Received: December 30, 2014
Accepted: March 30, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015027, DOI 10.4084/MJHID.2015.027
This article is available on PDF format at:

Mostafa Saadat
Department of Biology, College of Sciences, Shiraz University, Shiraz 71454, Iran

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Consanguineous marriage that defines
as a union between biologically related persons has a variety of known
deleterious correlations with factors that affect public health within
human populations. To investigate the association between the mean of
inbreeding coefficient(α) and
incidence of leukemia, the present ecological study on 68 countries was
carried out. Statistical analysis showed that the age-standardized
incidence rate of leukemia positively correlated with log10GNI per capita (r=0.699, df=66, P<0.001) and negatively correlated with log10α (r=-0.609, df=66, P<0.001). Controlling log10GNI per capita, a significant negative correlation between log10
and the age-standardized incidence rate of leukemia was observed
(r=-0.392, df=65, P=0.001). The countries were stratified according to
their annual GNI per capita, low and high-income countries with GNI per
capita less than and more than 10,000$, respectively. Statistical
analysis showed that in high-income countries, after controlling for log10GNI per capita, the correlation between the age-standardized incidence rate of leukemia and log10α
was still significant (r=-0.600, df=36, P<0.001). It should be noted
that there was no significant association between the age-standardized
mortality rate due to leukemia and log10α
(P>0.05). The present finding indicates that the rate of leukemia,
age-standardized for incidence, is lower in countries with a high
prevalence of consanguineous marriages. |
Introduction
Consanguineous marriage defined as a union between biologically
related persons has a long-standing social habit among some
populations. Its prevalence depends on many factors, such as
demographic, religious, cultural and socio-economic factors.[1-4]
Consanguinity
has a variety of known deleterious correlations with factors that
affect public health within human populations.[1,5-14] However, there
were some reports that described a negative association between
consanguinity and risk of diseases.[15-17] A negative association was
reported between the susceptibility to infection with HIV-1 and
inbreeding coefficient.[17] Based on an ecologic study the mean of
inbreeding coefficient (at the population level; α) is negatively
associated with age-standardized mortality rate due to breast cancer.
This means that the countries with a high level of consanguinity show a
low level of age-standardized mortality rates due to breast cancer.[15]
A significant relationship between parental consanguinity and clinical
response to chemotherapy among locally advanced breast cancer patients
has been reported.[16]
It is well established that genetic
components involved in the risk of several types of cancers, including
leukemia.[18-24] On the other hand, consanguinity increases the
homozygosity of the offspring. Therefore, for countries such as our
country, where the consanguineous marriage is common,[25] the
association between consanguinity and incidence of leukemia or
mortality due to leukemia is highly important for public health
programs. Therefore, the present ecological study was carried out.
Materials and methods
Data collection.
The age-standardized rate is the number of new cases or deaths per
100,000 persons per year. An age-standardized rate is the rate that a
population would have if it had a standard age structure.
Standardization is necessary when comparing several populations that
differ with respect to age because age has a powerful influence on the
risk of cancer. Data about age-standardized incidence rates and
age-standardized mortality rates for leukemia (per 100,000 persons per
year) were obtained from the WHO website.[26] The inbreeding
coefficient is the probability that an individual has received both
alleles of a pair from an identical ancestral. The mean of inbreeding
coefficient (α) values for the countries were obtained from the
website.[27] In the present study, we used gross national income per
capita (GNI, annual; at international dollars) as a confounding factor.
Data about GNI per capita for 2012 were obtained from the WHO
website.[28] Inclusion criteria for selection of countries were based
on the availability of the variables mentioned above (Table 1).
Statistical analysis.
Kolmogorov-Smirnov test indicates that the GNI per capita and α
have an abnormal distribution (For GNI per capita: Kolmogorov-Smirnov
Z-test=1.276, P=0.077; For α Kolmogorov-Smirnov Z-test=2.043,
P<0.001). Logarithmic transformation (log10) was used on GNI per capita (named log10GNI) and α (named log10α)
because they had skewed distributions, and the logarithmic
transformations brought them closer to a normal distribution.
Correlations between the variables having normal distribution or the
logarithmic transformation of the variables not showing normal
distribution were determined using the parametric Pearson's correlation
coefficient analysis. Multiple regression analysis was carried out.
Also, the partial correlation coefficient analysis was carried out.
Statistical analysis was performed using SPSS (version 11.5)
statistical software package. A probability of P<0.05 was considered
statistically significant. All statistical tests were two-sided.
Results
Table 1 represents the mean
of inbreeding coefficients, GNI per capita; age-standardized incidence
rate and age-standardized mortality rate in the study countries.
There was no significant association between the age-standardized mortality rate due to leukemia and log10α before and/or after controlling for the log10GNI per capita (Table 2).
However, it is not the same as a previous report that indicates that
the age-standardized mortality rate due to breast cancer is negatively
correlated with the mean of inbreeding coefficient (α).[15]
Statistical analysis showed that the age-standardized incidence of leukemia positively correlated with the log10GNI per capita (r=0.699, df=66, P<0.001) and negatively correlated with the log10α
(r=-0.609, df=66, P<0.001). In multiple regression analysis,
age-standardized incidence or age-standardized mortality from leukemia
were used as dependent variables and log10GNI and log10α were used as independent variables. It should be noted that logand log10αGNI
per capita had a significant correlation with each other (r=-0.531,
df=66, P<0.001). However, the multiple regression analysis showed
that there was no significant collinearity. Partial correlation
analysis was carried out in order to eliminate the effect of possible
confounding effect of GNI per capita on the association between the
age-standardized incidence rate of leukemia and log10α. After checking the log10GNI pern capita, the log10α showed a significant negative correlation with the age-standardized incidence of leukemia (r=-0.392, df=65, P=0.001).
We
noted a possible lack of reliable data from low-income countries.
Therefore, we stratified the countries according to their GNI per
capita, low and high-income countries with GNI per capita less than and
more than 10,000$, respectively. Statistical analysis showed that in
high-income countries, after controlling the log10GNI per capita, the correlation between the age-standardized incidence rate of leukemia and log10α was significant (r=-0.600, df=36, P<0.001).
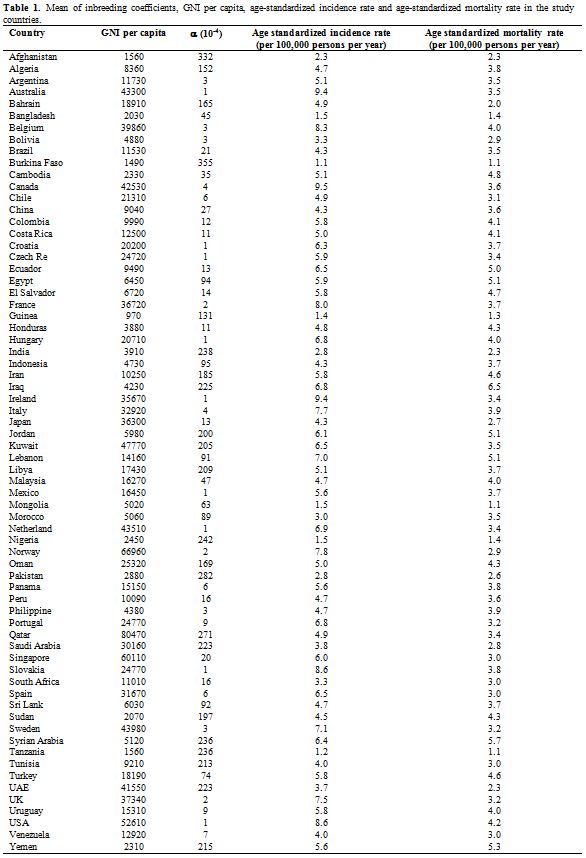 |
Table 1. Mean of inbreeding coefficients, GNI per capita, age-standardized incidence rate and age-standardized mortality rate in the study countries. |
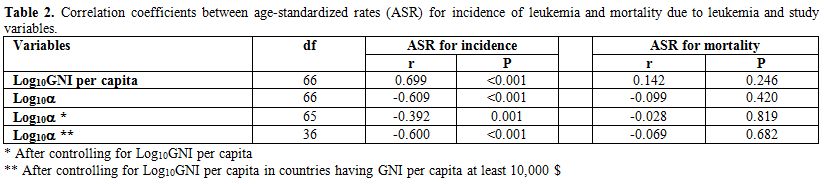 |
Table 2. Correlation coefficients between age-standardized rates (ASR) for incidence of leukemia and mortality due to leukemia and study variables. |
Discussion
The main finding of the present study is the negative association
between age-standardized incidence rate for leukemia and mean of
inbreeding coefficient (α). This means that countries are having a high
frequency of consanguinity, show a low level of age-standardized
incidence rates for leukemia. This finding is not easy to interpret.
A
significant positive association between level of inbreeding
coefficient (due to consanguineous marriages) and risk of cancers
(including leukemia) has been reported by some studies.[29-32] However,
several studies had shown that countries with high consanguinity
demonstrate lower age-standardized mortality rates and
incidence in breast cancer.[15,33,34]
It is well established
that the prevalence of consanguinity is mostly present in some
regions.[1,25,27,35,36] The prevalence of consanguineous marriages is
remarkably higher in many Asian and African countries compared with the
Western countries. On the hand, the data published by WHO,[26] show the
estimated age-standardized incidence of leukemia in the
Asian (3.9 per 100,000 persons per year) and African (3.0 per 100,000
persons per year) countries was significantly lower than in North
America (8.7 per 100,000 persons per year) and European countries (7.0
per 100,000 persons per year).
At present, it is very difficult
to establish how much the consanguinity, high in Eastern countries,
contributes to this difference or if this reflects only ethnic and
environmental factors.
We know that there are several types of
leukemia and genetic elements involved in the pathogenesis of each type
might be differing from the other types.[18-24] However, for estimating
the age-standardized incidence and mortality rates, all of leukemia
types were pooled.
The present study is an ecological study.
Other studies (such as case-control and cohort studies) are necessary
for concluding that a large proportion of death could attribute to
inbreeding due, in several countries, to the high prevalence of
consanguinity.
References
 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.