Management of Meningitis Caused by Multi Drug-Resistant Acinetobacter Baumannii: Clinical, Microbiological and Pharmacokinetic Results in a Patient Treated with Colistin Methanesulfonate
Elisabetta Schiaroli1, Maria Bruna Pasticci1, Maria Iris Cassetta2, Stefania Fallani2, Corrado Castrioto3, Matteo Pirro4, Andrea Novelli2, Lucia Henrici De Angelis5, Marco Maria D’Andrea5, Maria Lina Mezzatesta6, Franco Baldelli1 and Antonella Mencacci7
1 Unit of Infectious Diseases, Department of Medicine, University of Perugia, Perugia, Italy
2 Department of Health Science, University of Florence, Florence, Italy
3 Unit of Neurosurgery, Hospital Santa Maria della Misericordia, Perugia, Italy
4 Unit of of Internal Medicine, Department of Medicine, University of Perugia, Perugia, Italy
5 Department of Medical Biotechnologies, University of Siena, Siena, Italy
6 Department of Biomedical and Biotechnological Sciences, University of Catania, Catania Italy.
7.Unit of Microbiology, Department of Experimental Medicine and Biochemical Sciences, University of Perugia, Perugia, Italy
Corresponding author: Elisabetta Schiaroli, MD.
Unit of Infectious Diseases. Department of Medicine. University of
Perugia, Perugia, Italy. Hospital "Santa Maria della Misericordia".
Piazzale Menghini, 1 – 06156, Perugia, Italy. Tel: +39-075-5784375 Fax:
+39-075-5784346 . E-mail:
elisabettask@libero.it
Published: 11 October, 2015
Received: June 19, 2015
Accepted: September 4, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015055, DOI
10.4084/MJHID.2015.055
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
This paper reports on a 71-year-old
Caucasian male who underwent neurosurgery for an oligodendroglioma,
followed by a cranial-sinus fistula and cerebrospinal fluid rhinorrhea.
The clinical course was complicated due to an extensively
drug-resistant Acinetobacter baumannii meningitis. The patient was
treated with colistin methanesulfonate, intrathecal for 24 days and
intravenous for 46 days. In addition, the patient received meropenem
and teicoplanin to treat a urinary tract infection and a bacterial
aspiration pneumonia. Cerebrospinal fluid trough colistin levels
resulted above the MIC of A. baumannii. Colistin cerebrospinal fluid
concentration did not increase over the treatment period. Meningitis
was cured and A. baumannii eradicated. No side effects from the
antimicrobial therapy were observed.
In conclusion, this case
highlights the issues in treating infections caused by resistant Gram
negative bacteria and supports previous findings on the efficacy,
pharmacokinetic and tolerability of intravenous and intrathecal
colistin treatments.
|
Introduction
Over the last decade, extensively drug- resistant Gram-negative bacteria, including Acinetobacter baumannii, have become a serious cause of hospital-acquired infections. A. baumannii
has also emerged as a cause of central nervous system (CNS) infections,
which are often associated with the use of external cerebrospinal fluid
(CSF) catheters.[1-3] The treatment of these
infections can often be extremely complex due to antimicrobial
resistance and the inadequate antimicrobial concentration at the site
of infection.[1-6] The increased rate of infections
due to multi drug-resistant Gram-negative bacteria has been reported to
lead to a revival in the use of “forgotten” antibiotics, such as
colistin.[4,5]
Colistin, a polymyxin antibiotic
that is administered intravenously (IV) as colistin methanesulfonate,
is a prodrug that is converted in vivo and in vitro into its active
form colistin.[4,6] Colistin
methanesulfonate and colistin poorly cross the brain blood
barrier,[4,6] thus in order to treat CNS infections, colistin
methanesulfonate needs to be administered either intrathecally (IT) or
intraventricularly (IVT).[3,6]
The authors report on a case of meningitis caused by multi-drug resistant A. baumannii treated with IV and IT colistin.
Case Report
A 71-year-old Caucasian male was admitted to our hospital with
cerebrospinal fluid rhinorrhea one month after having undergone
neurosurgery for an oligodendroglioma. Ten days after admission (Table 1), the patient manifested acute meningitis caused by methicillin-resistant Staphylococcus aureus (MRSA) and Corynebacterium striatum,
treated with vancomycin IV 750 mg TID and imipenem IV 500 mg QD. At the
same time, the cranial-sinus fistula was repaired. The clinical course
was complicated by pneumonia and acute respiratory insufficiency
requiring assisted mechanical ventilation (Table 1).
A week later, the patient was extubated and re-admitted to the floor.
The following day, the patient manifested a low-grade fever and blood
tests evidenced increased leukocyte and neutrophil counts; whereas the
C-reactive protein (C-RP) and erythrocyte sedimentation rate (ESR)
values were 15.4 mg/dL (normal <0.5mg/dL) and 8 mm 1st
h (normal 1-30), respectively. Due to a persistent drowsiness and a
suspected hydrocephalus, an external CSF lumbar catheter was
positioned. The CSF from the catheter resulted having normal cell and
glucose values, and the microbiological investigations were negative.
Additionally, Enterobacter cloacae urinary infection was treated with meropenem (Table 1).
Five days later, the patient’s temperature rose to 38.8°C, the
leukocyte, neutrophil, C-RP and ERS values also increased, the patient
manifested a more depressed level of consciousness and the patient
complained of neck stiffness. Simultaneous CSF findings from the lumbar
catheter were consistent with acute Gram-negative bacterial meningitis.[7] In addition, the SeptiFast real-time PCR (SF) (Roche Diagnostics, Monza, Italy)[8] performed on the CSF sample from the lumbar catheter resulted positive for A. baumannii and K. pneumoniae,
while the CSF mass spectrometry by matrix-assisted laser
desorption/ionization time-of-light (MALDI-TOF) (Bruker Daltonics,
Bremen, Germany)[9] was negative. CSF culture yielded A. baumannii and a few colonies of K. pneumoniae,
both susceptible only to colistin. Antimicrobial therapy was
administrated: intravenous colistin methanesulfonate 4.500.000
International Unit (IU) (equal to 150 mg of colistin based activity)
BID (infused over 30’), meropenem 2 g TID, rifampin 600 mg OD and
teicoplanin 600 mg OD after the loading dose plus colistin
methanesulfonate IT 125.000 IU (equal to 4.16 mg of colistin based
activity) a day.[3,10] Rifampin had
to be discontinued soon after due to an allergic reaction. Two days
later, after three doses of IV colistin and a single dose of IT
colistin, a repeated culture of CSF, from both the lumbar catheter and
rachicentesis, evidenced A. baumannii.
Whenever IT colistin was administered (range of time ± 4h), the
catheter was kept closed for 3 hours after. CSF samples for laboratory
investigations and concentrations were collected from the lumbar
catheter before colistin was administered. Colistin concentrations were
evaluated on samples (stored at -20°C until testing) using an HPLC
method having fluorimetric detection and netilmicin as an internal
standard. Linear calibration curves were obtained by the concentrations
of colistin sulfate from 0.30 to 5.0 mg/L in plasma.[11]
On day four of therapy, the patient was without fever, CSF cell count
was decreased, and the culture resulted negative. After a total of 24
days of therapy, the lumbar catheter was removed, while a
lumbar-peritoneal catheter was positioned to treat a hydrocephalus that
had developed. Results of CSF findings are reported in Table 2.
IT colistin was discontinued while IV colistin, meropenem, and
teicoplanin were continued for a further 22 days, followed by meropenem
3 g and oral doxycycline 200 mg per day for another 11 days. During
this period, the patient was without fever but multiple episodes of
acute respiratory insufficiency occurred, along with alternatively
reduced or increased neutrophils values, C-RP values, and lung
infiltrates. Repeated bronchoscopic aspirations were performed, and a
percutaneous endoscopic gastrostomy (PEG) was positioned (Table 1). Repeated respiratory secretion cultures evidenced MRSA and K. pneumoniae resistant to colistin, but fosfomycin susceptible (Table 1). Despite fosfomycin therapy, the patient had a fatal episode of acute respiratory insufficiency leading to his death.
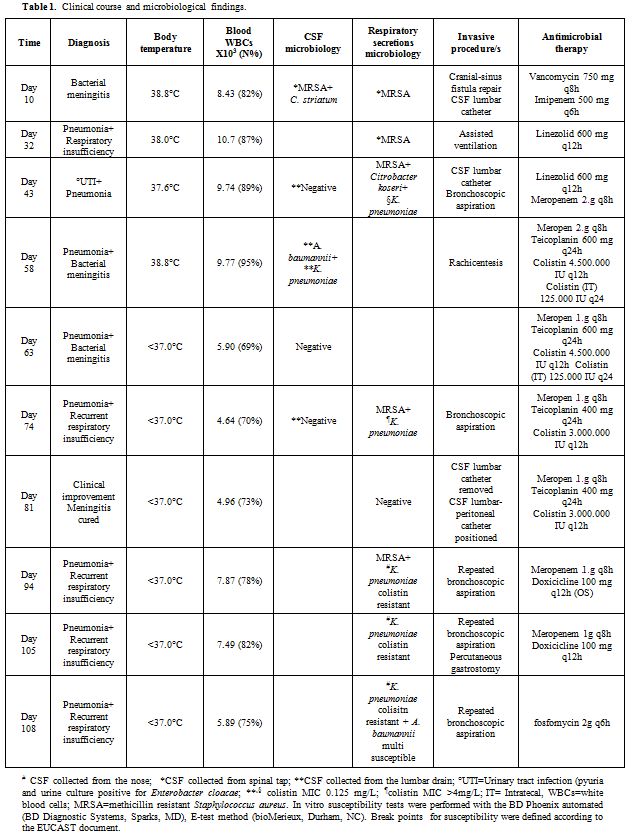 |
Table 1. Clinical course and microbiological findings. |
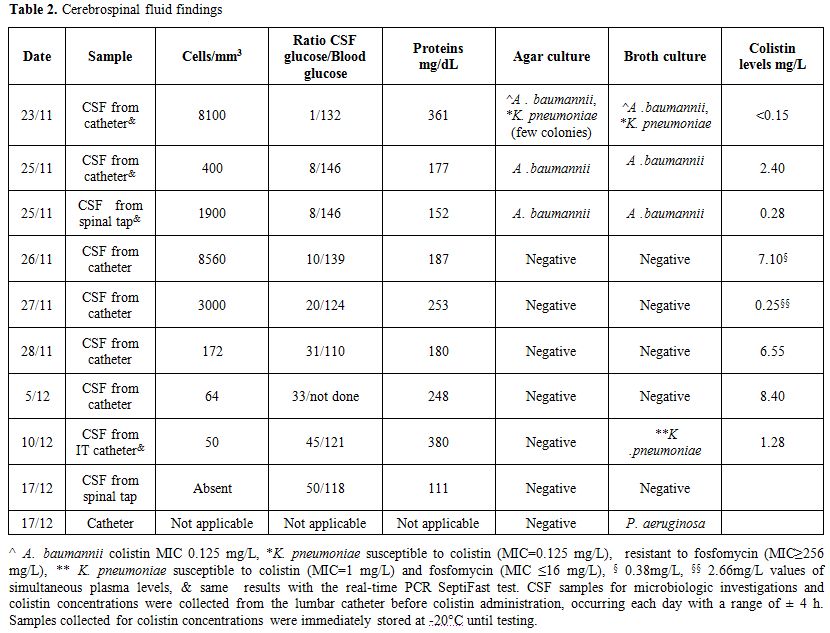 |
Table 2. Cerebrospinal fluid findings. |
Discussion
Over
the last decade, the frequency of CNS infections caused by
Gram-negative bacteria has increased from 12-27% of cases,[1-3] as well
as meningitis caused by A. baumannii. In our patient, clinical and microbiological findings supported a diagnosis of hospital acquired A. baumannii meningitis.[7] In fact, 1) A. baumannii
was detected by culture and SF in CSF samples obtained from both
rachicentesis and the lumbar catheter on the third day of treatment; 2)
A. baumannii DNA was detected by SF (data not shown) in the CSF from day 12 of treatment; 3) airways were colonized/infected with K. pneumoniae, leading us to deduce that the CSF could have been contaminated with this microorganism during collection. [3,7] To this regard, it is important to report that K. pneumoniae was cultured with A. baumannii from
the CSF taken on both day one when the patient had acute bacterial
meningitis and on day 17 of treatment when there was clinical
improvement and CFS laboratory parameters resulted normalized. Pseudomonas aeruginosa detected from the lumbar catheter was considered of no clinical relevance, given an absence of symptoms and CFS abnormalities.[7]
Culture
results, SF, and MALDI-TOF tests were performed. Microbial culture is
still considered the reference method for infection diagnosis. SF test
is a molecular based method used to detect bacteria from blood, but it
has also been applied to samples different from blood.[8]
One of the advantages of SF over culture is that the results of this
test can be available in less than 6 h, allowing for a prompt and more
accurate empiric therapy. Moreover, SF has a high sensitivity for
identifying microbial DNA in patients receiving antimicrobial therapy.[8]
However, the clinical significance of blood microbial DNA, even when
patients are septic in the absence of microorganism growth, is not well
defined.[12] As well, there are limited data on the reliability of performing SF on other biological samples.[8] MALDI-TOF is considered a reliable, rapid method for identifying bacterial strains from colonies on solid culture media[9]
and has also been employed to analyze clinical specimens such as urine
and CSF for direct bacterial identification. Nevertheless, for our
patient, the best results were obtained when the bacterial
concentration in the sample was ≥105CFU/mL.[9]Colistin
is defined as a concentration dependent bactericidal antibiotic,
therefore, according to Hara GH et al., peak levels seem to be more
predictive of clinical efficacy.[4] In vitro and in
vivo animal studies suggest that the area under the curve AUC/MIC and
Cmax/MIC ratio is the best predictor of antibacterial activity.
However, the pharmacodynamic parameters that best predict efficacy are
not well defined.[5,6] Overall, it has been suggested to maintain steady state levels ≥2 mg/L for effective therapy.[5,6]Considering the in vitro antimicrobial susceptibility of A. baumannii isolate
and the poor capacity of colistin methanesulfonate to cross the
blood brain barrier, colistin methanesulfonate was administered both
intravenously and intrathecally without a loading dose.[3,10] Overall, the treatment resulted being both effective and well tolerated. In
our patient, only trough CSF values were obtained, and on 3 different
days values below 2 mg/L were observed. The ratio between CSF
concentration and A. baumannii
MIC ranged between 2 and 70. This broad range could have been due to
the different collection times of CSF and/or CSF efflux fluctuations
through the external drainage.[6] Overall, the CSF colistin concentration did not increase over time, mirroring results by Imberti et. al.[6]
Regarding the Colistin blood levels without a loading dose, values
above ≥2 mg/L were registered on day 5 of therapy and a lower colistin
concentration in the respiratory secretions most likely favored the
selection of Colistin hetero-resistant K. pneumoniae isolates. Considering in vitro susceptibility results of K. pneumoniae
isolates to fosfomycin, it is plausible that the variable results
reported from our laboratory were due to MIC being close to the
susceptibility break point. When these K. pneumoniae
isolates were evaluated at a reference laboratory they were reported as
susceptible to fosfomycin, suggesting that laboratory fluctuation could
have induced variable susceptibility results. Furthermore, PFGE
analysis of these isolates showed an analogous pattern which was
similar to the international blaKPC-3-positive ST258b hybrid clone
(data not shown). It
has been suggested that CSF catheters need to be removed in order to
achieve recovery from a CNS infection. However, the exact time of CSF
catheter removal has yet to be clearly defined.[3,10]
In our case, the infection was controlled, and CSF cultures were
negative after 4 days of treatment, thus, we decided to keep the
external lumbar catheter in place. It was removed only after the
meningitis was cured, and a lumbar-peritoneal derivation could be
placed without a high risk of relapse. In
conclusion, this case highlights the issues involved in treating
infections caused by drug-resistant Gram-negative bacteria and supports
previous findings on the efficacy, pharmacokinetics and tolerability of
intravenous and intrathecal Colistin treatments.
Acknowledgments
We thank Thomas Charles Kilcline for his important editorial assistance.
References
- Wang KW, Chang WN, Huang CR, Tsai NW, Tsui HW, Wang
HC, Su TM, Rau CS, Cheng BC, Chang CS, Chuang YC, Liliang PC, Tsai YD,
Lu CH. Post-neurosurgical nosocomial bacterial meningitis in adults:
microbiology, clinical features, and outcomes. J Clin Neurosci
2005;12:647-650. http://dx.doi.org/10.1016/j.jocn.2004.09.017 PMid:16023857

- Kim
B-N, Peleg AY, Lodise TP, Lipman J, Nation R, Paterson DL. Management
of meningitis due to antibiotic-resistant Acinetobacter species. Lancet
2009;9:245-255. http://dx.doi.org/10.1016/S1473-3099(09)70055-6

- Karaiskos
I, Galani L, Bazika F, Katsouda E, Ioannidis I, Andreou A, Paskalis H,
Giamarellou H. Successful treatment of extensively drug-resistant
Acinetobacter baumannii ventricultitis and meningitis with
intraventricular colistin after application of a loading dose: a cases
series. Intern J Antimicrob Agent 2013;4:480-483. http://dx.doi.org/10.1016/j.ijantimicag.2013.02.010 PMid:23566531

- Hara
GH, Gould I, Endimiani A, Pardo PR, Daikos G, Hsueh PR, Mehtar S,
Petrikko G, Casellas JM, Daciuk L, Paciel D, Novelli A, Saginur R,
Pryluka D, Medina J, Savio E. Detection, treatment, and prevention of
carbapenemase-producing Enterobacteriaceae: recommendations from an
International Working Group. J Chemother 2013; 25:129-140. http://dx.doi.org/10.1179/1973947812Y.0000000062 PMid:23783137

- Daikos
GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Agrypoulou A,
Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis
A, Goukos D, Skoutelis A. C. Carbapenemase-producing Klebsiella
pneumoniae bloodstream infections: lowering mortality by antibiotic
combination schemes and the role of carbapenems. Antimicrob Agents
Chemother 2014;58:2322-2328. http://dx.doi.org/10.1128/AAC.02166-13 PMid:24514083 PMCid:PMC4023796

- Imberti
R, Cusato M, Accetta G, Marinò V, Procaccio F, Del Gaudio A, Iotti GA,
Regazzi M. Pharmacokinetics of colistin in cerebrospinal fluid after
intraventricular administartion of colistin methanesulfonate.
Antimicrob Agent Chemother 2012;56: 4416-4421. http://dx.doi.org/10.1128/AAC.00231-12 PMid:22687507 PMCid:PMC3421567

- Horan
TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health
care-associated infection and criteria for specific types of infections
in the acute care setting. Am J Infect Control 2008;36:309-332. http://dx.doi.org/10.1016/j.ajic.2008.03.002 PMid:18538699

- Mencacci
A, Leli C, Cardaccia A, Montagna P, Moretti A, Bietolini C, Meucci M,
Perito S, Cenci E, Bistoni F. Comparison of conventional culture with
SeptiFast real-time PCR for microbial pathogen detection in clinical
specimens other than blood. J Med Microbiol 2011;60:1774-1778. http://dx.doi.org/10.1099/jmm.0.034280-0 PMid:21835970

- Wang
MC, Lin WH, Yan JJ, Fang HY, Kuo TH, Tseng CC, Wu JJ. Early
identification of microorganisms in blood culture prior to the
detection of a positive signal in the BACTEC FX system using
matrix-assisted laser desorption/ionization–time of flight mass
spectrometry. J Microbiol Immunol Infect 2013;
doi:10.1016/j.jmii.2013.10.006. http://dx.doi.org/10.1016/j.jmii.2013.10.006

- Van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med 2011;362:146-154. http://dx.doi.org/10.1056/NEJMra0804573 PMid:20071704

- Li
J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. A simple
method for the assay of colistin in human plasma, using pre-column
derivation with 9-fluorenylmethyl chloroformate in solid-phase
extraction cartridges and reversed-phase high-performance liquid
chromatography. Chromatogr B Biomed Sci Appl. 2001;761:167-175. http://dx.doi.org/10.1016/S0378-4347(01)00326-7

- Chang
S-S, Hsieh W-H, Liu T-S, Lee SH, Wang CH, Chou HC, Yeo YH, Tseng CP,
Lee CC. Multiplex PCR system for rapid detection of pathogens in
patients with presumed sepsis-a systematic review and meta-analysis.
PLos ONE 2013;8:e62323-62333. http://dx.doi.org/10.1371/journal.pone.0062323 PMid:23734173 PMCid:PMC3667030
















