Chemotherapy-Induced Neutropenia in HIV Positive Patients with Lymphoma:
Comparison of Pegfilgrastim with Daily Filgrastim Administration
Luciana Teofili1, Immacolata Izzi2, Eugenia Rosa Nuzzolo1, Giancarlo Scoppettuolo2, Lorenza Torti1, Marianna
Rossi2 and Katleen de Gaetano Donati2
1Department of Hematology and 2Department of Infectious Diseases, Catholic University of Rome, Italy
Correspondence
to: Dr. Luciana Teofili, Hematology Department, Catholic University of Rome, Largo Gemelli 8, 00168, Rome, Italy. Tel: +39-06-3015-4180, Fax: +39-06-3055153. Email: mailto:lteofili@rm.unicatt.it
Published: October 3, 2012
Received: August 28, 2012
Accepted: September 20, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012062, DOI 10.4084/MJHID.2012.062
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
We
retrospectively compared the incidence of neutropenia in two groups of
HIV patients with lymphoma, who underwent chemotherapy supported by
once-per-cycle administration of pegfilgrastim or by daily subcutaneous
injection of filgrastim, respectively. Our findings indicate that
pegfilgrastim and filgastrim produce similar results in preventing both
neutropenia and febrile neutropenia.
Introduction
HIV infection leads to an increased risk of non-Hodgkin’s lymphoma (NHL) and, to a minor extent, of Hodgkin’s lymphoma (HL). The concomitant administration of highly active antiretroviral therapy (ART) to chemotherapy (CT) has greatly improved the outcome of these diseases.[1] Furthermore, the management of CT induced neutropenia with recombinant human granulocyte-colony stimulating factors (G-CSFs, i.e. filgrastim and lenograstim) rendered feasible intensive CT regimens, including dose-dense CT or high dose CT, also in HIV positive patients.[2,3] A pegylated form of filgrastim is today available: actually, the addition of the polyethylene glycol molecule increases the serum half-life of filgrastim, resulting in its prolonged activity.[3] Therefore, pegfilgrastim shows the advantage of a single injection per CT course as compared to repeated daily administration of filgrastim or lenograstim. The safety and efficacy of primary prophylaxis with all currently available G-CSFs in preventing febrile neutropenia (FN) and in supporting dose-dense therapy has been demonstrated in various malignancies and it is strongly recommended.[4] However, a recent meta-analysis demonstrated that all currently available G-CSFs significantly reduces the incidence of FN in comparison with patients not receiving growth factors, whereas pegfilgrastim in particular appeared the most effective one.[4] If so, the superiority of pegfilgrastim should be even more evident in the setting of AIDS associated tumors, since these patients have a particular high infectious risk. Nevertheless, to our knowledge, the efficacy of pegylated and non pegylated G-CSFs in HIV positive patients has never been investigated. In this retrospective study we compared the efficacy of pegfilgrastim and filgastrim in preventing neutropenia and fever in a series of HIV patients treated at our institution.
Patients and Methods
The study population consisted of a series of 8 HIV positive patients with diagnosis of HL (n = 4) and NHL (n = 4), consecutively observed at the department of Infectious Diseases of the Catholic University of Rome between October 2005 and July 2006. These patients received CT regimens supported by once-per-cycle administration of pegfilgrastim. As a control group, 13 HIV positive patients (5 with HL and 8 with NHL) matched for age, diagnosis and CT, who had been previously followed at the same institution, were evaluated. All patients in the control group received CT supported by daily subcutaneous injection of filgrastim. Chemotherapy for HL consisted of ABVD[7] in 3 patients with early stage HL, and of standard dose-BEACOPP[8] or EBVP[7] regimens in 6 patients with advanced stage-HL. Chemotherapy for NHL consisted of CHOP[7] in 9 patients with diffuse large B-cell lymphoma and of Magrath protocol[9] in 3 patients with Burkitt’s lymphoma. Considering that all patients were treated before 2006, none of them received Rituximab in association with CT. Both pegfilgrastim and filgrastim were administered 1 day after the completion of CT. In total, 23 courses of CT were evaluated in the pegfilgrastim group and 75 courses in the filgrastim group. The efficacy of pegfilgrastim and of filgrastim was assessed by evaluating the incidence of severe neutropenia (defined as neutrophil count less than 0.5 x 109/L), the number of FN episodes and the number of positive blood cultures occurred among all recorded CT courses. Statistical comparison of continuous variables was performed by the Mann-Whitney U-test. Comparison of categorical variables was performed by chi-square statistic, using the Fisher’s exact test. P values <0.05 were considered statistically significant.
Results
Clinical and laboratory findings of patients are shown in Table 1. No differences were found between pegfilgrastim or filgastrim groups of patients for sex and age distribution, stage of disease, Type of CT, and hematological parameters (Table 1). Moreover, the number of CD4+ cell count at diagnosis (evaluated as continuous variable) and the percentage of patients showing CD4+ cell count less than 200/μL were similar in both groups (Table 1). A similar proportion of patients in pegfilgrastim and filgrastim groups received ART in combination with CT (Table 1).
Table 2 shows the efficacy end points observed in pegfilgrastim- as compared to filgrastim-supported CT courses. Severe neutropenia was observed in 8 out of the 23 CT courses analyzed in the pegfilgrastim group, and in 32 out of the 75 CT courses analyzed in the filgrastim group (p=0.63, Table 2). The median duration of neutropenia was 5 days in both groups (p=0.80, Table 2). FN was recorded in 5 cases in pegfilgrastim group and in 27 cases in filgrastim group (p=0.14, Table 2). Accordingly, no differences were found between pegfilgrastim and filgrastim-supported-CT in the incidence of positive blood cultures (p=1.00, Table 2).
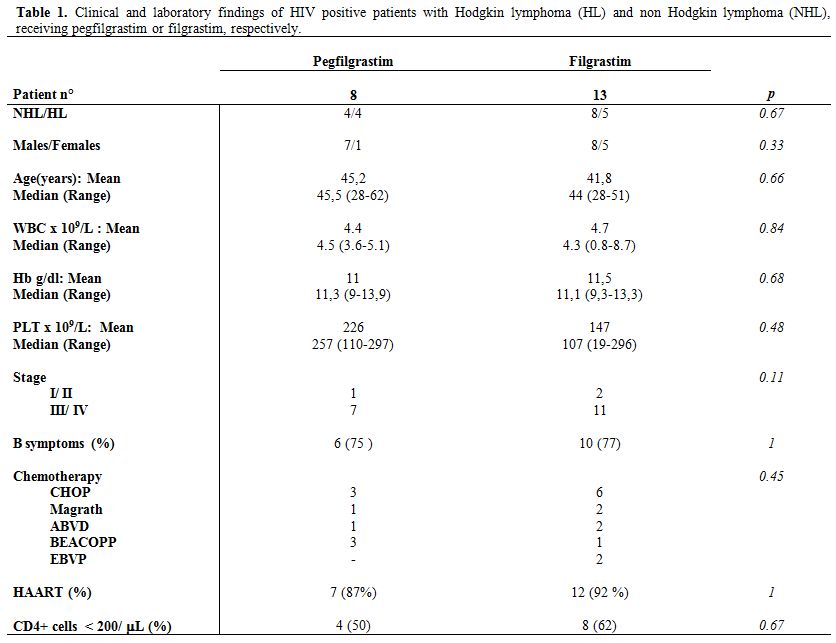
Table 1. Clinical and laboratory findings of HIV positive patients with Hodgkin lymphoma (HL) and non Hodgkin lymphoma (NHL), receiving pegfilgrastim or filgrastim, respectively.
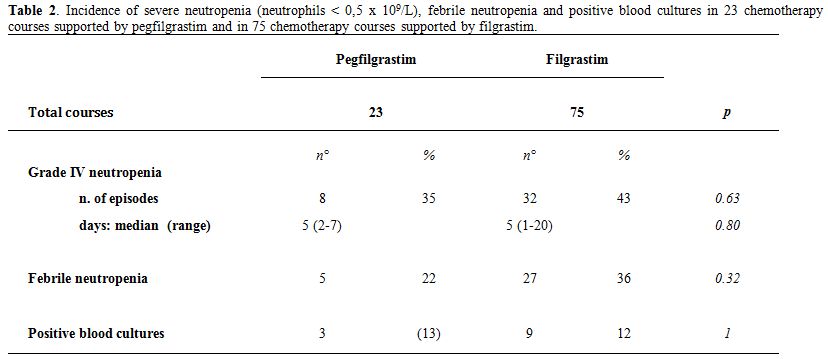
Table 2. Incidence of severe neutropenia (neutrophils < 0,5 x 109/L), febrile neutropenia and positive blood cultures in 23 chemotherapy courses supported by pegfilgrastim and in 75 chemotherapy courses supported by filgrastim.
Discussion
To compare the efficacy of pegylated and non-pegylated G-CSFs in the setting of HIV associated malignancies is an interesting issue to address. According to evidence-based clinical practice, primary prophylaxis with G-CSFs is recommended for CT regimens exceeding the 20% threshold of FN risk[4,10] and in dose-dense CT schedules.[11,12] The existence of concomitant conditions predisposing to prolonged neutropenia, such as other myelotoxic therapies or bone marrow involvement by lymphoma cells, may help to identify which patients are most likely to benefit from prophylactic G-CSFs.[4,10] In this respect, HIV positive persons undergoing CT for lymphoma represent a patients population at high risk for infection and FN. Actually, the diagnosis of lymphoma and HIV infection frequently occurs simultaneously[1] and lymphoid tumors often arise in most immunocompromised HIV patients.[13] In addition, the majority of HIV infected patients receives antiretroviral drugs and CT in combination: importantly, not only ART can have a direct hematologic toxicity[14] but, in some instances, it can affect the metabolism of antiproliferative drugs so enhancing their bone marrow toxicity.[15] Finally, lymphomas occurring in HIV positive patients, involve bone marrow more frequently than in general people.[16] Our study shows that pegfilgrastim and filgrastim exhibit similar efficacy in controlling both CT-induced neutropenia and FN episodes in HIV positive patients with HL and NHL. Notwithstanding the worth of our findings is limited by the retrospective design of the study, they can suggest some considerations. While filgrastim and lenogratim are administered by a course of daily injections, pegfilgrastim posses the important advantage of once-per-cycle administration. This is an important aspect considering that several data from low level studies derived from audit data and clinical practice suggests that some patients receive suboptimal daily G-CSFs4 and the use of pegfilgrastim prevents this problem. Likewise, however, it is important to consider the cost of care is rising progressively, and that it is partly due to unnecessary use of health care resources. To this purpose, the American Society of Clinical Oncology has recently stated, among other opportunities to improve care and reduce costs, that G-CSFs should be used only when the risk of FN, secondary to a recommended chemotherapy regimen is approximately 20% and equally effective treatment programs that do not require white cell stimulating factors are unavailable.[17] In line with these recommendation, pegfilgrastim support should be mainly tailored to a cost-effective care provision.[11,12] Nevertheless, in HIV positive patients, the simplified one-per cycle injection could result also in the reduced risk of infection by health care operators. This important topic should be considered an additional point to be clarified in studies performed on HIV infected patients.
HIV infection leads to an increased risk of non-Hodgkin’s lymphoma (NHL) and, to a minor extent, of Hodgkin’s lymphoma (HL). The concomitant administration of highly active antiretroviral therapy (ART) to chemotherapy (CT) has greatly improved the outcome of these diseases.[1] Furthermore, the management of CT induced neutropenia with recombinant human granulocyte-colony stimulating factors (G-CSFs, i.e. filgrastim and lenograstim) rendered feasible intensive CT regimens, including dose-dense CT or high dose CT, also in HIV positive patients.[2,3] A pegylated form of filgrastim is today available: actually, the addition of the polyethylene glycol molecule increases the serum half-life of filgrastim, resulting in its prolonged activity.[3] Therefore, pegfilgrastim shows the advantage of a single injection per CT course as compared to repeated daily administration of filgrastim or lenograstim. The safety and efficacy of primary prophylaxis with all currently available G-CSFs in preventing febrile neutropenia (FN) and in supporting dose-dense therapy has been demonstrated in various malignancies and it is strongly recommended.[4] However, a recent meta-analysis demonstrated that all currently available G-CSFs significantly reduces the incidence of FN in comparison with patients not receiving growth factors, whereas pegfilgrastim in particular appeared the most effective one.[4] If so, the superiority of pegfilgrastim should be even more evident in the setting of AIDS associated tumors, since these patients have a particular high infectious risk. Nevertheless, to our knowledge, the efficacy of pegylated and non pegylated G-CSFs in HIV positive patients has never been investigated. In this retrospective study we compared the efficacy of pegfilgrastim and filgastrim in preventing neutropenia and fever in a series of HIV patients treated at our institution.
Patients and Methods
The study population consisted of a series of 8 HIV positive patients with diagnosis of HL (n = 4) and NHL (n = 4), consecutively observed at the department of Infectious Diseases of the Catholic University of Rome between October 2005 and July 2006. These patients received CT regimens supported by once-per-cycle administration of pegfilgrastim. As a control group, 13 HIV positive patients (5 with HL and 8 with NHL) matched for age, diagnosis and CT, who had been previously followed at the same institution, were evaluated. All patients in the control group received CT supported by daily subcutaneous injection of filgrastim. Chemotherapy for HL consisted of ABVD[7] in 3 patients with early stage HL, and of standard dose-BEACOPP[8] or EBVP[7] regimens in 6 patients with advanced stage-HL. Chemotherapy for NHL consisted of CHOP[7] in 9 patients with diffuse large B-cell lymphoma and of Magrath protocol[9] in 3 patients with Burkitt’s lymphoma. Considering that all patients were treated before 2006, none of them received Rituximab in association with CT. Both pegfilgrastim and filgrastim were administered 1 day after the completion of CT. In total, 23 courses of CT were evaluated in the pegfilgrastim group and 75 courses in the filgrastim group. The efficacy of pegfilgrastim and of filgrastim was assessed by evaluating the incidence of severe neutropenia (defined as neutrophil count less than 0.5 x 109/L), the number of FN episodes and the number of positive blood cultures occurred among all recorded CT courses. Statistical comparison of continuous variables was performed by the Mann-Whitney U-test. Comparison of categorical variables was performed by chi-square statistic, using the Fisher’s exact test. P values <0.05 were considered statistically significant.
Results
Clinical and laboratory findings of patients are shown in Table 1. No differences were found between pegfilgrastim or filgastrim groups of patients for sex and age distribution, stage of disease, Type of CT, and hematological parameters (Table 1). Moreover, the number of CD4+ cell count at diagnosis (evaluated as continuous variable) and the percentage of patients showing CD4+ cell count less than 200/μL were similar in both groups (Table 1). A similar proportion of patients in pegfilgrastim and filgrastim groups received ART in combination with CT (Table 1).
Table 2 shows the efficacy end points observed in pegfilgrastim- as compared to filgrastim-supported CT courses. Severe neutropenia was observed in 8 out of the 23 CT courses analyzed in the pegfilgrastim group, and in 32 out of the 75 CT courses analyzed in the filgrastim group (p=0.63, Table 2). The median duration of neutropenia was 5 days in both groups (p=0.80, Table 2). FN was recorded in 5 cases in pegfilgrastim group and in 27 cases in filgrastim group (p=0.14, Table 2). Accordingly, no differences were found between pegfilgrastim and filgrastim-supported-CT in the incidence of positive blood cultures (p=1.00, Table 2).

Table 1. Clinical and laboratory findings of HIV positive patients with Hodgkin lymphoma (HL) and non Hodgkin lymphoma (NHL), receiving pegfilgrastim or filgrastim, respectively.

Table 2. Incidence of severe neutropenia (neutrophils < 0,5 x 109/L), febrile neutropenia and positive blood cultures in 23 chemotherapy courses supported by pegfilgrastim and in 75 chemotherapy courses supported by filgrastim.
Discussion
To compare the efficacy of pegylated and non-pegylated G-CSFs in the setting of HIV associated malignancies is an interesting issue to address. According to evidence-based clinical practice, primary prophylaxis with G-CSFs is recommended for CT regimens exceeding the 20% threshold of FN risk[4,10] and in dose-dense CT schedules.[11,12] The existence of concomitant conditions predisposing to prolonged neutropenia, such as other myelotoxic therapies or bone marrow involvement by lymphoma cells, may help to identify which patients are most likely to benefit from prophylactic G-CSFs.[4,10] In this respect, HIV positive persons undergoing CT for lymphoma represent a patients population at high risk for infection and FN. Actually, the diagnosis of lymphoma and HIV infection frequently occurs simultaneously[1] and lymphoid tumors often arise in most immunocompromised HIV patients.[13] In addition, the majority of HIV infected patients receives antiretroviral drugs and CT in combination: importantly, not only ART can have a direct hematologic toxicity[14] but, in some instances, it can affect the metabolism of antiproliferative drugs so enhancing their bone marrow toxicity.[15] Finally, lymphomas occurring in HIV positive patients, involve bone marrow more frequently than in general people.[16] Our study shows that pegfilgrastim and filgrastim exhibit similar efficacy in controlling both CT-induced neutropenia and FN episodes in HIV positive patients with HL and NHL. Notwithstanding the worth of our findings is limited by the retrospective design of the study, they can suggest some considerations. While filgrastim and lenogratim are administered by a course of daily injections, pegfilgrastim posses the important advantage of once-per-cycle administration. This is an important aspect considering that several data from low level studies derived from audit data and clinical practice suggests that some patients receive suboptimal daily G-CSFs4 and the use of pegfilgrastim prevents this problem. Likewise, however, it is important to consider the cost of care is rising progressively, and that it is partly due to unnecessary use of health care resources. To this purpose, the American Society of Clinical Oncology has recently stated, among other opportunities to improve care and reduce costs, that G-CSFs should be used only when the risk of FN, secondary to a recommended chemotherapy regimen is approximately 20% and equally effective treatment programs that do not require white cell stimulating factors are unavailable.[17] In line with these recommendation, pegfilgrastim support should be mainly tailored to a cost-effective care provision.[11,12] Nevertheless, in HIV positive patients, the simplified one-per cycle injection could result also in the reduced risk of infection by health care operators. This important topic should be considered an additional point to be clarified in studies performed on HIV infected patients.
References
- Vaccher E, Spina M, Talamini R, Zanetti M,
di Gennaro G, Nasti G,et al. Improvement of systemic human
immunodeficiency virus-related non-Hodgkin lymphoma outcome in the era
of highly active antiretroviral therapy (2003) Clin Infect Dis 37:
1556-64. http://dx.doi.org/10.1086/379517 PMid:14614680

- Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood 2012; 119:3245-55. http://dx.doi.org/10.1182/blood-2011-08-373738 PMid:22337719

- Michieli M, Mazzucato M, Tirelli U, De
Paoli P. Stem cell transplantation for lymphoma patients with HIV
infection. Cell Transplant. 2011; 20:351-70. http://dx.doi.org/10.3727/096368910X528076 PMid:20875226

- Lyman GH. Pegfilgrastim: a granulocyte
colony-stimulating factor with sustained duration of action (2005).
Expert Opin Biol Ther 5:1635-46. Review. http://dx.doi.org/10.1517/14712598.5.12.1635 PMid:16318427

- Aapro MS, Bohlius J, Cameron DA, Dal Lago
L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC,
Walewski J, Weber DC, Zielinski C; European Organisation for Research
and Treatment of Cancer. 2010 update of EORTC guidelines for the use of
granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphoproliferative disorders and solid tumours. Eur J Cancer.
2011;47:8-32. http://dx.doi.org/10.1016/j.ejca.2010.10.013 PMid:21095116

- Cooper KL, Madan J, Whyte S, Stevenson MD,
Akehurst RL Granulocyte colony-stimulating factors for febrile
neutropenia prophylaxis following chemotherapy: systematic review and
meta-analysis. BMC Cancer. 2011; 11:404. http://dx.doi.org/10.1186/1471-2407-11-404 PMid:21943360 PMCid:3203098

- Berretta M, Cinelli R, Martellotta F, Spina
M, Vaccher E, Tirelli U. (2003) Therapeutic approaches to AIDS-related
malignancies. Oncogene 22: 6646-59. http://dx.doi.org/10.1038/sj.onc.1206771 PMid:14528290

- Hartmann P, Rehwald U, Salzberger B,
Franzen C, Sieber M, Wohrmann A, et al. BEACOPP therapeutic regimen for
patients with Hodgkin's disease and HIV infection. Ann Oncol 2003; 14:
1562-9. http://dx.doi.org/10.1093/annonc/mdg408 PMid:14504059

- Mead GM, Sydes MR, Walewski J, Grigg A,
Hatton CS, Pescosta N, et al. An international evaluation of CODOX-M
and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results
of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002; 13:
1264-74. http://dx.doi.org/10.1093/annonc/mdf253 PMid:12181251

- Smith TJ, Khatcheressian J, Lyman GH, Ozer
H, Armitage JO, Balducci L, et al. 2006 update of recommendations for
the use of white blood cell growth factors: an evidence-based clinical
practice guideline. J Clin Oncol 2006; 24: 3187-205. http://dx.doi.org/10.1200/JCO.2006.06.4451 PMid:16682719

- Anderlini P, Champlin R. Use of filgrastim
for stem cell mobilisation and transplantation in high-dose cancer
chemotherapy. Drugs. 2002;62 Suppl 1:79-88. http://dx.doi.org/10.2165/00003495-200262001-00006 PMid:12479596

- Kobbe G, Bruns I, Fenk R, Czibere A, Haas
R. Pegfilgrastim for PBSC mobilization and autologous haematopoietic
SCT. Bone Marrow Transplant. 2009;43:669-77. http://dx.doi.org/10.1038/bmt.2009.59 PMid:19308043

- Grulich AE, Wan X, Law MG, Milliken ST,
Lewis CR, Garsia RJ, et al. B-cell stimulation and prolonged immune
deficiency are risk factors for non-Hodgkin's lymphoma in people with
AIDS. AIDS 2000; 14: 133-40. http://dx.doi.org/10.1097/00002030-200001280-00008 PMid:10708283

- Pluda JM, Mitsuya H, Yarchoan R.
Hematologic effects of AIDS therapies. Hematol Oncol Clin North Am
1991; 5: 229-48. PMid:1708759

- Cingolani A, Torti L, Pinnetti C, de
Gaetano Donati K, Murri R, Tacconelli E, Larocca LM, Teofili L.
Detrimental clinical interaction between ritonavir-boosted protease
inhibitors and vinblastine in HIV-infected patients with Hodgkin's
lymphoma. AIDS. 2010; 24: 2408-12 PMid:20671541

- Diamond C, Taylor TH, Im T, Anton-Culver
H. Presentation and outcomes of systemic non-Hodgkin's lymphoma: a
comparison between patients with acquired immunodeficiency syndrome
(AIDS) treated with highly active antiretroviral therapy and patients
without AIDS. Leuk Lymphoma 2006; 47: 1822-9. http://dx.doi.org/10.1080/10428190600658688

- Schnipper LE, Smith TJ, Raghavan D,
Blayney DW, Ganz PA, Mulvey TM, Wollins DS American Society of Clinical
Oncology identifies five key opportunities to improve care and reduce
costs: the top five list for oncology. J Clin Oncol. 2012;30:1715-24. http://dx.doi.org/10.1200/JCO.2012.42.8375 PMid:22493340
