Clinical Aspects and Therapy of Sporadic Burkitt Lymphoma
Livio Pagano, Morena Caira, Caterina Giovanna Valentini and Luana Fianchi
Istituto di Ematologia, Università Cattolica S. Cuore, Roma, Italy
Published: December 29, 2009
Received: December 23, 2009
Accepted: December 27, 2009
Medit J Hemat Infect Dis 2009, 1(2): e2009030, DOI 10.4084/MJHID.2009.030
This article is available from: http://www.mjhid.org/article/view/5249
Abstract
Burkitt’s lymphoma is a highly aggressive mature B-cell neoplasm consisting of endemic, sporadic, and immunodeficiency-associated variants, that share many morphologic and immunophenotypic features. It is characterized by a high proliferation rate and propensity for extranodal sites such as gastrointestinal tract and reproductive organs. Brief-duration, high-intensity chemotherapy regimens including aggressive central nervous system prophylaxis have had remarkable success in the treatment of this disease in the sporadic form, with very high complete remission rate and overall survival in adults. Although Burkitt's lymphoma is extremely chemosensitive, biologically targeted therapies should be developed, because current treatment options are suboptimal for patients with poor prognostic features or with relapsed disease.
Burkitt's lymphoma (BL) is a small non-cleaved cell lymphoma with a high proliferation rate and characteristic molecular changes involving the c-MYC oncogene. It is a clinically distinct and aggressive disease, that frequently involves extranodal sites, such as the gastrointestinal tract and the central nervous system (CNS), so that it requires urgent treatment.
In the WHO classification three clinical variants are recognized: endemic, sporadic and immunodeficiency-associated [1]. The three subtypes are identical according to histological pattern, and they all possess chromosomal rearrangements of the c-MYC oncogene, that contributes to lymphomagenesis altering the mechanisms of cell cycle regulation, cellular differentiation, apoptosis, cellular adhesion, and metabolism.
BL is common in children, accounting for 40-50% of childhood non-Hodgkin's lymphomas (NHL) in non-endemic areas [2,3].
These data have been recently update by a study about sporadic childhood BL incidence in United States during 1992-2005, reporting over this period 296 cases of children ages(da eliminare) 0–14 years-old, accounting for approximately 30% of childhood NHL. The distribution of the cases indicated an early age onset (3–5 years) and a predominance in boys (79%) and in non-Hispanic Whites (81%), suggesting that male sex and factors correlated with race may be risk factors for sporadic BL [4].
These results were confirmed in another analysis conducted by the same Authors about age-specific incidence pattern for BL in US over the years 1973-2005. In this study a novel tri/bimodal incidence patterns for BL emerged, which showed disparities by gender but not race. In fact a notable finding was distinct trimodal age-specific BL incidence patterns among males, with three separate incidence peaks near ages 10, 40, and 75 years, respectively. Among females, the pediatric and the geriatric peaks were remarkable, but not the adult one. BL incidence rates were significantly higher among males for pediatric and adult BL, but only marginally for geriatric patients [5].
BL also occurs in adults, with the sporadic form accounting for 1–2% only(da eliminare) of all adult NHL in the western Europe and in the United States [6].
Clinical aspects
Unique clinical features have been described among the 3 different variants of BL, although there is considerable overlap among them.
The sporadic form is mostly characterized by abdominal tumours, with no specific geographic or climatic distribution [6]. It tends to arise in the lymphoid tissues of the gut and the upper respiratory tract, often presenting as masses in the Waldeyer ring or the terminal ileum, or even with massive abdominal involvement. It could be associated with EBV in approximately 30% of cases.
Symptoms of sporadic BL are usually aspecific: abdominal pain, nausea, vomiting, bowel obstruction, gastrointestinal bleeding have been reported. Bowel or mesenteric lymphonodes are frequent intra-abdominal localizations, but also kidney, pancreas, liver, spleen, breast, or ovarian involvements can occur. At diagnosis, patients may have bulky disease and elevated levels of lactate dehydrogenase and uric acid. Involvement of bone marrow and central nervous system (CNS) is reported in 30-38% and 13-17% of adults, respectively [7-9]. Bone marrow involvement is more commonly seen in progressive disease. In fact, Burkitt's leukemia is essentially considered a presentation of advanced stage of BL, and it includes patients with acute lymphoblastic leukemia (1–2%), and circulating blasts that morphologically and histologically resemble BL’s cells.
Treatment
Before the advent of high intensity chemotherapy, BL was associated with poor outcomes, probably because of its high proliferative rate. The introduction of high intensity regimens has significantly changed the prognosis of this disease. At present BL appears to be curable in a high proportion of cases, when treated with aggressive multiagent- based chemotherapy regimens.
Evolution of treatment strategies:
The optimal strategies for BL evolved over the last years, with the growing knowledge of the biological characteristics of the disease, such as the rapid double time of the tumor, the propensity for extranodal sites, the high chemosensitivity, and the potential for CNS relapses.
Characteristic of BL is the chemo-sensitivity to single agents [1–3,6]. In fact, using cyclophosphamide (CTX) alone, complete response (CR) rates of more than 70% [3] were obtained; however the high rates of relapse after such treatment have induced to employ treatment with cyclical CTX, followed by two cycles of combination therapy with vincristine (VCR), methotrexate (MTX) and/or cytosine arabinoside (Ara-C) in the event of relapsed disease. Despite the good results in patients with limited disease (up to 90% survival at 100 weeks), most of patients with CNS or bone marrow involvement relapsed and died [3,6].
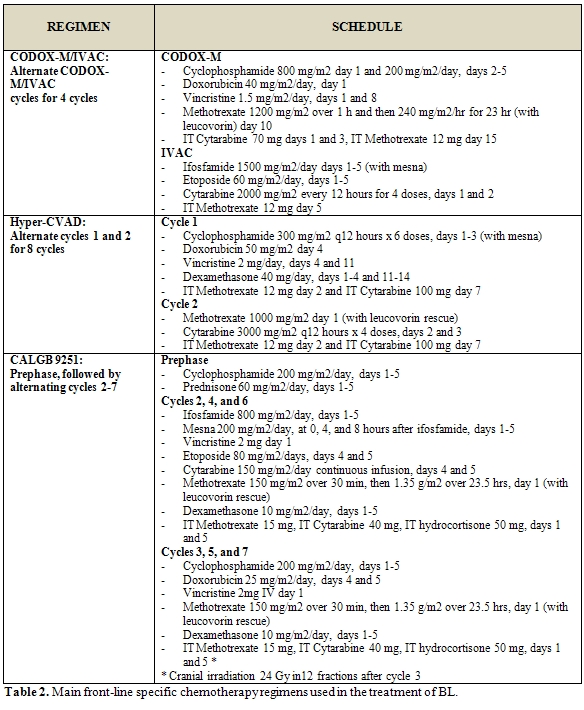
Chemotherapy approaches in adults (table 1) have been largely adapted from pediatric regimens [7-9]. The main regimens for the front-line therapy of BL are reported in table 2.
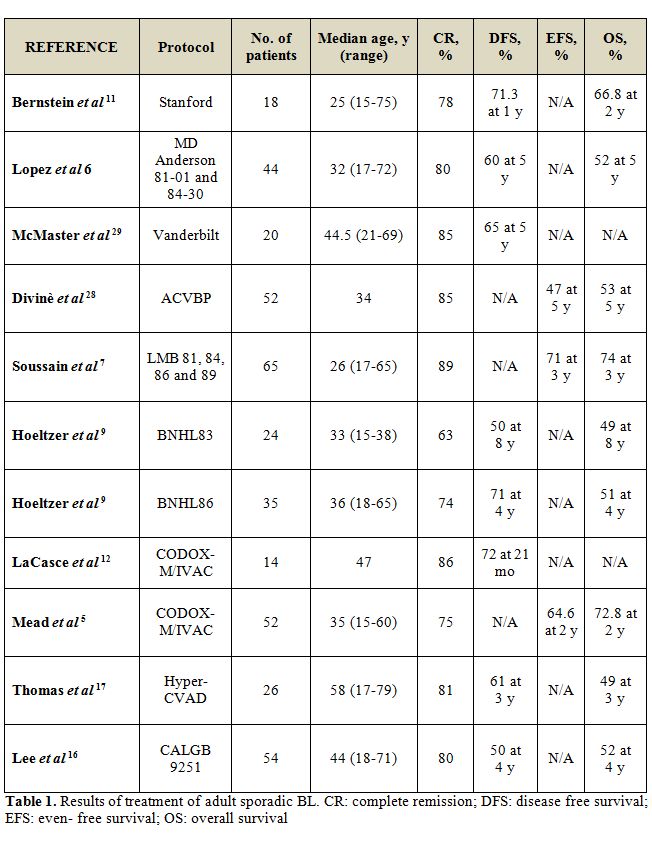
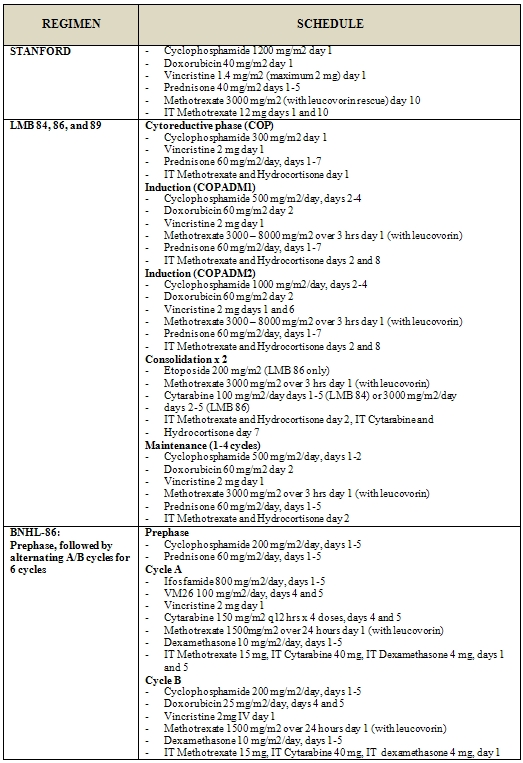
In 1995 and 1996, two reports documented the success of adapting the pediatric French LMB and the German BFM regimens to the treatment of adult BL [9, 11]. In a retrospective review 65 adults were treated according to the pediatric LMB 81, 84, 86, and 89 regimens, consisting of an initial cytoreductive phase, using CTX and prednisone to reduce the tumour burden and to minimize the risk of tumor lysis, followed by 2 induction cycles, 1 to 2 consolidation cycles, and 1 to 4 maintenance cycles. In these series 58 patients (89%) achieved a CR with a 3-year OS of 74%, even if most patients had advanced-stage disease or evidence of leukemic involvement; 7 of 12 patients who presented with CNS involvement remained disease free up to 56 months after therapy [9]. A prospective study of the LMB protocol in adults confirmed the retrospective findings, with a CR rate of 83% and a 2-year OS of 66% [12].
After the success in pediatric BL with the BFM protocols, the German Multicenter Study Group for the treatment of adult ALL (GMALL) developed 2 protocols, B-NHL 83 and B-NHL 86, for the treatment of adult Burkitt’s leukemia [11]. Similarly to the LMB trials, these studies included a cytoreductive pre-phase to minimize the risk of tumour lysis, after which 6 cycles of alternating chemotherapy regimens were given, with fractionated CTX, MTX, and low-dose Ara-C in each of these alternating cycles. In the B-NHL 86 regimen the dose of MTX was escalated to 1500 mg/m2 and ifosfamide was added to the B-NHL 83 regimen. Results were comparable to those noted in the French LMB trials, with 4- to 8-year OS reaching 49-51% [11].
McMaster et al treated 20 patients with 2 intensive inpatient induction courses of high-dose CTX, MTX (200 mg/m2), bleomycin, VCR, and doxorubicin achieving CR in 85% of patients, with 5-year DFS of 60% [8]. The Stanford group obtained similar results with a regimen containing high-dose CTX (1500 mg/m2) and mid-cycle high-dose MTX (3000 mg/m2) administered over 6 to 9 cycles. With this regimen, 2-year OS reached 66.8%; however, the best responses were noted in patients with limited-stage disease (a single extra-abdominal tumour site or a completely resected intra-abdominal disease), where 2-year OS was 100%, compared with 53.8% in the advanced setting [13]. The experiences with dose-dense regimens culminated in the scheme reported by Magrath and colleagues [10]. Pediatric and young adult patients with not-cleaved small lymphocitic lymphomas, when high risk, were treated alternating cycles with Cyclophosfamide (CTX), Vincristine (VCR), doxorubicin, high-dose Methotrexate (MTX )i.v. and intrathecal (CODOX-M) and cicles including
ifosfamide, etoposide, high-dose Ara-C i.v. and intrathecal (IVAC regimen), and when low risk received three cycles of CTX, VCR, doxorubicin and high-dose MTX (CODOX-M) [10 ].
This study demonstrated that short courses of intensive therapy had excellent response rates, with 92% 2-year EFS both for children and adults with small non-cleaved lymphoma. Unfortunately, the toxicities reported from many of these intensive regimens are significant, including neurotoxicities from intrathecal therapy, hematological toxicity and severe mucositis. These data referred to a selected group of young patients (median age 25 years); a subsequent international validation of this first impressing data came from an international cohort of patients [7].
The current approaches to Burkitt’s lymphoma:
Over the past few years, more focus has been placed on identifying efficacious but less toxic regimens. Several trials aimed at reducing MTX-associated toxicity, while maintaining the treatment's efficacy. Utilizing a modified Magrath’s regimen, one small study treated adults with reduced doses of systemic MTX and intrathecal Ara-C, and altered the fractionated schedule for the CTX. This resulted in a significant decrease in neurotoxicity and mucositis, and there were no treatment-associated deaths. Overall, the 2-year EFS was 64%, but 100% for low-risk patients (normal LDH and focal, smaller volume tumour burden) and 60% for high-risk patients [14]. This study proved that some patients can be effectively treated with lower intensity regimens, with reduced drug toxicity. The inferior survival of high-risk patients may reflect the change in regimen, but it may also be explained by other factors, different in the two studies. For example, the median age was 46 years in the latter study, compared with 24 years for the original study by Magrath et al. It has became increasingly clear that age is a significant prognostic factor related to survival, as older patients do not tolerate the chemotherapy as well as the younger, or do not have the same good response. This fact could be explained by the innate differences in the biology of the tumours. The results of a FAB/LMB96 trial for pediatric intermediate-risk NHL patients revealed that it is possible to reduce treatment for early responding patients. In this trial, the intermediate-risk patients (defined as non-resected, stage I/II and CNS negative advanced-stage III/IV), who have had an early response to therapy (> 20% response at day 7), could be treated with reduced doses of CTX and doxorubicin without a significant decrement in EFS and in overall survival, comparing to the original trial. This approach should reduce the risk of future toxicities such as cardiac disease or infertility [15,16]. With the Hyper-CVAD regimen, a modified Murphy regimen used to treat adult Burkitt leukemia at MD Anderson, 81% of patients achieved a CR, with a 3-year OS of 49%. Notably, this study contained a much older population of patients (median age, 58 years) than that reported in other trials, and patients 60 years or older had an inferior outcome (3-year OS of 17% versus 77%) [17].
The CALGB regimen contains a cytoreductive phase, followed by 3 cycles, each of 2 different regimens administered every 3 weeks [18]. In 54 evaluable patients, CR is 80%, with 4-year DFS of 50%. However, severe neurologic toxicity was observed in 10 of 74 patients enrolled on this trial, attributed to the combination of high-dose MTX (1500 mg/m2), triple intrathecal chemotherapy, and whole brain irradiation (24 Gy) used for CNS prophylaxis. The cranial radiation was subsequently eliminated for patients without bone marrow involvement at presentation and the rate of neurologic events decreased. In the CALGB study, only 32% of patients older than 50 years were able to complete 6 to 7 cycles of treatment, compared with 79% of younger patients. Mortality (21% versus 9%), disease progression (32% versus 3%), and toxicity (16% versus 9%) were noted to be higher in those patients older than 50 years [8]. The higher rate of relapse in elderly patients with BL implies that these poor outcomes may not simply be related to treatment-related toxicity.
Thomas et al noted an increased incidence of complex cytogenetic abnormalities in older patients, including bcl-2 gene rearrangements, which may contribute to a more aggressive phenotype [19]. A prospective study of dose-modified CODOX-M/IVAC was recently conducted in patients with sporadic BL, defined using cytogenetic and immunophenotypic criteria; immunophenotype and fluorescent in situ hybridization (FISH) were used to separate BL from other aggressive B-cell lymphomas. Compared with the previous trial LY06 with full-dose MTX (6.7 g/m(2) [5], there was a reduction in toxicity with comparable outcomes in patients treated dose-modified CODOX-M (MTX, dose 3 g/m(2)) with or without IVAC, according to risk group [20].
Monoclonal antibodies and new drugs:
It seems interesting the use of monoclonal antibodies and other biological reagents as adjuvant therapy in BL. These include agents such as anti-CD20 monoclonal antibody (Rituximab). It has been used most extensively with CTX, VCR, doxorubicin and dexamethasone [17,21] or with ifosfamide, carboplatin, etoposide in children with refractory BL [22].
A recent study of 31 patients treated with hyper-fractionated CTX, VCR, doxorubicin, and dexamethasone (hyper-CVAD) regimen plus rituximab demonstrated a significant increase in overall survival, EFS and disease-free survival (89, 80 and 88%, respectively), when compared with historical patients treated with CTX, VCR, doxorubicin and dexamethasone alone [17].
Interestingly, preliminary results of 19 patients (median age 29 years, with 53% advanced stage III/IV) with BL treated using dose-adjusted etoposide, prednisone, VCR, CTX and doxorubicin and rituximab (a known effective therapeutic regimen for the treatment of DLBCL); remission was obtained in all patients, without any relapses reported, with an average follow-up of 28 months. In addition, the therapy was administered in an outpatient, on the basis of the minimal side effects reported [21].
Other biological agents include monoclonal antibodies such as anti-CD22 (epratuzumab), in study for the treatment of NHL. Respect to rituximab, it has a different mechanism of action, but they could be synergistic in inducing cellular apoptosis [23].
Novel treatment options are developed basing on the rapidly growing knowledge about the molecular biology of this disease. In early development are epigenetic regulators such as histone deacetylases inhibitors and DNA methyltransferases inhibitors. Among them, Depsipeptide is a histone deacetylases inhibitor under investigation, because it has been shown to have an additive cytotoxic effect with many of the standard chemotherapies used for the treatment of lymphomas and leukemias (including BL) [24].
Other agents can theoretically be used to target oncogenes, such as small peptide nucleic acids. A recent study showed that BL developing in severe combined immunodeficient mice can be inhibited by a peptide nucleic acid complementary to regulatory intronic sequences, reducing c-myc production. Boffa and colleagues demonstrated a significant reduction in tumour size and progression of disease after the use of small peptide nucleic acids complementary to a regulatory sequence for c-myc [25,26].
Hemopoietic stem cell transplantation:
The role of stem cell transplantation in the treatment of BL has been explored over the past 12 years. Several studies have focused on the potential benefit of high-dose chemotherapy followed by autologous stem cell transplantation, with some promising results in response rates and overall survival [27,28]. A recent phase II study for adult patients by van Imhoff et al [29] utilized an up-front short intensive chemotherapy course followed by autologous stem cell transplantation: this scheme resulted in equivalent, or slightly better, 5-year EFS and overall survival compared with current chemotherapy regimens. One of the limitations for the study was the low number of patients with bone marrow involvement, compared with other studies utilizing only intensive chemotherapy. It has been well documented that bone marrow involvement is a known poor prognostic indicator for BL [30,31].
In addition, there have been reports of retrospective evaluations of allogeneic transplantation for BL. The theoretical benefits include the removal of the possibility of tumour contamination, and the still controverse graft versus lymphoma effect. Such reports show lower relapse rates for patients receiving allogeneic transplantation when compared with recipients of autologous transplantation, but unfortunately with higher rates of transplant-related mortality [32]. At the same time, there have been case-reports of allogeneic transplantations leading to complete response and long survival of individual patients [33,34]. The existence of a significant graft versus lymphoma effect from allogeneic stem cell transplantation is still widely debated [33,35].
At this time the role of hemopoietic stem cell transplant for BL is difficult to be defined.
Comment
and conclusions:
The response of BL to different chemotherapic approaches represents a
very interesting point. In the past it was thought to be an
incurable
disease in adults because of its high proliferative rates, but the
incorporation in treatment regimens of several active agents,
particularly of the cyclophosphamide and the high-dose methotrexate,
improved the outcome of these patients, reaching a very high cure rate.
A maintenance phase does not seem to add an amelioration of
outcome,
while the prognosis of patients has further improved after the addition
of rituximab to chemotherapy regimens. The use of hemopoietic stem cell
transplantation remains experimental, and it should be reserved for
younger patients with refractory or resistant disease.
References
- Wright DH. What is Burkitt's lymphoma and
when is it endemic? Blood 1999; 93:758.

- Murphy SB, Fairclough DL, Hutchison RE,
Berard CW. Non-Hodgkin's lymphomas of childhood: an analysis of the
histology, staging, and response to treatment of 338 cases at a single
institution. J Clin Oncol 1989; 7:186–193.

- Wilson JF, Jenkin RD, Anderson JR, Chilcote
RR, Coccia P, Exelby PR, Kersey J, Kjeldsberg CR, Kushner J, Meadows A,
Sheehan WW, Siegel S, Sposto R, Leikin S, Hammond D. Studies on
the pathology of non-Hodgkin's lymphoma of childhood. I. The role of
routine histopathology as a prognostic factor. A report from the
Children's Cancer Study Group. Cancer 1984; 53:1695–1704.

- Mbulaiteye SM, Biggar RJ, Bhatia K, Linet
MS, Devesa SS. Sporadic Childhood Burkitt Lymphoma Incidence in the
United States During 1992–2005. Pediatr Blood Cancer 2009; 53:366–370.

- Mbulaiteye SM, Anderson WF, Bhatia K,
Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence
patterns for Burkitt lymphoma in the United States, 1973-2005. Int J
Cancer. 2009 Oct 6. [Epub ahead of print]

- Diebold J, Jaffe E, Raphael M, Warnke R. Burkitt lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, eds. Pathology and Genetics of Tumors of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001: 181-184.
- Mead GM, Sydes MR, Walewski J, Grigg A,
Hatton CS, Pescosta N, Guarnaccia C, Lewis MS, McKendrick J, Stenning
SP, Wright D; UKLG LY06 collaborators. An international evaluation of
CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma:
results of United Kingdom Lymphoma Group LY06 study. Ann Oncol.
2002;13: 1264-1274.

- McMaster M, Greer J, Greco A, Johnson D,
Wolff S, Hainsworth J. Effective treatment of small-non-cleaved-cell
lymphoma with high-intensity, brief-duration chemotherapy. J Clin
Oncol. 1991;9: 941-946.

- Soussain C, Patte C, Ostronoff M, Delmer A,
Rigal-Huguet F, Cambier N, Leprisé PY, François S, Cony-Makhoul P,
Harousseau JL. Small noncleaved cell lymphoma and leukemia in adults. A
retrospective study of 65 adults treated with the LMB pediatric
protocols. Blood. 1995;85: 664-674.

- Magrath I, Adde M, Shad A, Venzon D,
Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA,
Horak ID. Adults and children with small noncleaved-cell lymphoma have
a similar excellent outcome when treated with the same chemotherapy
regimen. J Clin Oncol 1996; 14:925–934.

- Hoelzer D, Gökbuget N, Arnold R, Büchner
T, Freund M, Gassmann W, Heil G, Hiddemann W, Löffler H, Lipp T, Ludwig
WD, Maschmeyer G, Thiel E, Messerer D. Improved outcome in adult B-cell
acute lymphoblastic leukemia. Blood 1996; 87:495–508.

- Diviné M, Casassus P, Koscielny S, Bosq J,
Sebban C, Le Maignan C, Stamattoulas A, Dupriez B, Raphaël M, Pico JL,
Ribrag V. Burkitt lymphoma in adults: a prospective study of 72
patients treated with an adapted pediatric LMB protocol. Ann Oncol
2005; 16:1928–1935.

- Bernstein JI, Coleman CN, Strickler JG,
Dorfman RF, Rosenberg SA. Combined modality therapy for adults with
small noncleaved cell lymphoma (Burkitt's and non-Burkitt's types). J
Clin Oncol 1986; 4:847–858.

- LaCasce A, Howard O, Lib S, Fisher D, Weng
A, Neuberg D, Shipp M. Modified magrath regimens for adults with
Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased
toxicity. Leuk Lymphoma 2004; 45:761–767.

- Patte C, Auperin A, Gerrard M, Michon J,
Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K,
Cairo MS; FAB/LMB96 International Study Committee. Results of the
randomized international FAB/LMB96 trial for intermediate risk B-cell
non-Hodgkin's lymphoma in children and adolescents: it is possible to
reduce treatment for the early responding patients. Blood
2007;109(7):2773-2780.

- Di Nicola M, Carlo-Stella C, Mariotti J,
Devizzi L, Massimino M, Cabras A, Magni M, Matteucci P, Guidetti A,
Gandola L, Gianni AM. High response rate and manageable toxicity with
an intensive, short-term chemotherapy programme for Burkitt's lymphoma
in adults. Br J Haematol 2004; 126:815–820.

- Thomas DA, Faderl S, O'Brien S,
Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S,
Wierda WG, Pierce SA, Shan J, Brandt M, Hagemeister FB, Keating MJ,
Cabanillas F, Kantarjian H. Chemoimmunotherapy with hyper-CVAD plus
rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma
or acute lymphoblastic leukemia. Cancer 2006; 106:1569–1580.

- Lee EJ, Petroni GR, Schiffer CA, Freter
CE, Johnson JL, Barcos M, Frizzera G, Bloomfield CD, Peterson BA.
Brief-duration high-intensity chemotherapy for patients with small
noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results
of Cancer and Leukemia Group B study 9251. J Clin Oncol. 2001; 19:
4014-4022.

- Thomas DA, Cortes J, O'Brien S, Pierce S,
Faderl S, Albitar M, Hagemeister FB, Cabanillas FF, Murphy S, Keating
MJ, Kantarjian H. Hyper-CVAD program in Burkitt's type adult acute
lymphoblastic leukemia. J Clin Oncol. 1999;17: 2461-2470.

- Mead GM, Barrans SL, Qian W, Walewski J,
Radford JA, Wolf M, Clawson SM, Stenning SP, Yule CL, Jack AS. A
prospective clinicopathologic study of dose-modified CODOX-M/IVAC in
patients with sporadic Burkitt lymphoma defined using cytogenetic and
immunophenotypic criteria (MRC/NCRI LY10 trial). Blood
2008;112(6):2248-60.

- Wilson WH, Dunleavy K, Pittaluga S, Hegde
U, Grant N, Steinberg SM, Raffeld M, Gutierrez M, Chabner BA, Staudt L,
Jaffe ES, Janik JE. Phase II study of dose-adjusted EPOCH and rituximab
in untreated diffuse large B-cell lymphoma with analysis of germinal
center and post-germinal center biomarkers. J Clin Oncol. 2008 Jun
1;26(16):2717-24

- Griffin TC, Weitzman S, Weinstein H, Chang
M, Cairo M, Hutchison R, Shiramizu B, Wiley J, Woods D, Barnich M,
Gross TG. A study of rituximab and ifosfamide, carboplatin, and
etoposide chemotherapy in children with recurrent/refractory B-cell
(CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic
leukemia: a report from the Children's Oncology Group. Pediatr Blood
Cancer. 2009 52(2):177-181.

- Carnahan J, Stein R, Qu Z, Hess K, Cesano
A, Hansen HJ, Goldenberg DM. Epratuzumab, a CD22-targeting recombinant
humanized antibody with a different mode of action from rituximab. Mol
Immunol 2007; 44:1331–1341.

- Kano Y, Akutsu M, Tsunoda S, Izumi T,
Kobayashi H, Mano H, Furukawa Y. Cytotoxic effects of histone
deacetylase inhibitor FK228 (depsipeptide, formally named FR901228) in
combination with conventional antileukemia/lymphoma agents against
human leukemia/lymphoma cell lines. Invest New Drugs 2007; 25:31–40.

- Boffa LC, Cutrona G, Cilli M, Matis S,
Damonte G, Mariani MR, Millo E, Moroni M, Roncella S, Fedeli F,
Ferrarini M. Inhibition of Burkitt's lymphoma cells growth in SCID mice
by a PNA specific for a regulatory sequence of the translocated c-myc.
Cancer Gene Ther 2007; 14:220–226.

- Boffa LC, Cutrona G, Cilli M, Mariani MR,
Matis S, Pastorino M, Damonte G, Millo E, Roncella S, Ferrarini M.
Therapeutically promising PNA complementary to a regulatory sequence
for c-myc: pharmacokinetics in an animal model of human Burkitt's
lymphoma. Oligonucleotides 2005; 15:85–93.

- Nademanee A, Schmidt GM, O'Donnell MR,
Snyder DS, Parker PA, Stein A, Smith E, Lipsett JA, Sniecinski I,
Margolin K, Somlo G, Joyce C. Niland JC, Blume KC, Forman SJ. High-dose
chemoradiotherapy followed by autologous bone marrow transplantation as
consolidation therapy during first complete remission in adult patients
with poor-risk aggressive lymphoma: a pilot study. Blood 1992;
80:1130–1134.

- Freedman AS, Takvorian T, Neuberg D, Mauch
P, Rabinowe SN, Anderson KC, Soiffer RJ, Spector N, Grossbard M,
Robertson MJ. Autologous bone marrow transplantation in poor-prognosis
intermediate-grade and high-grade B-cell non-Hodgkin's lymphoma in
first remission: a pilot study. J Clin Oncol 1993; 11:931–936.

- Van Imhoff GW, van der Holt B, MacKenzie
MA, Ossenkoppele GJ, Wijermans PW, Kramer MH, van 't Veer MB, Schouten
HC, van Marwijk Kooy M, van Oers MH, Raemaekers JM, Sonneveld P,
Meulendijks LA, Kluin PM, Kluin-Nelemans HC, Verdonck LF. Short
intensive sequential therapy followed by autologous stem cell
transplantation in adult Burkitt, Burkitt-like and lymphoblastic
lymphoma. Leukemia 2005; 19:945–952.

- Diviné M, Lepage E, Brière J, Pautier P,
Dupriez B, Lederlin P, Mineur P, Tilly H, Blanc M, Audhuy B, Herbrecht
R, Coiffier B, Reyes F. Is the small noncleaved-cell lymphoma
histologic subtype a poor prognostic factor in adult patients? A
case-controlled analysis. The Groupe d'Etude des Lymphomes de l'Adulte.
J Clin Oncol 1996; 14:240–248.

- Lopez TM, Hagemeister FB, McLaughlin P,
Velasquez WS, Swan F, Redman JR, Rodriguez MA, Tucker SL, Silvermintz
K, Johnson J. Small noncleaved cell lymphoma in adults: superior
results for stages I–III disease. J Clin Oncol 1990; 8:615–622.

- Peniket AJ, Ruiz de Elvira MC, Taghipour
G, Cordonnier C, Gluckman E, de Witte T, Santini G, Blaise D, Greinix
H, Ferrant A, Cornelissen J, Schmitz N, Goldstone AH. An EBMT registry
matched study of allogeneic stem cell transplants for lymphoma:
allogeneic transplantation is associated with a lower relapse rate but
a higher procedure-related mortality rate than autologous
transplantation. Bone Marrow Transplant 2003; 31:667–678.

- Ungkanont A, Mongkonsritrakoon W, Jootar
S, Srichaikul T. Allogeneic stem cell transplantation in a patient with
refractory Burkitt's lymphoma using nonmyeloablative conditioning
regimen. Bone Marrow Transplant 2000; 26:1351–1354.

- Weinthal JA, Goldman SC, Lenarsky C.
Successful treatment of relapsed Burkitt's lymphoma using unrelated
cord blood transplantation as consolidation therapy. Bone Marrow
Transplant 2000; 25:1311–1313.

- Grigg AP, Seymour JF. Graft versus
Burkitt's lymphoma effect after allogeneic marrow transplantation. Leuk
Lymphoma 2002; 43:889–892.
