borato Viana M,
Valadares Roquete ML et al Sicke cell disease: a clinica and
histopathologic study of the liver in living children. J Pediatr
Hematol Oncol. 2002;24.125-129

- Harmatz P, Butensky E, Quirolo K et al.
Severity of iron overload in patients with sickle cell disease
receiving chronic red blood cell transfusions. Blood 2000; 96:76-79.

- Singh H, Pradhan M, Singh RL et al. High
frequency of hepatitis B virus infection in patients with
beta-thalassemia receiving multiple transfusions. Vox Sang 2003; 84:
292–99.

- Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13-21.
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-55
- Papatheodoridis GV, Manolakopoulos S,
Archimandritis AJ. Current treatment indications and strategies in
chronic hepatitis B virus infection. World J Gastroenterol.
2008;14:6902-10.

- Uysal Z, Cin S, Arcasoy A, Akar N.
Interferon treatment of hepatitis B and C in beta-thalassemia. Pediatr
Hematol Oncol 1995; 12: 87–89.

- Feld JJ, Wong DK, Heathcote EJ. Endpoints
of therapy in chronic hepatitis B. Hepatology. 2009;49:S96-S102.

- Bisharat N, Omari H, Lavi I et al.
Risk of Infection and Death Among Post-splenectomy Patients. Journal of
Infection 2001; 43:182-186

- Lynch AM, Kapila R. Overwhelming
postsplenectomy infection. Infect Dis Clin North Am 1996;10:693–707.

- Sumaraju V, Smith LG, Smith SM.
Infectious complications in asplenic hosts. Infect Dis Clin North Am
2001;15:551–565.

- Adamkiewicz TV, Silk BJ, Howgate J et al.
Effectiveness of the 7-valent pneumococcal conjugate vaccine in
children with sickle cell disease in the first decade of life.
Pediatrics 2008;121:562-569.

- British Committee for Standards in
Haematology. Davies JM, Barnes R, Milligan D. Working Party of the
Haematology/Oncology Task Force. Update of guidelines for the
prevention and treatment of infection in patients with an absent or
dysfunctional spleen. Clin Med 2002;2:440–443.

- American Academy of Pediatrics. Immunocompromised children-Asplenic children. The red book, report of the committee on infectious diseases, 25th edn. Elk Grove Village, IL, USA: American Academy of Pediatrics; 2000
- American Academy of Pediatrics. Committee on Infectious Diseases. Policy statement: Recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 2000;106:362–366
- Jugenburg M, Haddock G, Freedman MH et
al. The morbidity and mortality of pediatric splenectomy: Does
prophylaxis make a difference? J Pediatr Surg 1999;34:1064–1067

- Price VE, Dutta S, Banchette VS et al.
The prevention and treatment of bacterial infections in children with
asplenia or hyposplenia: practice considerations at the hospital for
sick children, Toronto. Pediatr Blood Cancer 2006;46:597-603

- Resende V, Petroianu A. Functions of the
splenic remnant after subtotal splenectomy for treatment of severe
splenic injuries. Am J Surg 2003;185:311–315.

- Rice HE, Oldham KT, Hillery CA, Skinner
MA, O'Hara SM, Ware RE : Clinical and hematologic benefits of partial
splenectomy for congenital hemolytic anemias in children. Ann Surg.
2003 Feb;237(2):281-8
