Perspectives
Similarities
and Differences Between Therapy-Related and Elderly Acute Myeloid
Leukemia.Francesco D’Alò, Luana Fianchi, Emiliano Fabiani, Marianna Criscuolo, Mariangela Greco, Francesco Guidi, Livio Pagano, Giuseppe Leone and Maria Teresa Voso.
1Istituto di
Ematologia,
Università Cattolica Sacro Cuore, Rome, Italy.
Correspondence
to:
Dr Maria Teresa Voso. Istituto di Ematologia, Università Cattolica del
Sacro Cuore. L.go A. Gemelli, 1, 00168 Rome, Italy. Phone:
+39-0630154180; Fax: +39-0635503777; E-mail: mtvoso@rm.unicatt.it
Published: November 28, 2011
Received: July 7, 2011
Accepted: October 7, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011052, DOI 10.4084/MJHID.2011.052
This article is available from: http://www.mjhid.org/article/view/8876
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0), which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Acute
myeloid leukemia (AML) is a clonal disorder of the hematopoietic stem
cell, typical of the elderly, with a median age of over 60 years at
diagnosis. In AML, older age is one of the strongest independent
adverse prognostic factor, associated with decreased complete response
rate, worse disease-free and overall survival, with highest rates of
treatment related mortality, resistant disease and relapse, compared to
younger patients. Outcomes are compromised in older patients not only
by increased comorbidities and susceptibility to toxicity from therapy,
but it is now recognized that elderly AML has peculiar biologic
characteristics with a negative impact on treatment response.
In older individuals prolonged exposure to environmental carcinogens may be the basis for similarities to therapy-related myeloid malignancies (t-MN), which result from toxic effects of previous cytotoxic treatments on hematopoietic stem cells. Age is itself a risk factor for t-MN, which are more frequent in elderly patients, where also a shorter latency between treatment of primary tumor and t-MN has been reported. t-MN following chemotherapy with alkylating agents and elderly AML frequently present MDS-related cytogenetic abnormalities, including complex or monosomal karyotype, and a myelodysplastic phase preceding the diagnosis of overt leukemia. Similarly, t-MN and elderly-AML share common molecular abnormalities, such as reduced frequency of NPM1, FLT3 and CEBPA mutations and increased MDR1 expression.
Given the unfavorable prognosis of elderly and t-MN and the similar clinical and molecular aspects, this is a promising field for implementation of new treatment protocols including alternative biological drugs.
In older individuals prolonged exposure to environmental carcinogens may be the basis for similarities to therapy-related myeloid malignancies (t-MN), which result from toxic effects of previous cytotoxic treatments on hematopoietic stem cells. Age is itself a risk factor for t-MN, which are more frequent in elderly patients, where also a shorter latency between treatment of primary tumor and t-MN has been reported. t-MN following chemotherapy with alkylating agents and elderly AML frequently present MDS-related cytogenetic abnormalities, including complex or monosomal karyotype, and a myelodysplastic phase preceding the diagnosis of overt leukemia. Similarly, t-MN and elderly-AML share common molecular abnormalities, such as reduced frequency of NPM1, FLT3 and CEBPA mutations and increased MDR1 expression.
Given the unfavorable prognosis of elderly and t-MN and the similar clinical and molecular aspects, this is a promising field for implementation of new treatment protocols including alternative biological drugs.
Introduction
Acute myeloid leukemia (AML) is mainly a disease of older adults. Worldwide, AML affects approximately 3 to 4 individuals in 100,000 per year and represents 90% of acute leukemia in adults, while it is rare in children. The AML incidence rises rapidly after 50 years, with a median age at diagnosis of 67 years.[1]
Therapy-related AML (t-AML) accounts for 10 to 20% of AML cases in adults. The incidence of t-AML is increasing due to longer life expectancy of cancer survivors. Therapy-related myeloid neoplasms (t-MN), including t-AML and therapy-related myelodisplastic syndromes, are now recognized as distinct nosographic entities according to the 2008 WHO classification of tumors of Hematopoietic and Lymphoid Tissues (2008).[2,3]
Age is a risk factor also for t-AML, which occurs at a higher median age compared to de novo AML.[4,5] Interestingly, patients with breast cancer treated with adjuvant chemo/radiotherapy who develop t-MN are older at breast cancer diagnosis (mean age 60.2 years versus 54.5 years; P = 0.01),6 and older age at the primary cancer diagnosis has been associated to shorter latency to t-MN development.[7] History of multiple cancer and familial history of neoplastic diseases represent additional risk factors for t-MN.[6]
This review will focus on similarities between elderly and therapy-related AML, both from the clinical and biological point of view. These diseases are commonly associated to worse prognosis related both to tumor biology and host-related factors. Tumor biology is characterized by adverse prognostic signature affecting cytogenetics, molecular genetics, epigenetics, GEP, and multidrug resistance (Table 1). Similarly, compared to de novo AML in younger patients, elderly patients and those with t-MN more often present significant comorbidities, reduced tolerance to treatment, higher incidence of treatment-related complications and are commonly excluded from treatment protocols.
In this review, we will use the terms “AML in older patients” and “elderly AML” to indicate AML occurring in individual aged 60 years or more. Changes of leukemia features will be described according to patients’ age considering different age intervals. Nevertheless, aging is a continuum of changes affecting not only individuals, but also several aspects of diseases. In particular, leukemia biological and clinical features change gradually with aging without any sharp distinction or any universally accepted temporal watershed to define distinct disease subsets.
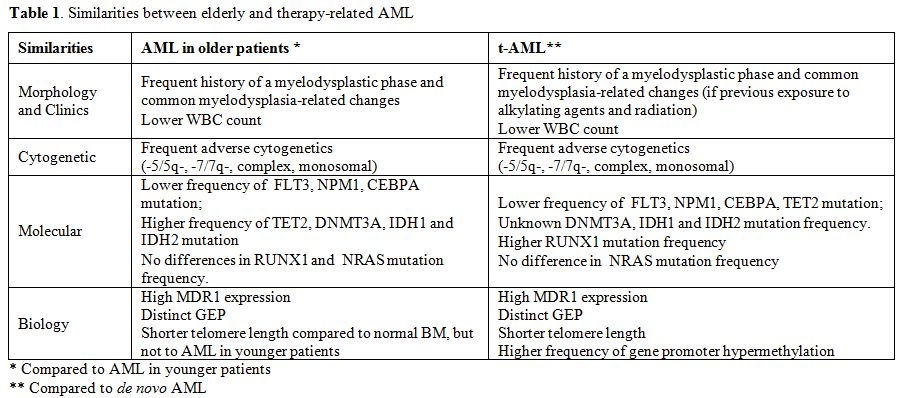
Table 1. Similarities between elderly and therapy-related AML.
Morphological and Cytogenetic Aspects. Morphological/laboratory characteristics as well as clinical course present several similarities between t-AML and elderly AML. Cytological and cytochemical differences have been described between “young” and elderly AML, indicating a less differentiated phenotype for “elderly” blasts.[8] Elderly AML usually presents with lower white blood cell (WBC) counts compared to younger patients (12,510/μl versus 19,800/μl, P < .001).[9] Similarly, t-AML have lower WBC count at diagnosis, compared to de novo AML (7400/μl versus 12500/μl, respectively, P = 0.003).[4]
The WHO Classification of Myeloid Malignancies identifies AML with myelodysplasia-related changes (AML-MRC) as a distinct AML subclass when at least one of the following prerequisites is fulfilled: a history of secondary AML arising from a previous MDS or MDS/MPN, AML with MDS-related cytogenetic abnormalities, and/or AML with multilineage dysplasia (MLD).[3] The presence of MDS-related cytogenetic abnormalities and/or a MDS or MDS/MPN history is associated to older age (69.8 years versus 65.6 years, P = 0.011), lower median WBC count (6300/ μl versus 13800/μl, P < 0.001) and worse median EFS (10.7 months versus 16.9 months; P = 0.005) and OS (16.8 months versus not reached; P = 0.001).[10] This confirms the impact of a previous history of MDS or of typical cytogenetics, as one of the main biological factors in AML.
Two distinct t-AML subtypes have been described according to the previous cytotoxic therapy.[2,3] Those t-AML occurring 5-10 years after the exposure to alkylating agents and/or ionizing radiation present several similarities with elderly AML. These patients commonly present a myelodysplastic phase (t-MDS) and bone marrow failure with one or multiple cytopenias before evolving into overt leukemia. This subset of t-AML is commonly associated with MDS-related cytogenetic abnormalities, such as monosomies or partial deletions of chromosome 5 and/or 7. On the contrary, a smaller subset of t-AML occurring at 1-5 years after exposure to topoisomerase II inhibitors, does not have a myelodysplastic phase but presents as overt leukemia and is often associated with balanced chromosomal translocations. It could be hypothesized that limited amounts of genetic and epigenetic events could be implicated in the pathogenesis of the latter type of t-AML, while t-MN following alkylating agents requires a longer latency period and probably a multistep accumulation of genetic and epigenetic events that finally result in overt leukemia.[2,3]
Cytogenetics has been indicated as the most powerful outcome predictor in AML, independent of age and other potential confounders.11 Elderly AML and t-AML share common cytogenetic aspects. Elderly AML is associated to increasing incidence of unfavorable complex karyotypes and a decreasing incidence of favorable balanced chromosomal translocations.[9,12,13] In a large study evaluating the age-specific incidence of cytogenetic abnormalities in 2555 patients with AML between 21 and 70 years of age, adverse cytogenetic profiles were disproportionately seen in elderly patients, while balanced translocations decreased and unbalanced aberrations increased with age.[12] Similarly, in 1284 patients with AML aged 18 to 85 years, a lower incidence of favorable karyotypes and a higher incidence of unfavorable complex karyotypes were seen in patients aged over 60 years.[9]
Monosomal kayotype (MK), defined as 2 or more distinct monosomies or a single monosomy in the presence of other structural abnormalities, identifies a distinct subset of AML with an extremely poor prognosis and a 4-year OS of 3-4%, even worse than that of unfavorable cytogenetics .[14,15] The proportion of patients with MK-AML including -5/5q- and -7/7q- increases with age, from 4% in patients younger than 30 years to 20% in those older 60 years of age or more.[15]
In the same line, t-AML frequently present abnormal karyotype (75% versus 51%, P < .0001) and adverse cytogenetics (39% versus 19%, P < .0001). The frequency of favorable-risk abnormalities is similar in t-AML and de novo AML, while the intermediate risk category is less frequent in t-AML. Common poor risk cytogenetic abnormalities significantly enriched in t-AML group include t(9;11), -5/5q-, -7/7q-, abnormalities (17p), complex karyotype and monosomal karyotype.[4] A trend toward a higher representation of trisomy 8 outside a complex karyotype has also been reported in t-AML.[4]
Recurrent Molecular Abnormalities.
Heterogeneous data have been published about the prevalence of NMP1 mutation in elderly AML. On the other hand, FLT3 internal tandem duplications (ITD) were shown to be less common in elderly compared to younger AML patients (23% and 37%).16 Both NPM1 mutation and FLT3 ITD have been reported to be under-represented in elderly normal karyotype AML compared to younger patients (52.1% and 66.4% for NPM1 mutation; 26.6% and 37.2% for FLT3-ITD, respectively).[9] The frequency of both NPM1 mutations and FLT3-ITD are significantly lower in t-AML compared to de novo AML, indicating that secondary leukemogenesis might follow mechanisms different from those seen in de novo AML. Nevertheless, in cytogenetically normal AML, no difference was found in the incidence and distribution of mutated NPM1 and FLT3-ITD in t-AML and de novo AML.[4]
TET2 mutations occur in 23% of AML patients and are associated with older age (P < .001). The prevalence of TET2 mutations gradually increased with age, from 7% in adults younger than 30 to 32% in patients aged 70 years or older (P < .001).21 Mutation of at least one copy of the TET2 gene was detected in 49/247 (19.8%) patients with secondary acute myeloid leukemia, including both AML with MRC (n=201) or therapy-related (n=46) leukemias. In this study, TET2 mutations were significantly less frequent in therapy-related (8.7%) than MRC (22.3%; P = 0.035) AML.[18]
DNMT3A mutations occur in 22% of AML patients, are highly enriched in the group of patients with an intermediate-risk cytogenetic profile, and are associated to shorter overall survival. Patients carrying DNMT3A mutations have a higher median age at the diagnosis compared to unmutated patients.[19] There are no data on the incidence of DNMT3A mutation in t-AML.
Isocitrate dehydrogenases IDH1 and IDH2 mutations, which lead to accumulation of 2-hydroxyglutarate in neoplastic cells, are recurrent in AML patients (about 16% of AML patients), particularly in those with normal cytogenetics and NPM1 mutated, and have an unfavorable impact on outcome. Patients carrying IDH mutations present a higher median age at diagnosis compared to IDH wild-type patients.[20,21]
CEBPA mutations occur in 6-15% of AML patients and are more common in patients with normal kayotype. Double CEBPA-mutant have a better prognosis compared to wild-type and single mutant. Frequency of double CEBPA mutation decrease by increasing age.[22] CEBPA mutations are uncommon in t-MN.[23] RUNX1 mutations occur in 5.6% of AML patients with no difference in frequency among different age groups.[24] RUNX1 mutation are common in t-MN (15.7% in a cohort of 140 patients) and are commonly associated to previous therapy with alkylating agents and chromosome 7 deletion.25 NRAS mutations occur in 10.3% of AML patients with no differences according to age and between de novo and therapy-related AML.[26] WT1 mutations occur in 6.8% of AML patients and are closely associated with younger age27 and are uncommon in t-MDS/AML.[28]
Disease Behaviour and Biology.
Several adjunctive molecular features make elderly and therapy-related AML biologically different from de novo AML in younger patients, involving multidrug resistance, telomere shortening, gene expression profiling and gene promoter methylation.
P-glycoprotein (Pgp) is a membrane transporter encoded by the multidrug resistance (ABCB1, MDR1) gene, which traps hydrophobic drugs, including key chemotherapeutic drugs in the plasma membrane of cells and effluxes them using an ATP-dependent process. High Pgp expression is detected in about 50% of AML blast samples, is more common in older (71%), than in younger cohorts (35%) and is associated with poor response to chemotherapy.[29,30] Similarly to elderly AML, secondary leukemias present higher Pgp expression compared to de novo AML (61 versus 37, P < 0.001),31 which may lead to reduced responsiveness to chemotherapy and chemoresistance.
Telomeres are specialized structures composed of TTAGGG repeats and associated proteins located at the end of eukaryotic chromosomes. Telomere repeats are lost with each cell division, eventually leading to genetic instability and cellular senescence when telomeres become critically short. Age-adjusted telomere length in AML patients is significantly shorter than in matched healthy controls. Surprisingly, patients younger than 60 years had significantly shorter age-adjusted telomere length than patients older than 60 years (median: -3.4 vs. -1.7 telomere fluorescence units, TFU; P<0.001), while age-adjusted telomere length was found to be significantly shorter in patients displaying an aberrant karyotype than in patients with a normal karyotype (median -3.0 vs. -2.3 TFU; p=0.03)..32 The development of t-MDS/AML has been associated with and preceded by markedly altered telomere dynamics in hematopoietic cells. Accelerated telomere shortening in patients developing t-MDS/AML is associated to genomic instability and may contribute to leukemic transformation.[33] In experimental models, irradiation and chemotherapy were able to induce persistent and progressive telomere shortening and DNA damage accrual, reproducing the effects of aging in the hematopoietic system.[34,35]
Elderly AML also display a distinct gene expression profiling (GEP) compared to younger AML patients. In a cohort of 525 adult AML, the transcriptome of the 175 oldest patients (median age 59.4 years) compared to that of the 175 youngest patients (median age 31.1 years) revealed 969 differentially expressed probe sets, of whom 477 were up-regulated and 492 down-regulated with increasing age. The Gene Ontology analysis of 145 “Up-with-Age” as well as 257 “Down-with-Age” probe sets revealed that genes involved in biologic processes, as regulation of I-κB kinase NF-κB cascade and immune response were up-regulated with increasing age. On the other hand, biologic processes as establishment or maintenance of chromatin architecture, regulation of cyclin-dependent protein kinase activity, and cell matrix and cell-substrate adhesion were found to be downregulated with increasing age. In particular, among differentially-expressed genes, p16INK4A, encoded by the tumor-suppressor gene CDKN2A, is down-regulated in elderly AML samples, while it is normally induced during the physiologic aging of hematopoietic stem cells.[36] Similarly, the analysis of oncogenic signaling pathway showed that older patients have a lower probability of E2F and PI3-kinase pathway activation, but a higher probability of RAS, TNF, Src, and EPI pathway activation. Using genomic-derived signatures of anthracycline-sensitivity, elderly patients presented a uniformly anthracycline resistant signature irrespective of tumor biology, while younger patients were much more likely to be sensitive to adriamycin.[37]
According to GEP, two distinct t-AML subgroups have been identified: the first group associated with del5q/-5 shows a higher expression of genes involved in cell cycle control (CCNA2, CCNE2, CDC2), checkpoints (BUB1), or growth (MYC), and loss of expression of the gene encoding IFN consensus sequence-binding protein (ICSBP). The second t-AML group is characterized by down-regulation of transcription factors involved in early hematopoiesis (TAL1, GATA1, and EKLF) and overexpression of proteins involved in signaling pathways in myeloid cells (FLT3) and cell survival (BCL2). Moreover CD34+ cells from patients with t-AML present a more immature profile than normal CD34+ cells.[38]
Epigenetic changes, in particular DNA methylation, are recognized as significant contributors to leukemogenesis, and in particular in therapy-related diseases. By a single gene analysis of promoter hypermetylation of several tumor-suppressor genes, BRCA1 and DAPK1 were found more frequently hypermethylated in therapy-related than in de novo AML, while no significant differences were found according to patients’ age.[39-42] In 356 patients studied for concurrent methylation of several promoters, t-MDS/AML were significantly more frequently hypermethylated in 2 or more promoter regions than de novo MDS or AML (17% vs 11.5% and 9.5%, p=0.035).[42] Moreover, the methylation profile of 14,000 promoters in MDS and secondary AML have showed that these diseases display more extensive aberrant DNA methylation involving thousands of genes than normal CD34+ bone marrow cells or de novo AML blasts. This aberrant methylation affected particular chromosomal regions, occurred more frequently in Alu-poor genes, and included prominent involvement of genes involved in the WNT and MAPK signaling pathways.[43]
Treatment Outcome.
The most significant similarity between elderly and t-AML is the poor prognosis, compared to younger and de novo AML.
Older age is a major adverse prognostic factor for AML in general. Surveillance, Epidemiology, and End Results (SEER) statistics from 1996 to 2002 show 5-year relative survival rates of 34.4% for adults aged under 65 and 4.3% for those over 65 years.[13] A retrospective analysis of 968 patients with previously untreated AML in 5 Southwest Oncology Group trials compared treatment outcome by stratifying patients into 4 different age groups (<56, 56–65, 66–75, and >75 years). The CR rates decreased from 64%, to 46%, 39%, and 33%, respectively, while the median overall survival was 18.8, 9.0, 6.9, and 3.5 months, respectively. In responding patients, median disease-free survival was 21.6, 7.4, 8.3, and 8.9 months, respectively.44 In the same analysis, age had a modest effect in patients with an excellent performance status (PS), but for patients with a PS of 2 or 3, age had a dramatic effect, with 82% of patients older than 75 and a PS of 3 dying within 30 days from induction start .[44]
In the last three decades significant improvement in survival has been mainly observed in younger AML patients. In a large Swedish population-based cohort study including 9729 AML patients diagnosed between 1973 and 2005, one-year survival increased across all age categories, but the improvement in 5- and 10-year AML survival was confined to patients younger than 80 years and was most prominent in the young (<60 years) patient population.[45]
Similar to elderly AML, patients with t-AML are often precluded from intensive treatment due to frequent comorbidities and cumulative toxicities, related to previous cytotoxic treatments. Also when appropriately treated as de novo AML, t-AML still has worse outcome. According to the analysis of 6 prospective multicenter treatment trials of the German-Austrian AMLStudy Group (AMLSG), 4-year relapse-free survival was 24.5% in t-AML and 39.5% in de novo AML and 4-year overall survival was 25.5% and 37.9% (age-stratified log-rank test P = .001 for both). In multivariate analysis, t-AML remained an adverse prognostic factor for death in complete remission, but not relapse, and overall survival in younger intensively treated patients. On the other hand it predicted relapse, but not death in complete remission, in older, less intensively treated patients. In more intensively-treated younger adults, treatment-related toxicity had a major negative impact on outcome, possibly reflecting cumulative toxicity of cancer treatment.[4]
An analysis matched for age, ECOG PS and additional cytogenetic abnormalities, compared de novo and therapy related Core Binding Factor (CFB) AML, carrying the recurrent chromosomal aberrations inv16 or t(8;21). An inferior treatment outcome was observed in patients with t-AML, with a median overall survival of 100 weeks compared to 376 weeks in de novo CBF AML (P = .001).[46] The negative prognostic role of t-AML in inv16 AML has been confirmed also by the AMLSG prospective trials.4 On the other hand, favorable karyotype t-AML, including t(15;17) and t(8;21), treated according to standard protocols, had an outcome similar to de novo cases, indicating the dominant prognostic role of good karyotypes.[4]
Treatment Implications.
Besides biological factors associated to a more aggressive disease behavior, host related factors, including performance and functional status, cumulative toxicities and comorbity significantly impact on prognosis, treatment tolerability and complications. Both elderly and therapy-related AML are associated with reduced bone marrow hematopoietic cell reserve, due to physiological aging-related bone marrow impairment or to the effect of previous cytotoxic treatment, respectively. This hampers patients to overcome intensive treatment or makes recovery time longer than that of younger patients with de novo AML, so that most patients have to interrupt treatment after induction chemotherapy due to complications. Notwithstanding, t-AML patients with good performance status, favorable karyotypes and without significant comorbidities should still take advantage from standard therapy, since response rates are similar to younger patients with de novo AML. All these aspects should accurately be taken into account during treatment decision-making.
Before starting treatment, elderly leukemic patients should undergo comprehensive geriatric assessment (CGA) to identify problems that may interfere with cancer treatment, as recommended by the NCCN guidelines.[13] Comorbidity burden significantly impact on response rate, treatment-related toxicity and survival. The use of standardized comorbidity assessment tools can help to stratify patients and identify those who are less likely to benefit from standard therapies. Both the Charlson Comorbidity Index (CCI) and the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) were able to identify patients with higher score associated with an adverse prognosis.[48,49]
Evaluation of physical function provides information independent of comorbidity in older patients with AML. Score systems, such as the Eastern Cooperative Oncology Group (ECOG) and the Karnofsky performance status scales and the Basic Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scales, can detect subclinical disability that might predict tolerability and response.[13]
Although the optimal therapy for older patients with AML remains debated, there is some evidence to guide decision making for individual patients. Older adults with newly diagnosed AML who present with any of the following characteristics are more likely to experience toxicity and less likely to benefit from standard induction chemotherapy: an ECOG score >2, significant comorbidity (CCI score >1, HCT-CI score >2), and poor risk cytogenetics. It would be reasonable to offer these patients supportive care alone, low-intensity therapy, or a clinical trial investigating novel agents. Alternatively, older adults with good functional status (ECOG score < 2, no impairment in IADLs), minimal comorbidity, and good-risk cytogenetics are likely to benefit from standard curative therapies regardless of chronologic age. The optimal treatment for the large majority of older adults falling between these two extremes is unclear.[5,13]
Similarly to elderly AML, patients with t-AML should be accurately evaluated for performace status, comorbidities, status of the primary disease and presence of complications from primary therapy, as well as for cytogenetic abnormalities. Patients with therapy-related acute promyelocytic leukemia (APL) should be treated as patients with de novo APL. Patients with t-AML associated with other favorable karyotypes should receive standard induction chemotherapy followed by high-dose cytarabine consolidation. Patients with indermediate and unfavorable cytogenetics who have an HLA-matched donor should be considered for allogeneic HCT. Supportive care or low dose chemotherapy remain the gold standard for patients with poor performance status.[50,51]
New investigational treatment approaches are under consideration for t-AML and elderly AML patients, including hypomethylating agents which induce significant response rates with limited toxicity in patients with unfavorable biologic factors and not susceptible of intensive chemotherapy.[5,13,50,51]
Conclusions.
The different biology of elderly AML favors the hypothesis of the involvement of specific pathogenetic pathways in this disease, similar to therapy-related AML. De novo AML in younger patients, especially those with “balanced” karyotypes, are thought to result from a limited number of mutational events restricting the diversity of leukemia subclones and leaving many cell functions relatively intact. In contrast, AML in the elderly may more often be the result of a string of mutational events leading to multiple leukemic subclones with the opportunity to develop multiple mechanisms of chemoresistance.[52]
Both elderly and t-AML take their origin by the physiological or treatment-induced aging process of bone marrow hematopoietic system and microenvironment, associated to reduced efficiency of DNA repair system, reduced hematopoietic reserve and immune surveillance, and prolonged exposure to carcinogens (environmental or cytotoxic agents and irradiation, respectively).
New therapeutic development are needed, because actual therapy strategies are limited due both to host-related factors responsible of poor tolerance to standard chemotherapy, and adverse biological features associated to treatment resistance.
Acute myeloid leukemia (AML) is mainly a disease of older adults. Worldwide, AML affects approximately 3 to 4 individuals in 100,000 per year and represents 90% of acute leukemia in adults, while it is rare in children. The AML incidence rises rapidly after 50 years, with a median age at diagnosis of 67 years.[1]
Therapy-related AML (t-AML) accounts for 10 to 20% of AML cases in adults. The incidence of t-AML is increasing due to longer life expectancy of cancer survivors. Therapy-related myeloid neoplasms (t-MN), including t-AML and therapy-related myelodisplastic syndromes, are now recognized as distinct nosographic entities according to the 2008 WHO classification of tumors of Hematopoietic and Lymphoid Tissues (2008).[2,3]
Age is a risk factor also for t-AML, which occurs at a higher median age compared to de novo AML.[4,5] Interestingly, patients with breast cancer treated with adjuvant chemo/radiotherapy who develop t-MN are older at breast cancer diagnosis (mean age 60.2 years versus 54.5 years; P = 0.01),6 and older age at the primary cancer diagnosis has been associated to shorter latency to t-MN development.[7] History of multiple cancer and familial history of neoplastic diseases represent additional risk factors for t-MN.[6]
This review will focus on similarities between elderly and therapy-related AML, both from the clinical and biological point of view. These diseases are commonly associated to worse prognosis related both to tumor biology and host-related factors. Tumor biology is characterized by adverse prognostic signature affecting cytogenetics, molecular genetics, epigenetics, GEP, and multidrug resistance (Table 1). Similarly, compared to de novo AML in younger patients, elderly patients and those with t-MN more often present significant comorbidities, reduced tolerance to treatment, higher incidence of treatment-related complications and are commonly excluded from treatment protocols.
In this review, we will use the terms “AML in older patients” and “elderly AML” to indicate AML occurring in individual aged 60 years or more. Changes of leukemia features will be described according to patients’ age considering different age intervals. Nevertheless, aging is a continuum of changes affecting not only individuals, but also several aspects of diseases. In particular, leukemia biological and clinical features change gradually with aging without any sharp distinction or any universally accepted temporal watershed to define distinct disease subsets.

Table 1. Similarities between elderly and therapy-related AML.
Morphological and Cytogenetic Aspects. Morphological/laboratory characteristics as well as clinical course present several similarities between t-AML and elderly AML. Cytological and cytochemical differences have been described between “young” and elderly AML, indicating a less differentiated phenotype for “elderly” blasts.[8] Elderly AML usually presents with lower white blood cell (WBC) counts compared to younger patients (12,510/μl versus 19,800/μl, P < .001).[9] Similarly, t-AML have lower WBC count at diagnosis, compared to de novo AML (7400/μl versus 12500/μl, respectively, P = 0.003).[4]
The WHO Classification of Myeloid Malignancies identifies AML with myelodysplasia-related changes (AML-MRC) as a distinct AML subclass when at least one of the following prerequisites is fulfilled: a history of secondary AML arising from a previous MDS or MDS/MPN, AML with MDS-related cytogenetic abnormalities, and/or AML with multilineage dysplasia (MLD).[3] The presence of MDS-related cytogenetic abnormalities and/or a MDS or MDS/MPN history is associated to older age (69.8 years versus 65.6 years, P = 0.011), lower median WBC count (6300/ μl versus 13800/μl, P < 0.001) and worse median EFS (10.7 months versus 16.9 months; P = 0.005) and OS (16.8 months versus not reached; P = 0.001).[10] This confirms the impact of a previous history of MDS or of typical cytogenetics, as one of the main biological factors in AML.
Two distinct t-AML subtypes have been described according to the previous cytotoxic therapy.[2,3] Those t-AML occurring 5-10 years after the exposure to alkylating agents and/or ionizing radiation present several similarities with elderly AML. These patients commonly present a myelodysplastic phase (t-MDS) and bone marrow failure with one or multiple cytopenias before evolving into overt leukemia. This subset of t-AML is commonly associated with MDS-related cytogenetic abnormalities, such as monosomies or partial deletions of chromosome 5 and/or 7. On the contrary, a smaller subset of t-AML occurring at 1-5 years after exposure to topoisomerase II inhibitors, does not have a myelodysplastic phase but presents as overt leukemia and is often associated with balanced chromosomal translocations. It could be hypothesized that limited amounts of genetic and epigenetic events could be implicated in the pathogenesis of the latter type of t-AML, while t-MN following alkylating agents requires a longer latency period and probably a multistep accumulation of genetic and epigenetic events that finally result in overt leukemia.[2,3]
Cytogenetics has been indicated as the most powerful outcome predictor in AML, independent of age and other potential confounders.11 Elderly AML and t-AML share common cytogenetic aspects. Elderly AML is associated to increasing incidence of unfavorable complex karyotypes and a decreasing incidence of favorable balanced chromosomal translocations.[9,12,13] In a large study evaluating the age-specific incidence of cytogenetic abnormalities in 2555 patients with AML between 21 and 70 years of age, adverse cytogenetic profiles were disproportionately seen in elderly patients, while balanced translocations decreased and unbalanced aberrations increased with age.[12] Similarly, in 1284 patients with AML aged 18 to 85 years, a lower incidence of favorable karyotypes and a higher incidence of unfavorable complex karyotypes were seen in patients aged over 60 years.[9]
Monosomal kayotype (MK), defined as 2 or more distinct monosomies or a single monosomy in the presence of other structural abnormalities, identifies a distinct subset of AML with an extremely poor prognosis and a 4-year OS of 3-4%, even worse than that of unfavorable cytogenetics .[14,15] The proportion of patients with MK-AML including -5/5q- and -7/7q- increases with age, from 4% in patients younger than 30 years to 20% in those older 60 years of age or more.[15]
In the same line, t-AML frequently present abnormal karyotype (75% versus 51%, P < .0001) and adverse cytogenetics (39% versus 19%, P < .0001). The frequency of favorable-risk abnormalities is similar in t-AML and de novo AML, while the intermediate risk category is less frequent in t-AML. Common poor risk cytogenetic abnormalities significantly enriched in t-AML group include t(9;11), -5/5q-, -7/7q-, abnormalities (17p), complex karyotype and monosomal karyotype.[4] A trend toward a higher representation of trisomy 8 outside a complex karyotype has also been reported in t-AML.[4]
Recurrent Molecular Abnormalities.
Heterogeneous data have been published about the prevalence of NMP1 mutation in elderly AML. On the other hand, FLT3 internal tandem duplications (ITD) were shown to be less common in elderly compared to younger AML patients (23% and 37%).16 Both NPM1 mutation and FLT3 ITD have been reported to be under-represented in elderly normal karyotype AML compared to younger patients (52.1% and 66.4% for NPM1 mutation; 26.6% and 37.2% for FLT3-ITD, respectively).[9] The frequency of both NPM1 mutations and FLT3-ITD are significantly lower in t-AML compared to de novo AML, indicating that secondary leukemogenesis might follow mechanisms different from those seen in de novo AML. Nevertheless, in cytogenetically normal AML, no difference was found in the incidence and distribution of mutated NPM1 and FLT3-ITD in t-AML and de novo AML.[4]
TET2 mutations occur in 23% of AML patients and are associated with older age (P < .001). The prevalence of TET2 mutations gradually increased with age, from 7% in adults younger than 30 to 32% in patients aged 70 years or older (P < .001).21 Mutation of at least one copy of the TET2 gene was detected in 49/247 (19.8%) patients with secondary acute myeloid leukemia, including both AML with MRC (n=201) or therapy-related (n=46) leukemias. In this study, TET2 mutations were significantly less frequent in therapy-related (8.7%) than MRC (22.3%; P = 0.035) AML.[18]
DNMT3A mutations occur in 22% of AML patients, are highly enriched in the group of patients with an intermediate-risk cytogenetic profile, and are associated to shorter overall survival. Patients carrying DNMT3A mutations have a higher median age at the diagnosis compared to unmutated patients.[19] There are no data on the incidence of DNMT3A mutation in t-AML.
Isocitrate dehydrogenases IDH1 and IDH2 mutations, which lead to accumulation of 2-hydroxyglutarate in neoplastic cells, are recurrent in AML patients (about 16% of AML patients), particularly in those with normal cytogenetics and NPM1 mutated, and have an unfavorable impact on outcome. Patients carrying IDH mutations present a higher median age at diagnosis compared to IDH wild-type patients.[20,21]
CEBPA mutations occur in 6-15% of AML patients and are more common in patients with normal kayotype. Double CEBPA-mutant have a better prognosis compared to wild-type and single mutant. Frequency of double CEBPA mutation decrease by increasing age.[22] CEBPA mutations are uncommon in t-MN.[23] RUNX1 mutations occur in 5.6% of AML patients with no difference in frequency among different age groups.[24] RUNX1 mutation are common in t-MN (15.7% in a cohort of 140 patients) and are commonly associated to previous therapy with alkylating agents and chromosome 7 deletion.25 NRAS mutations occur in 10.3% of AML patients with no differences according to age and between de novo and therapy-related AML.[26] WT1 mutations occur in 6.8% of AML patients and are closely associated with younger age27 and are uncommon in t-MDS/AML.[28]
Disease Behaviour and Biology.
Several adjunctive molecular features make elderly and therapy-related AML biologically different from de novo AML in younger patients, involving multidrug resistance, telomere shortening, gene expression profiling and gene promoter methylation.
P-glycoprotein (Pgp) is a membrane transporter encoded by the multidrug resistance (ABCB1, MDR1) gene, which traps hydrophobic drugs, including key chemotherapeutic drugs in the plasma membrane of cells and effluxes them using an ATP-dependent process. High Pgp expression is detected in about 50% of AML blast samples, is more common in older (71%), than in younger cohorts (35%) and is associated with poor response to chemotherapy.[29,30] Similarly to elderly AML, secondary leukemias present higher Pgp expression compared to de novo AML (61 versus 37, P < 0.001),31 which may lead to reduced responsiveness to chemotherapy and chemoresistance.
Telomeres are specialized structures composed of TTAGGG repeats and associated proteins located at the end of eukaryotic chromosomes. Telomere repeats are lost with each cell division, eventually leading to genetic instability and cellular senescence when telomeres become critically short. Age-adjusted telomere length in AML patients is significantly shorter than in matched healthy controls. Surprisingly, patients younger than 60 years had significantly shorter age-adjusted telomere length than patients older than 60 years (median: -3.4 vs. -1.7 telomere fluorescence units, TFU; P<0.001), while age-adjusted telomere length was found to be significantly shorter in patients displaying an aberrant karyotype than in patients with a normal karyotype (median -3.0 vs. -2.3 TFU; p=0.03)..32 The development of t-MDS/AML has been associated with and preceded by markedly altered telomere dynamics in hematopoietic cells. Accelerated telomere shortening in patients developing t-MDS/AML is associated to genomic instability and may contribute to leukemic transformation.[33] In experimental models, irradiation and chemotherapy were able to induce persistent and progressive telomere shortening and DNA damage accrual, reproducing the effects of aging in the hematopoietic system.[34,35]
Elderly AML also display a distinct gene expression profiling (GEP) compared to younger AML patients. In a cohort of 525 adult AML, the transcriptome of the 175 oldest patients (median age 59.4 years) compared to that of the 175 youngest patients (median age 31.1 years) revealed 969 differentially expressed probe sets, of whom 477 were up-regulated and 492 down-regulated with increasing age. The Gene Ontology analysis of 145 “Up-with-Age” as well as 257 “Down-with-Age” probe sets revealed that genes involved in biologic processes, as regulation of I-κB kinase NF-κB cascade and immune response were up-regulated with increasing age. On the other hand, biologic processes as establishment or maintenance of chromatin architecture, regulation of cyclin-dependent protein kinase activity, and cell matrix and cell-substrate adhesion were found to be downregulated with increasing age. In particular, among differentially-expressed genes, p16INK4A, encoded by the tumor-suppressor gene CDKN2A, is down-regulated in elderly AML samples, while it is normally induced during the physiologic aging of hematopoietic stem cells.[36] Similarly, the analysis of oncogenic signaling pathway showed that older patients have a lower probability of E2F and PI3-kinase pathway activation, but a higher probability of RAS, TNF, Src, and EPI pathway activation. Using genomic-derived signatures of anthracycline-sensitivity, elderly patients presented a uniformly anthracycline resistant signature irrespective of tumor biology, while younger patients were much more likely to be sensitive to adriamycin.[37]
According to GEP, two distinct t-AML subgroups have been identified: the first group associated with del5q/-5 shows a higher expression of genes involved in cell cycle control (CCNA2, CCNE2, CDC2), checkpoints (BUB1), or growth (MYC), and loss of expression of the gene encoding IFN consensus sequence-binding protein (ICSBP). The second t-AML group is characterized by down-regulation of transcription factors involved in early hematopoiesis (TAL1, GATA1, and EKLF) and overexpression of proteins involved in signaling pathways in myeloid cells (FLT3) and cell survival (BCL2). Moreover CD34+ cells from patients with t-AML present a more immature profile than normal CD34+ cells.[38]
Epigenetic changes, in particular DNA methylation, are recognized as significant contributors to leukemogenesis, and in particular in therapy-related diseases. By a single gene analysis of promoter hypermetylation of several tumor-suppressor genes, BRCA1 and DAPK1 were found more frequently hypermethylated in therapy-related than in de novo AML, while no significant differences were found according to patients’ age.[39-42] In 356 patients studied for concurrent methylation of several promoters, t-MDS/AML were significantly more frequently hypermethylated in 2 or more promoter regions than de novo MDS or AML (17% vs 11.5% and 9.5%, p=0.035).[42] Moreover, the methylation profile of 14,000 promoters in MDS and secondary AML have showed that these diseases display more extensive aberrant DNA methylation involving thousands of genes than normal CD34+ bone marrow cells or de novo AML blasts. This aberrant methylation affected particular chromosomal regions, occurred more frequently in Alu-poor genes, and included prominent involvement of genes involved in the WNT and MAPK signaling pathways.[43]
Treatment Outcome.
The most significant similarity between elderly and t-AML is the poor prognosis, compared to younger and de novo AML.
Older age is a major adverse prognostic factor for AML in general. Surveillance, Epidemiology, and End Results (SEER) statistics from 1996 to 2002 show 5-year relative survival rates of 34.4% for adults aged under 65 and 4.3% for those over 65 years.[13] A retrospective analysis of 968 patients with previously untreated AML in 5 Southwest Oncology Group trials compared treatment outcome by stratifying patients into 4 different age groups (<56, 56–65, 66–75, and >75 years). The CR rates decreased from 64%, to 46%, 39%, and 33%, respectively, while the median overall survival was 18.8, 9.0, 6.9, and 3.5 months, respectively. In responding patients, median disease-free survival was 21.6, 7.4, 8.3, and 8.9 months, respectively.44 In the same analysis, age had a modest effect in patients with an excellent performance status (PS), but for patients with a PS of 2 or 3, age had a dramatic effect, with 82% of patients older than 75 and a PS of 3 dying within 30 days from induction start .[44]
In the last three decades significant improvement in survival has been mainly observed in younger AML patients. In a large Swedish population-based cohort study including 9729 AML patients diagnosed between 1973 and 2005, one-year survival increased across all age categories, but the improvement in 5- and 10-year AML survival was confined to patients younger than 80 years and was most prominent in the young (<60 years) patient population.[45]
Similar to elderly AML, patients with t-AML are often precluded from intensive treatment due to frequent comorbidities and cumulative toxicities, related to previous cytotoxic treatments. Also when appropriately treated as de novo AML, t-AML still has worse outcome. According to the analysis of 6 prospective multicenter treatment trials of the German-Austrian AMLStudy Group (AMLSG), 4-year relapse-free survival was 24.5% in t-AML and 39.5% in de novo AML and 4-year overall survival was 25.5% and 37.9% (age-stratified log-rank test P = .001 for both). In multivariate analysis, t-AML remained an adverse prognostic factor for death in complete remission, but not relapse, and overall survival in younger intensively treated patients. On the other hand it predicted relapse, but not death in complete remission, in older, less intensively treated patients. In more intensively-treated younger adults, treatment-related toxicity had a major negative impact on outcome, possibly reflecting cumulative toxicity of cancer treatment.[4]
An analysis matched for age, ECOG PS and additional cytogenetic abnormalities, compared de novo and therapy related Core Binding Factor (CFB) AML, carrying the recurrent chromosomal aberrations inv16 or t(8;21). An inferior treatment outcome was observed in patients with t-AML, with a median overall survival of 100 weeks compared to 376 weeks in de novo CBF AML (P = .001).[46] The negative prognostic role of t-AML in inv16 AML has been confirmed also by the AMLSG prospective trials.4 On the other hand, favorable karyotype t-AML, including t(15;17) and t(8;21), treated according to standard protocols, had an outcome similar to de novo cases, indicating the dominant prognostic role of good karyotypes.[4]
Treatment Implications.
Besides biological factors associated to a more aggressive disease behavior, host related factors, including performance and functional status, cumulative toxicities and comorbity significantly impact on prognosis, treatment tolerability and complications. Both elderly and therapy-related AML are associated with reduced bone marrow hematopoietic cell reserve, due to physiological aging-related bone marrow impairment or to the effect of previous cytotoxic treatment, respectively. This hampers patients to overcome intensive treatment or makes recovery time longer than that of younger patients with de novo AML, so that most patients have to interrupt treatment after induction chemotherapy due to complications. Notwithstanding, t-AML patients with good performance status, favorable karyotypes and without significant comorbidities should still take advantage from standard therapy, since response rates are similar to younger patients with de novo AML. All these aspects should accurately be taken into account during treatment decision-making.
Before starting treatment, elderly leukemic patients should undergo comprehensive geriatric assessment (CGA) to identify problems that may interfere with cancer treatment, as recommended by the NCCN guidelines.[13] Comorbidity burden significantly impact on response rate, treatment-related toxicity and survival. The use of standardized comorbidity assessment tools can help to stratify patients and identify those who are less likely to benefit from standard therapies. Both the Charlson Comorbidity Index (CCI) and the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) were able to identify patients with higher score associated with an adverse prognosis.[48,49]
Evaluation of physical function provides information independent of comorbidity in older patients with AML. Score systems, such as the Eastern Cooperative Oncology Group (ECOG) and the Karnofsky performance status scales and the Basic Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scales, can detect subclinical disability that might predict tolerability and response.[13]
Although the optimal therapy for older patients with AML remains debated, there is some evidence to guide decision making for individual patients. Older adults with newly diagnosed AML who present with any of the following characteristics are more likely to experience toxicity and less likely to benefit from standard induction chemotherapy: an ECOG score >2, significant comorbidity (CCI score >1, HCT-CI score >2), and poor risk cytogenetics. It would be reasonable to offer these patients supportive care alone, low-intensity therapy, or a clinical trial investigating novel agents. Alternatively, older adults with good functional status (ECOG score < 2, no impairment in IADLs), minimal comorbidity, and good-risk cytogenetics are likely to benefit from standard curative therapies regardless of chronologic age. The optimal treatment for the large majority of older adults falling between these two extremes is unclear.[5,13]
Similarly to elderly AML, patients with t-AML should be accurately evaluated for performace status, comorbidities, status of the primary disease and presence of complications from primary therapy, as well as for cytogenetic abnormalities. Patients with therapy-related acute promyelocytic leukemia (APL) should be treated as patients with de novo APL. Patients with t-AML associated with other favorable karyotypes should receive standard induction chemotherapy followed by high-dose cytarabine consolidation. Patients with indermediate and unfavorable cytogenetics who have an HLA-matched donor should be considered for allogeneic HCT. Supportive care or low dose chemotherapy remain the gold standard for patients with poor performance status.[50,51]
New investigational treatment approaches are under consideration for t-AML and elderly AML patients, including hypomethylating agents which induce significant response rates with limited toxicity in patients with unfavorable biologic factors and not susceptible of intensive chemotherapy.[5,13,50,51]
Conclusions.
The different biology of elderly AML favors the hypothesis of the involvement of specific pathogenetic pathways in this disease, similar to therapy-related AML. De novo AML in younger patients, especially those with “balanced” karyotypes, are thought to result from a limited number of mutational events restricting the diversity of leukemia subclones and leaving many cell functions relatively intact. In contrast, AML in the elderly may more often be the result of a string of mutational events leading to multiple leukemic subclones with the opportunity to develop multiple mechanisms of chemoresistance.[52]
Both elderly and t-AML take their origin by the physiological or treatment-induced aging process of bone marrow hematopoietic system and microenvironment, associated to reduced efficiency of DNA repair system, reduced hematopoietic reserve and immune surveillance, and prolonged exposure to carcinogens (environmental or cytotoxic agents and irradiation, respectively).
New therapeutic development are needed, because actual therapy strategies are limited due both to host-related factors responsible of poor tolerance to standard chemotherapy, and adverse biological features associated to treatment resistance.
References
- Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H. Hematology. Basic principles and practice. 5th Ed., Philadelphia. Churchill Livingstone Elsevier. 2009; 933-63.
- Leone G, Pagano L, Ben-Yehuda D, Voso MT.
Therapy-related leukemia and myelodysplasia: susceptibility and
incidence. Haematologica. 2007; 92:1389-98.
http://dx.doi.org/10.3324/haematol.11034 PMid:17768113

- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Ed., Lyon. IARC Press. 2008.
- Kayser S, Döhner K, Krauter J, Köhne CH,
Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K,
Rummel M, Nachbaur D, Schlegelberger B, Göhring G, Späth D, Morlok C,
Zucknick M, Ganser A, Döhner H, Schlenk RF; German-Austrian AMLSG. The
impact of therapy-related acute myeloid leukemia (AML) on outcome in
2853 adult patients with newly diagnosed AML. Blood. 2011; 117:
2137-45. http://dx.doi.org/10.1182/blood-2010-08-301713
PMid:21127174

- Laubach J, Rao AV. Current and emerging
strategies for the management of acute myeloid leukemia in the elderly.
Oncologist. 2008; 13:1097-108.
http://dx.doi.org/10.1634/theoncologist.2008-0100 PMid:18922830

- Padmanabhan A, Baker JA, Zirpoli G, Sait
SN, Ford LA, Moysich KB, Baer MR. Acute myeloid leukemia and
myelodysplastic syndrome following breast cancer: increased frequency
of other cancers and of cancers in multiple family members. Leuk Res.
2008; 32:1820-3. http://dx.doi.org/10.1016/j.leukres.2008.03.032
PMid:18468682

- Smith SM, Le Beau MM, Huo D, Karrison T,
Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA.
Clinical-cytogenetic associations in 306 patients with therapy-related
myelodysplasia and myeloid leukemia: the University of Chicago series.
Blood. 2003; 102:43-52. http://dx.doi.org/10.1182/blood-2002-11-3343
PMid:12623843

- Hassan HT, Rees JK. Relation between age
and blast cell differentiation in acute myeloid leukaemia patients.
Oncology. 1990; 47:439-42. http://dx.doi.org/10.1159/000226865
PMid:2216301

- Büchner T, Berdel WE, Haferlach C,
Haferlach T, Schnittger S, Müller-Tidow C, Braess J, Spiekermann K,
Kienast J, Staib P, Grüneisen A, Kern W, Reichle A, Maschmeyer G, Aul
C, Lengfelder E, Sauerland MC, Heinecke A, Wörmann B, Hiddemann W.
Age-related risk profile and chemotherapy dose response in acute
myeloid leukemia: a study by the German Acute Myeloid Leukemia
Cooperative Group. J Clin Oncol. 2009; 27:61-9. PMid:19047294

- Miesner M, Haferlach C, Bacher U, Weiss T,
Macijewski K, Kohlmann A, Klein HU, Dugas M, Kern W, Schnittger S,
Haferlach T. Multilineage dysplasia (MLD) in acute myeloid leukemia
(AML) correlates with MDS-related cytogenetic abnormalities and a prior
history of MDS or MDS/MPN but has no independent prognostic relevance:
a comparison of 408 cases classified as "AML not otherwise specified"
(AML-NOS) or "AML with myelodysplasia-related changes" (AML-MRC).
Blood. 2010; 116:2742-51. http://dx.doi.org/10.1182/blood-2010-04-279794
PMid:20581309

- Grimwade D, Walker H, Harrison G, Oliver
F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH;
Medical Research Council Adult Leukemia Working Party. The predictive
value of hierarchical cytogenetic classification in older adults with
acute myeloid leukemia (AML): analysis of 1065 patients entered into
the United Kingdom Medical Research Council AML11 trial. Blood. 2001;
98:1312-20. http://dx.doi.org/10.1182/blood.V98.5.1312
PMid:11520776

- Bacher U, Kern W, Schnittger S, Hiddemann
W, Haferlach T, Schoch C. Population-based age-specific incidences of
cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;
90:1502-10. PMid:16266897

- Klepin HD, Balducci L. Acute myelogenous
leukemia in older adults. Oncologist. 2009; 14:222-32. http://dx.doi.org/10.1634/theoncologist.2008-0224
PMid:19282349

- Breems DA, Van Putten WL, De Greef GE, Van
Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, Nieuwint A,
Jotterand M, Hagemeijer A, Beverloo HB, Löwenberg B. Monosomal
karyotype in acute myeloid leukemia: a better indicator of poor
prognosis than a complex karyotype. J Clin Oncol. 2008; 26:4791-7. http://dx.doi.org/10.1200/JCO.2008.16.0259
PMid:18695255

- Medeiros BC, Othus M, Fang M, Roulston D,
Appelbaum FR. Prognostic impact of monosomal karyotype in young adult
and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG)
experience. Blood. 2010; 116:2224-8. http://dx.doi.org/10.1182/blood-2010-02-270330
PMid:20562328

- Beran M, Luthra R, Kantarjian H, Estey E.
FLT3 mutation and response to intensive chemotherapy in young adult and
elderly patients with normal karyotype. Leuk Res. 2004; 28:547-50. http://dx.doi.org/10.1016/j.leukres.2003.09.016
PMid:15120929

- Metzeler KH, Maharry K, Radmacher MD,
Mrózek K, Margeson D, Becker H, Curfman J, Holland KB, Schwind S,
Whitman SP, Wu YZ, Blum W, Powell BL, Carter TH, Wetzler M, Moore JO,
Kolitz JE, Baer MR, Carroll AJ, Larson RA, Caligiuri MA, Marcucci G,
Bloomfield CD. TET2 mutations improve the new European LeukemiaNet risk
classification of acute myeloid leukemia: a Cancer and Leukemia Group B
study. J Clin Oncol. 2011; 29:1373-81. http://dx.doi.org/10.1200/JCO.2010.32.7742
PMid:21343549

- Kosmider O, Delabesse E, Mansat-Demas V,
Cornillet-Lefebvre P, Blanchet O, Delmer A, Recher C, Raynaud SD,
Bouscary D, Viguie F, Lacombe C, Bernard OA, Ifrah N, Dreyfus F,
Fontenay M. TET2</> mutations in secondary acute myeloid
leukemias: a French retrospective study. Haematologica. 2011;
96:1059-1063. http://dx.doi.org/10.3324/haematol.2011.040840
PMid:21508122 PMCid:3128227

- Ley TJ, Ding L, Walter MJ, McLellan MD,
Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, Harris
CC, Lichti CF, Townsend RR, Fulton RS, Dooling DJ, Koboldt DC, Schmidt
H, Zhang Q, Osborne JR, Lin L, O'Laughlin M, McMichael JF, Delehaunty
KD, McGrath SD, Fulton LA, Magrini VJ, Vickery TL, Hundal J, Cook LL,
Conyers JJ, Swift GW, Reed JP, Alldredge PA, Wylie T, Walker J, Kalicki
J, Watson MA, Heath S, Shannon WD, Varghese N, Nagarajan R, Westervelt
P, Tomasson MH, Link DC, Graubert TA, DiPersio JF, Mardis ER, Wilson
RK. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;
363:2424-33. http://dx.doi.org/10.1056/NEJMoa1005143
PMid:21067377
PMCid:3201818

- Paschka P, Schlenk RF, Gaidzik VI, Habdank
M, Krönke J, Bullinger L, Späth D, Kayser S, Zucknick M, Götze K, Horst
HA, Germing U, Döhner H, Döhner K. IDH1 and IDH2 mutations are frequent
genetic alterations in acute myeloid leukemia and confer adverse
prognosis in cytogenetically normal acute myeloid leukemia with NPM1
mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;
28:3636-43. http://dx.doi.org/10.1200/JCO.2010.28.3762
PMid:20567020

- Abbas S, Lugthart S, Kavelaars FG, Schelen
A, Koenders JE, Zeilemaker A, van Putten WJ, Rijneveld AW, Löwenberg B,
Valk PJ. Acquired mutations in the genes encoding IDH1 and IDH2 both
are recurrent aberrations in acute myeloid leukemia: prevalence and
prognostic value. Blood. 2010; 116:2122-6. http://dx.doi.org/10.1182/blood-2009-11-250878
PMid:20538800

- Green CL, Koo KK, Hills RK, Burnett AK,
Linch DC, Gale RE. Prognostic significance of CEBPA mutations in a
large cohort of younger adult patients with acute myeloid leukemia:
impact of double CEBPA mutations and the interaction with FLT3 and NPM1
mutations. J Clin Oncol. 2010; 28:2739-47. http://dx.doi.org/10.1200/JCO.2009.26.2501
PMid:20439648

- Christiansen DH, Andersen MK, Desta F,
Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine
kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related
myelodysplasia and acute myeloid leukemia. Leukemia. 2005; 19:2232-40. http://dx.doi.org/10.1038/sj.leu.2404009
PMid:16281072

- Gaidzik VI, Bullinger L, Schlenk RF,
Zimmermann AS, Röck J, Paschka P, Corbacioglu A, Krauter J,
Schlegelberger B, Ganser A, Späth D, Kündgen A, Schmidt-Wolf IG, Götze
K, Nachbaur D, Pfreundschuh M, Horst HA, Döhner H, Döhner K. RUNX1
mutations in acute myeloid leukemia: results from a comprehensive
genetic and clinical analysis from the AML study group. J Clin Oncol.
2011; 29:1364-72. http://dx.doi.org/10.1200/JCO.2010.30.7926
PMid:21343560

- Christiansen DH, Andersen MK,
Pedersen-Bjergaard J. Mutations of AML1 are common in therapy-related
myelodysplasia following therapy with alkylating agents and are
significantly associated with deletion or loss of chromosome arm 7q and
with subsequent leukemic transformation. Blood. 2004; 104:1474-81. http://dx.doi.org/10.1182/blood-2004-02-0754
PMid:15142876

- Bacher U, Haferlach T, Schoch C, Kern W,
Schnittger S. Implications of NRAS mutations in AML: a study of 2502
patients. Blood. 2006; 107:3847-53. http://dx.doi.org/10.1182/blood-2005-08-3522
PMid:16434492

- Hou HA, Huang TC, Lin LI, Liu CY, Chen CY,
Chou WC, Tang JL, Tseng MH, Huang CF, Chiang YC, Lee FY, Liu MC, Yao M,
Huang SY, Ko BS, Hsu SC, Wu SJ, Tsay W, Chen YC, Tien HF. WT1 mutation
in 470 adult patients with acute myeloid leukemia: stability during
disease evolution and implication of its incorporation into a survival
scoring system. Blood. 2010; 115:5222-31. http://dx.doi.org/10.1182/blood-2009-12-259390
PMid:20368469

- Christiansen DH, Pedersen-Bjergaard J.
Internal tandem duplications of the FLT3 and MLL genes are mainly
observed in atypical cases of therapy-related acute myeloid leukemia
with a normal karyotype and are unrelated to type of previous therapy.
Leukemia. 2001; 15:1848-51. http://dx.doi.org/10.1038/sj.leu.2402246
PMid:11753604

- Leith CP, Kopecky KJ, Chen IM, Eijdems L,
Slovak ML, McConnell TS, Head DR, Weick J, Grever MR, Appelbaum FR,
Willman CL. Frequency and clinical significance of the expression of
the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in
acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;
94:1086-99. PMid:10419902

- Leith CP, Kopecky KJ, Godwin J, McConnell
T, Slovak ML, Chen IM, Head DR, Appelbaum FR, Willman CL. Acute myeloid
leukemia in the elderly: assessment of multidrug resistance (MDR1) and
cytogenetics distinguishes biologic subgroups with remarkably distinct
responses to standard chemotherapy. A Southwest Oncology Group study.
Blood. 1997; 89:3323-9. PMid:9129038

- Seedhouse CH, Grundy M, White P, Li Y,
Fisher J, Yakunina D, Moorman AV, Hoy T, Russell N, Burnett A, Pallis
M; National Cancer Research Network. Sequential influences of
leukemia-specific and genetic factors on p-glycoprotein expression in
blasts from 817 patients entered into the National Cancer Research
Network acute myeloid leukemia 14 and 15 trials. Clin Cancer Res. 2007;
13:7059-66. http://dx.doi.org/10.1158/1078-0432.CCR-07-1484
PMid:18056183

- Hartmann U, Brümmendorf TH, Balabanov S,
Thiede C, Illme T, Schaich M. Telomere length and hTERT expression in
patients with acute myeloid leukemia correlates with chromosomal
abnormalities. Haematologica. 2005; 90:307-16. PMid:15749662

- Chakraborty S, Sun CL, Francisco L, Sabado
M, Li L, Chang KL, Forman S, Bhatia S, Bhatia R. Accelerated telomere
shortening precedes development of therapy-related myelodysplasia or
acute myelogenous leukemia after autologous transplantation for
lymphoma. J Clin Oncol. 2009; 27:791-8. http://dx.doi.org/10.1200/JCO.2008.17.1033
PMid:19124806 PMCid:2645091

- Buttiglieri S, Ruella M, Risso A, Spatola
T, Silengo L, Avvedimento EV, Tarella C. The aging effect of
chemotherapy on cultured human mesenchymal stem cells. Exp Hematol.
2011; in press. http://dx.doi.org/10.1016/j.exphem.2011.08.009
PMid:21864489

- Rübe CE, Fricke A, Widmann TA, Fürst T,
Madry H, Pfreundschuh M, Rübe C. Accumulation of DNA damage in
hematopoietic stem and progenitor cells during human aging. PLoS One.
2011; 6:e17487. http://dx.doi.org/10.1371/journal.pone.0017487
PMid:21408175 PMCid:3049780

- de Jonge HJ, de Bont ES, Valk PJ,
Schuringa JJ, Kies M, Woolthuis CM, Delwel R, Veeger NJ, Vellenga E,
Löwenberg B, Huls G. AML at older age: age-related gene expression
profiles reveal a paradoxical down-regulation of p16INK4A mRNA with
prognostic significance. Blood. 2009; 114:2869-77. http://dx.doi.org/10.1182/blood-2009-03-212688
PMid:19667402

- Rao AV, Valk PJ, Metzeler KH, Acharya CR,
Tuchman SA, Stevenson MM, Rizzieri DA, Delwel R, Buske C, Bohlander SK,
Potti A, Löwenberg B. Age-specific differences in oncogenic pathway
dysregulation and anthracycline sensitivity in patients with acute
myeloid leukemia. J Clin Oncol. 2009; 27:5580-6. http://dx.doi.org/10.1200/JCO.2009.22.2547
PMid:19858393

- Qian Z, Fernald AA, Godley LA, Larson RA,
Le Beau MM. Expression profiling of CD34+ hematopoietic stem/
progenitor cells reveals distinct subtypes of therapy-related acute
myeloid leukemia. Proc Natl Acad Sci U S A. 2002; 99:14925-30. http://dx.doi.org/10.1073/pnas.222491799
PMid:12417757 PMCid:137521

- Voso MT, Scardocci A, Guidi F, Zini G, Di
Mario A, Pagano L, Hohaus S, Leone G. Aberrant methylation of
DAP-kinase in therapy-related acute myeloid leukemia and
myelodysplastic syndromes. Blood. 2004; 103:698-700. http://dx.doi.org/10.1182/blood-2003-07-2249
PMid:14504087

- Scardocci A, Guidi F, D'Alo' F, Gumiero D,
Fabiani E, Diruscio A, Martini M, Larocca LM, Zollino M, Hohaus S,
Leone G, Voso MT. Reduced BRCA1 expression due to promoter
hypermethylation in therapy-related acute myeloid leukaemia. Br J
Cancer. 2006; 95:1108-13. http://dx.doi.org/10.1038/sj.bjc.6603392
PMid:17047656 PMCid:2360697

- Voso MT, D'Alò F, Greco M, Fabiani E,
Criscuolo M, Migliara G, Pagano L, Fianchi L, Guidi F, Hohaus S, Leone
G. Epigenetic changes in therapy-related MDS/AML. Chem Biol Interact.
2010; 184:46-9. http://dx.doi.org/10.1016/j.cbi.2009.10.013
PMid:19874806

- Greco M, D'Alò F, Scardocci A, Criscuolo
M, Fabiani E, Guidi F, Di Ruscio A, Migliara G, Pagano L, Fianchi L,
Chiusolo P, Hohaus S, Leone G, Voso MT. Promoter methylation of DAPK1,
E-cadherin and thrombospondin-1 in de novo and therapy-related myeloid
neoplasms. Blood Cells Mol Dis. 2010; 45:181-5. http://dx.doi.org/10.1016/j.bcmd.2010.05.008
PMid:20655775

- Figueroa ME, Skrabanek L, Li Y, Jiemjit A,
Fandy TE, Paietta E, Fernandez H, Tallman MS, Greally JM, Carraway H,
Licht JD, Gore SD, Melnick A. MDS and secondary AML display unique
patterns and abundance of aberrant DNA methylation. Blood. 2009;
114:3448-58. http://dx.doi.org/10.1182/blood-2009-01-200519
PMid:19652201 PMCid:2765680

- Appelbaum FR, Gundacker H, Head DR, Slovak
ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute
myeloid leukemia. Blood. 2006; 107:3481-5. http://dx.doi.org/10.1182/blood-2005-09-3724
PMid:16455952
PMCid:1895766

- Derolf AR, Kristinsson SY, Andersson TM,
Landgren O, Dickman PW, Björkholm M. Improved patient survival for
acute myeloid leukemia: a population-based study of 9729 patients
diagnosed in Sweden between 1973 and 2005. Blood. 2009; 113:3666-72. http://dx.doi.org/10.1182/blood-2008-09-179341
PMid:19020306

- Borthakur G, Lin E, Jain N, Estey EE,
Cortes JE, O'Brien S, Faderl S, Ravandi F, Pierce S, Kantarjian H.
Survival is poorer in patients with secondary core-binding factor acute
myelogenous leukemia compared with de novo core-binding factor
leukemia. Cancer. 2009; 115:3217-21. http://dx.doi.org/10.1002/cncr.24367
PMid:19441109

- Derolf AR, Kristinsson SY, Andersson TM,
Landgren O, Dickman PW, Björkholm M. Improved patient survival for
acute myeloid leukemia: a population-based study of 9729 patients
diagnosed in Sweden between 1973 and 2005. Blood. 2009; 113:3666-72. http://dx.doi.org/10.1182/blood-2008-09-179341
PMid:19020306

- Etienne A, Esterni B, Charbonnier A,
Mozziconacci MJ, Arnoulet C, Coso D, Puig B, Gastaut JA, Maraninchi D,
Vey N. Comorbidity is an independent predictor of complete remission in
elderly patients receiving induction chemotherapy for acute myeloid
leukemia. Cancer. 2007; 109:1376-83. http://dx.doi.org/10.1002/cncr.22537
PMid:17326052

- Giles FJ, Borthakur G, Ravandi F, Faderl
S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S,
Albitar M, Cortes J, Kantarjian H. The haematopoietic cell
transplantation comorbidity index score is predictive of early death
and survival in patients over 60 years of age receiving induction
therapy for acute myeloid leukaemia. Br J Haematol. 2007; 136:624-7. http://dx.doi.org/10.1111/j.1365-2141.2006.06476.x
PMid:17223919

- Godley LA, Larson RA. Therapy-related
myeloid leukemia. Semin Oncol. 2008; 35:418-29.
http://dx.doi.org/10.1053/j.seminoncol.2008.04.012 PMid:18692692
PMCid:2600445

- Leone G, Fianchi L, Voso MT.
Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011; in press http://dx.doi.org/10.1097/CCO.0b013e32834bcc2a
PMid:21918440

- Rossi DJ, Bryder D, Zahn JM, Ahlenius H,
Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie
hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;
102:9194-9. http://dx.doi.org/10.1073/pnas.0503280102
PMid:15967997
PMCid:1153718
