Received: October 21, 2013
Accepted: April 27, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014034, DOI 10.4084/MJHID.2014.034
This article is available on PDF format at:

Kolakkadan Hasaf Mubeen, Clement Wilfred Devadoss, Rau Aarathi Rangan, Monnappa Gitanjali, Shetty Prasanna and VP Sunitha
Department
of Pathology, M.S Ramaiah Medical College & Teaching Hospital,
Bangalore. India

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Malaria
is one of the most pervasive parasitic diseases ever known to mankind
affecting nearly 300 million people every year. The need for rapid
diagnosis of malaria in tropical and subtropical malaria endemic areas
is on the rise. In this study, we evaluated the usefulness of
hematology autoanalyzers, Sysmex XE-2100 & XT-2000i in the
presumptive diagnosis of malaria. Our study shows that abnormalities in
WBC/BASO scattergram when combined with presence of thrombocytopenia
had a high sensitivity and positive predictive value in the presumptive
diagnosis of Plasmodium vivax (P.vivax) malaria.
|
Introduction
The
World Health Organization estimates that half the world’s population is
at risk of malaria, with an estimated 200-300 million people developing
clinical malaria every year.[1]
According to World
Malaria Report (2013) nearly half (273 million) of the high-risk
population outside Africa resides in India[2]
Karnataka is located in the southern peninsular region of India and was
once considered as a high transmission zone for malaria but due to the
implementation of rigorous control measures the malaria incidence in
the state has fallen significantly. However urban malaria has continued
to be a problem in the cities of Bangalore and Mangalore in Karnataka
is due to the migration of people from high risk rural areas.
In 2011, 24487 malaria cases were reported in the state of Karnataka of
which 21842 (89.19%) cases were infected by Plasmodium vivax
species, indicating that P.
vivax
is the predominant species in this region. Demographic data from these
cases reveal that age group 21-30 years was most affected.[3]
Light microscopy is considered as the gold standard approach in
diagnosis of malaria, but it requires time and expertise. Many rapid
diagnostic tests (RDT) have emerged recently to overcome these factors
however these are expensive and not routinely available.[4,5]
As Complete Blood Count (CBC) is a baseline investigation ordered for
patients with fever, there has been a growing focus on the utility of
hematology analyzers in the presumptive diagnosis of malarial
infection.
In the year 1999, Hancheid et al. reported the usefulness of automated
hematology analyzer Cell Dyn 3500 (Abbott, Santa Clara, CA) in
diagnosis of malaria even in the absence of clinical suspicion.[6]
This study was conducted to evaluate the utility of automated
hematology analyzers, Sysmex XE-2100 & XT-2000i (Kobe, Japan)
in
the diagnosis of the malarial parasite in conjunction with peripheral
smear examination, by evaluating various scattergram abnormalities.
Materials and Methods
Routine blood samples from both outpatient and inpatient departments
were analyzed during March 2013 to August 2013. Samples were collected
in K2 EDTA tubes (Becton Dickinson, USA) and complete blood count
analysis was done either on Sysmex XE-2100 or XT-2000i. Peripheral
smear examination was done for all the cases.
Both analyzers use flow cytometry using a semiconductor laser to
categorize WBCs based on the forward and side scattered light
information of cells. Forward scattered light analyses the size and the
side scattered light analyses the granularity of the WBCs. This data is
depicted on coloured depictions namely WBC-DIFF and WBC/BASO
scattergrams.
WBC-DIFF scattergram (WBC 4-part differential): RBCs are completely
lysed with lysing solution “STROMATOLYSER-4DL”, at the same time this
reagent acts on WBC membranes and makes them partially permeable.
Following this, a fluorescent dyeing solution “STROMATOLYSER-4DS” is
added to allow the fluorescent dye to enter the WBC through its
permeable membranes and stain the DNA and RNA. Following this reaction,
the inbuilt flow cytometer detects the forward and side scatter
information based on which WBC-DIFF scattergram is obtained. By
analyzing this scattergram, the analyzer gives a 4 part differential
count, viz lymphocytes, monocytes, eosinophils and other granulocytes
(neutrophils plus basophils).[7]
WBC/BASO scattergram: This scattergram is obtained by lysing RBCs with
a lysing reagent “STROMATOLYSER-FB” which selectively suppresses
degranulation of basophils. Following this reaction, the sample is
analysed by flow cytometry to detect forward and side scattered light
information to give a WBC/BASO scattergram. The analyzer gives a total
WBC count and basophil count based on this scattergram.[8]
Figure 1
shows a normal WBC-DIFF & WBC/BASO scattergram.
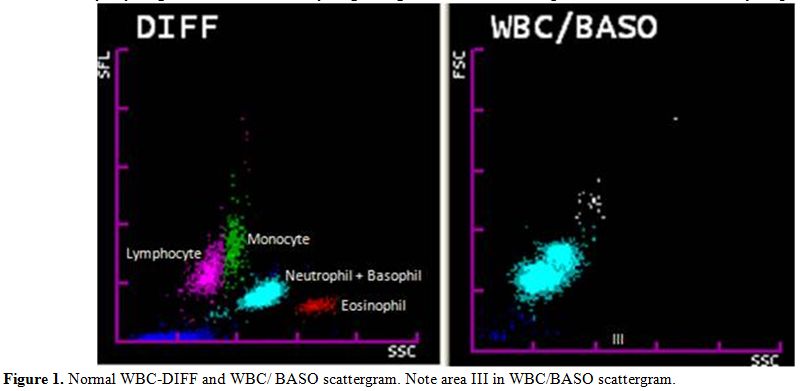 | Figure 1. Normal WBC-DIFF and WBC/ BASO scattergram. Note area III in WBC/BASO scattergram. |
Various
changes in the WBC scattergram (WBC-DIFF & WBC/BASO) like
graying
of WBC clusters, merging of clusters, multiple clustering, abnormal
blue coded events and other changes if any, were analyzed
simultaneously for all these cases.
Hematological parameters like hemoglobin (Hb), total leucocyte count
(TLC), differential count (DC) and platelet count of all the malaria
positive cases were collected.
Smears were reviewed for all the cases which showed abnormal
scattergram. Sensitivity, specificity, Positive Predictive Value (PPV)
and Negative Predictive Value (NPV) was calculated for both WBC-DIFF
& WBC/BASO scattergrams using Galen and Gambino method.
Results
During March 2013 to August 2013, we received 2610 peripheral smears
for malarial parasite detection from patients who presented with fever,
chills and rigors. Other uncommon presenting symptoms were vague
abdominal pain, myalgia and headache. However, 2 cases presented with
symptoms of Acute Renal Failure. Of the 2610 cases, 1730 were males and
880 were females with age ranging between 10-65 years.
Out of 2610 cases, 45 (n=45) cases were found to be malaria positive,
of which 35 were males and 10 were females with age ranging from 15-65
years. Forty cases were positive for Plasmodium vivax
and five cases were positive for Plasmodium
falciparumP.
vivax to P.
falciparum ratio of 8:1.
Out of the 40 P. vivax
cases,
five cases were reported negative by initial peripheral smear
examination, but these cases showed scattergram abnormalities and were
diagnosed as positive on repeat peripheral smear. These missed cases
were found to have very low parasite index. All the P. falciparum
cases were diagnosed on peripheral smear examination. Total number of
smear negative cases were 2565 during this period. Both WBC-DIFF and
WBC/BASO scattergrams were analyzed in all the 2610 cases. Distribution
of cases is given in figure
2.
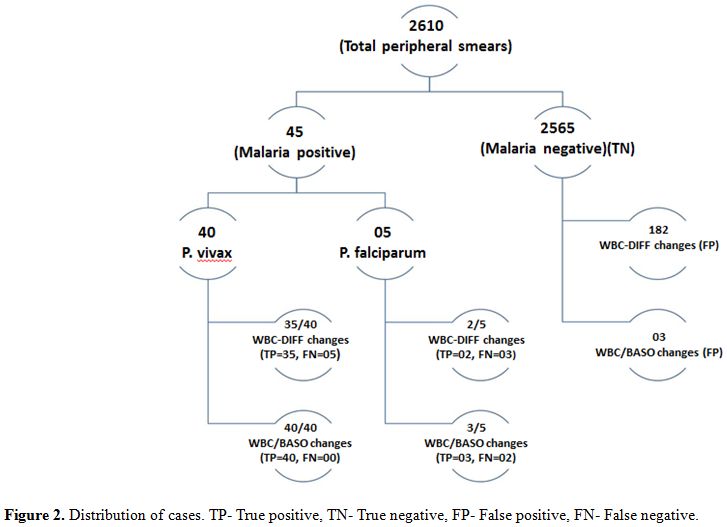 | Figure 2. Distribution of cases. TP- True positive, TN- True negative, FP- False positive, FN- False negative. |
WBC-DIFF
scattergram. WBC-DIFF abnormalities were found in 37 out
of 45 malaria positive cases. In P.
vivax group (n=40) 35 out of 40 cases showed various
abnormities with a sensitivity of 87.5%. In P. falciparum
group (n=5) 2 out of 5 cases showed WBC-DIFF abnormalities with a
sensitivity of 40%. The overall specificity of WBC-DIFF plot in
malarial detection was found to be 93.3%. PPV for P. vivax and P. falciparum was
16.13% and 1.09% respectively. NPV for both groups was found to be
99.8%. (Table 1)
Various changes noted in WBC-DIFF scattergram were merging of
neutrophil and eosinophil clusters (44.5%), multiple neutrophil or
eosinophil clusters (32%), graying of neutrophil and eosinophil
clusters (27%), prominent blue coded events between/above or below the
neutrophil and eosinophil clusters (22%) and large eosinophil clusters
(10%) (Figure 3).
These changes were noted singly or in combination.
WBC-DIFF abnormalities were also noted in 182 out of 2565 malaria
negative cases. This included 95 new born blood samples, 46 cases of
leukemia, 37 cases of other solid organ malignancies on chemotherapy
and 4 cases of hemolytic anemia.
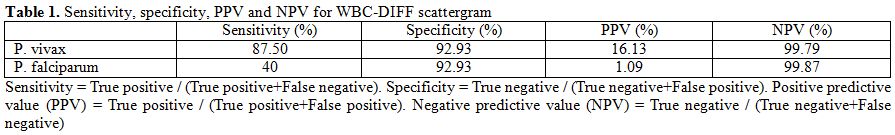 | Table 1. Sensitivity, specificity, PPV and NPV for WBC-DIFF scattergram. |
WBC/BASO
scattergram. WBC/BASO channel abnormalities were found in
43 out of 45 cases. In P.
vivax
group all the cases (n=40) showed one single most consistent change:
isolated prominent blue coded events in area III or late area II and
III in the WBC/BASO scattergram with a sensitivity of 100% (Figure 4). In P. falciparum
group, 3 out of 5 cases showed this change with a sensitivity of 60%.
Overall specificity of WBC/BASO graph in malaria detection was found to
be 98.9%. PPV for P.
vivax and P.
falciparum was 93.02% and 50% respectively. NPV for P. vivax and P. falciparum was
100% and 99.9% respectively. (Table
2)
Of the 5 cases of P.
vivax which
were missed on peripheral smear, fine but faint blue coded dots ranging
in number from 7 to 15 were found in area III of WBC/BASO channel (Figure 5A). This
gave us the opportunity to review the smears and perform rapid card
tests to give a final diagnosis of malaria.
Blue coded events were noted in 3 malaria negative cases out of which
two cases had hemolytic disease of newborn and one was a case of
thalassemia on treatment. These were confirmed to be malaria negative
by a repeat smear and a negative card test. In these cases, in contrast
to the malaria positive cases, the blue coding extended from area I to
III (Figure 5B).
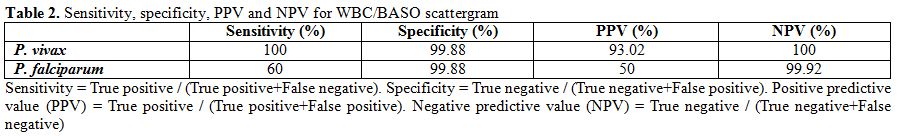 | Table 2. Sensitivity, specificity, PPV and NPV for WBC/BASO scattergram. |
Other
findings. Thrombocytopenia was seen in 44 out of 45
malaria positive cases with platelet count ranging from 12- 200 x 103/µl with a
mean of 53 x 103/µl
(SD 31.9). Pseudoeosinophilia which is defined as a difference in
automated and manual eosinophil count of >5%, was noted in 7 out
of
45 cases with a sensitivity of a mere 15.5%. It was also noted that all
the scattergram abnormalities reversed after two days of initiation of
antimalarial treatment.
Discussion
In tropical and endemic countries where malaria is highly prevalent,
there is a need for a rapid, cost effective and efficient method for
screening the blood samples. Various new methods of malaria detection
like quantitative buffy coat assay, antigen coated dipstick tests,
rapid diagnostic card tests and polymerase chain reaction have come up
in recent past.[9,10] But these
tests are limited by
their high cost and limited feasibility. Due to these factors
Romanowsky stained peripheral smear examination has remained as ‘gold
standard’ in malaria diagnosis but the quality of malaria microscopy is
far from satisfactory in most countries.[11]
There has been a growing interest in the use of automated hematology
analyzers in the presumptive diagnosis of malaria.[12]
Earliest of such studies has been done by Hanscheid et al.[6]
on Cell Dyn3500 (Abott Diagnostics, USA), proving the efficacy of
automated hematology analyzers in malaria detection using the principle
of flow cytometry.
Sysmex XE-2100 & XT-2000i uses flow cytometry in conjunction
with
fluorescence properties of leucocytes to generate various WBC
parameters, a few abnormalities of which can give a clue to the
diagnosis of malaria.
Zuluaga et al.[13] showed in their
study that area III blue coded events in WBC/BASO scattergram for P.vivax had a
sensitivity and specificity of 97% and 94% respectively using Sysmex
XE-2100 autoanalyzer. For P.
falciparum
the sensitivity and specificity was found to be 60% and 67%
respectively. These findings were similar to that of the present study.
Also, the presence of >8 blue coded events in area III of
WBC/BASO
plot when combined with the presence of thrombocytopenia increased the
sensitivity of malaria detection.These findings are similar to the
findings of Zuluaga et al.[13]
The WBC-DIFF plot abnormalities arise due to the neutrophils and
eosinophils which have ingested the malarial pigment. WBC/BASO
abnormalities are caused due to the red cells and reticulocytes which
contain parasites and pigment.[12]
Yoo et al.[14] reported in their
study that
psuedoeosinophilia and WBC scattergram abnormalities had sensitivity of
46.2% and specificity of 99.7%. Huh et al.[15]
also
reported psuedoeosinophilia in 38% of malaria cases. In the present
study psuedoeosinophilia was found in only 7 cases (15.5%).
Psuedoeosinophilia is thought to be caused by the neutrophils which
contain hemozoin pigment which are erroneously plotted in the
eosinophil area. Pseudoeosinophilia was not a consistent finding in our
cases, in contrary to previously reported studies and this could be due
to a low parasitic index/load.
We also found 3 false positive cases in the WBC/BASO scattergram, but
these cases showed blue coded events extending from area I to area III
in contrast to isolated area III blue coded events in malaria positive
cases. In our study, WBC scattergrams showed lower sensitivity and PPV
in P. falciparum
detection similar to studies conducted by Jain et al.[12]
and Zuluaga et al.[13] This could
also be attributed to the low number of P. falciparum cases
in the current study as P.
vivax is the dominant malarial parasite in this part of
the country.[16] However, larger
studies from P.
falciparum
predominant areas are required to further comment on the usefulness of
analyzers in diagnosis of the same. In our study, abnormalities in
WBC/BASO scattergram proved to be the most consistent finding in
positive cases. Moreover, 5 cases of P. vivax which were
missed on peripheral smear showed WBC/BASO plot abnormalities.
Thrombocytopenia was present in 97.7% of malaria positive cases in our
study, which was similar to the findings by Abro et al. and Chandra et
al.[17,18] As the number of
malaria cases presenting
with isolated thrombocytopenia is very high, these cases should be
specifically screened for malaria infestation.[19]
Last but not the least, this method was found to be the most cost
effective diagnostic tool as CBC analysis costs 3-4 USD when compared
to Malarial card test which costs nearly 8-10 USD in our setup.
Conclusions
We found the WBC/BASO scattergram abnormalities to be useful in the
presumptive diagnosis of P.
vivax
when combined with presence of thrombocytopenia. This helps the
pathologists and technicians who handle these autoanalyzers to pick up
all suspicious cases and subsequently confirm the same on a peripheral
smear and with other rapid diagnostic tests.
Acknowledgement
Dr. K.C Mahadeva, Professor & Head of the Department of Pathology, for his encouragement and granting permission to conduct this study. M.S Ramaiah Medical College & Teaching Hospital.
References


















[TOP]