Bacterial Infections Following Splenectomy for Malignant and Nonmalignant Hematologic Diseases
Giuseppe Leone1 and Eligio Pizzigallo2
1Istituto di Ematologia, Università Cattolica del Sacro Cuore, Roma. 2Università “G. d’Annunzio”, Chieti. (Italy)
Published: October 13, 2015
Received: September 25, 2015
Accepted: October 3, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015057, DOI
10.4084/MJHID.2015.057
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Splenectomy, while often
necessary in otherwise healthy patients after major trauma, finds its
primary indication for patients with underlying malignant or
nonmalignant hematologic diseases. Indications of splenectomy for
hematologic diseases have been reducing in the last few years, due to
improved diagnostic and therapeutic tools. In high-income countries,
there is a clear decrease over calendar time in the incidence of all
indication splenectomy except nonmalignant hematologic diseases.
However, splenectomy, even if with different modalities including
laparoscopic splenectomy and partial splenectomy, continue to be a
current surgical practice both in nonmalignant hematologic diseases,
such as Immune Thrombocytopenic Purpura (ITP), Autoimmune Hemolytic
Anemia (AIHA), Congenital Hemolytic Anemia such as Spherocytosis,
Sickle Cell Anemia and Thalassemia and Malignant Hematological Disease,
such as lymphoma. Today millions of people in the world are
splenectomized. Splenectomy, independently of its cause, induces an
early and late increase in the incidence of venous thromboembolism and
infections. Infections remain the most dangerous complication of
splenectomy. After splenectomy, the levels of antibody are preserved
but there is a loss of memory B cells against pneumococcus and tetanus,
and the loss of marginal zone monocytes deputed to immunological
defense from capsulated bacteria. Commonly, the infections strictly
correlated to the absence of the spleen or a decreased or absent
splenic function are due to encapsulated bacteria that are the most
virulent pathogens in this set of patients. Vaccination with
polysaccharide and conjugate vaccines again Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis
should be performed before the splenectomy. This practice reduces but
does not eliminate the occurrence of overwhelming infections due to
capsulated bacteria. At present, most of infections found in
splenectomized patients are due to Gram-negative (G-) bacteria. The
underlying disease is the most important factor in determining the
frequency and severity of infections. So, splenectomy for malignant
diseases has the major risk of infections.
|
Introduction
A 73-year-old man, affected by
splenic lymphoma with massive splenomegaly underwent to elective
splenectomy at seventy. He has been suffering from splenic lymphoma for
ten years and on therapy with Chlorambucil and Rituximab; the
indication for splenectomy was an enormous spleen resistant to chemo -
immunotherapy and a mild thrombocytopenia.[1] He received the 23-valent
pneumococcal polysaccharide vaccine (PNEUMOVAX 23) after surgery that
he repeated two years later. Three years after his surgery, he calls
his primary care doctor because he has fever and cephalgia. What is the
appropriate management? Patients splenectomized for a hematologic
disease are at major risk than subjects splenectomized for trauma?
Which prophylactic measures and which therapy are indicated?
Splenectomy,
while often necessary in otherwise healthy patients after major
trauma,[2-3] find its primary indication for patients with an
underlying malignant or nonmalignant hematologic diseases (Table 1).[2-12]
Rarely spleen rupture can occur spontaneously, more frequently in a
pathological spleen for infectious or/and hematologic diseases[13-17]
and in patients on anticoagulation.[18] People without risk factors or
previously diagnosed disease can, even if rarely,[19] undergo to
splenic rupture for minor trauma o if treated with high dose of growth
factors for stem cell harvest.[19] Furthermore, functional asplenia,
due to auto infarction, frequently develops in subjects with sickle
cell anemia[16] Also, hyposplenism states are common in patients with
chronic graft-versus-host disease after stem-cell transplantation,
severe celiac disease, and untreated human immunodeficiency virus
infection.[20]
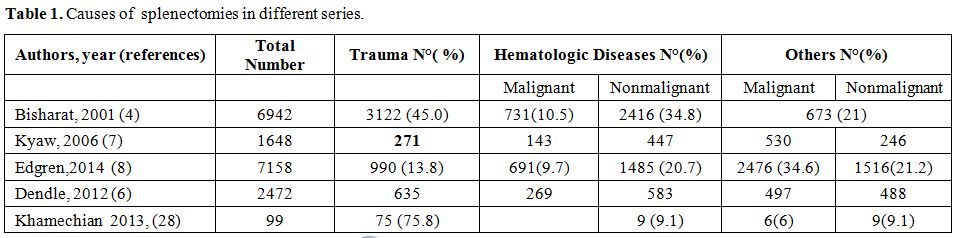 |
Table 1. Causes of splenectomies in different series.
|
Indications of splenectomy for hematologic diseases have
been reducing in the last few years, due to improved diagnostic and
therapeutic tools (Figure 1,2).[8]
Reduction of splenectomy is even more evident after trauma since
splenic preservation has become a well-reported and accepted
principle.[8,21] Splenectomy for cancer staging is infrequently
performed,[2] and no longer requested for Hodgkin Disease (HD) staging,
as in the past,.[22] The introduction of rituximab has reduced the
necessity of splenectomy for some lymphoproliferative diseases,[1]
hemolytic anemia and ITP.[12] At present the splenectomy sometimes can
also be avoid by treating resistant ITP patients with
thrombopoietin-receptor agonists.[12,23] All these data infer that the
indications for splenectomy continue to evolve, with a progressive
reduction, more evident after trauma and in malignant hematologic
diseases.
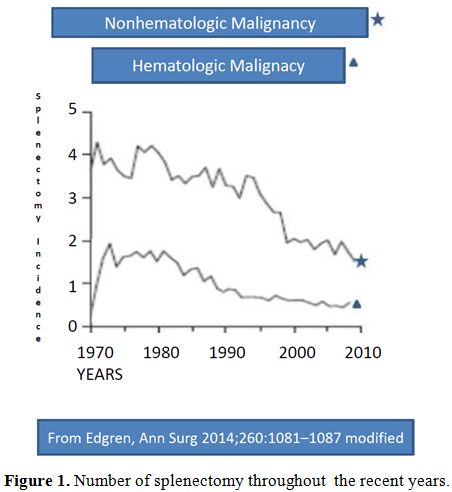 |
Figure
1. Number of splenectomy throughout the recent years. |
|
|
Figure
2. Number of splenectomy throughout the recent years. |
However,
splenectomy, even if with different modalities including laparoscopic
splenectomy and partial splenectomy,[24,25] continue to be a current
surgical practice. Approximately 25,000 surgical splenectomies are
performed annually in the United States;[26] and, the total number of
asplenic persons in the United States is currently estimated at 1
million, including 70,000 to 100,000 persons with sickle cell
disease.[27] Data in the other countries are not available. In clinical
practice splenectomy is performed worldwide for different reasons
according to the prevalence of different pathologies, circumstances and
availability of drugs, found in every country (Table 1).
In
high-income countries, like USA, Australia, Europe at present, the
proportion of splenectomy secondary to trauma represents the 15-30% of
all cases (Table 1).[2,5,7,8]
This percentage is lowering, Some years ago (2001) Bisharat reported a
percentage of splenectomy due to trauma in 50% of adults and 30% of
children.[4] However, in high-income countries there is a clear
decrease over calendar time in the incidence of splenectomy for all
indications except nonmalignant hematologic diseases (Figure 1 and 2).[8]
In the low-income country and war period, the proportion of trauma splenectomy could be higher.
Khamechian[28] report in Iran a percentage of 75% of trauma splenectomy and Deodhar report similar results in India (Table 1).[29]
Among
the non-traumatic splenectomy hematologic indications are prevalent but
differ in the various countries and the different series. In Europe and
USA the prevalent indications of splenectomy are represented by the
lymphoproliferative diseases (more frequently in the hospitals with
prevalent oncological patients, such as the Memorial Sloan-Kettering
Cancer Center, New York, USA[9] and by the ITP (more frequently in the
General Hospitals, as reported by two important series of American
College of Surgeons[10] and by the Swedish Study.[8] In Asia and in
Africa hemoglobin disorders are the prevalent indication for
splenectomy (Table 2).[30,31]
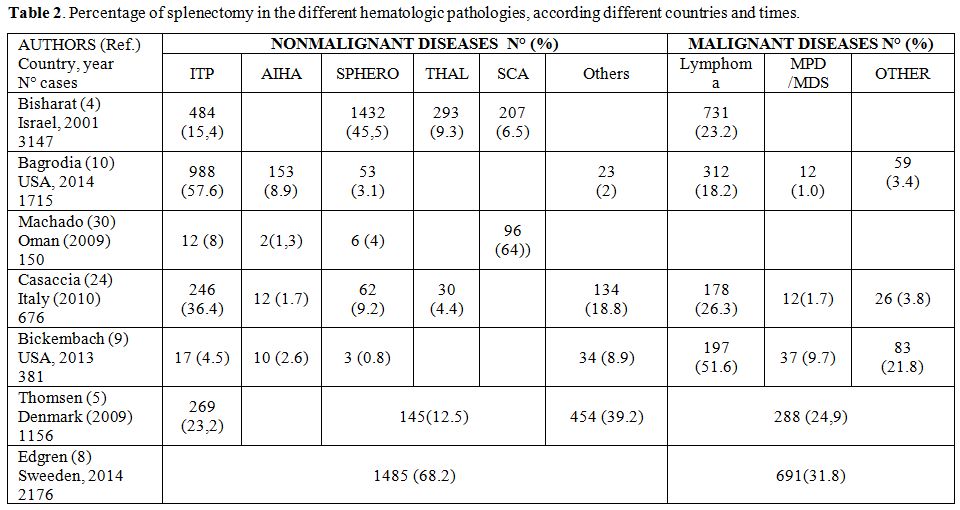 |
Table 2. Percentage of splenectomy in the different hematologic pathologies, according different countries and times. |
Programmed splenectomy is made more and more frequently by
laparoscopy, which is mostly utilized for benign spleen-related
diseases.[24,32] However at variance with European and USA experience,
in the Chinese and Asian series, portal hypertension and hypersplenism,
secondary to cirrhosis is an important cause of splenectomy.[32,33] The
study of Wang et Coll.[32] retrospectively reviewed 302 consecutive
patients who underwent laparoscopic splenectomy. 65% of patients had a
benign spleen-related disease, 14% a malignant spleen-related disease
and 21% portal hypertension. In a similar Italian series portal
hypertension does not appear as cause of splenectomy.[24]
Complications of Splenectomy
Splenectomy,
independently of its cause, induces an early and late increase in the
incidence of venous thromboembolism and infections.[4-12,34,35] The
previous pathology influences the incidence of both
complications.[35,36] Therefore, the comparison should be made with a
matched indication cohort.[5] However, in any case, infection remains
the most noxious complication of splenectomy.[4,8,34,35]
Infections
Commonly, the infections strictly correlated to the absence of the
spleen or a decreased or absent splenic function are due to
encapsulated bacteria that are the most virulent pathogens in this set
of patients.[2-8] They can produce a serious fulminant illness, called
overwhelming post-splenectomy infection (OPSI), that carries a high
mortality rate.[4,8,36,37,38] However, in the years, the bacterial
pattern of splenectomy sepsis have been changing. The most important
capsulated pathogen is Streptococcus pneumoniae (Str. Pneumoniae), but Haemophilus influenza (H. Influenzae) and Neisseria meningitidis (N. meningitidis)
are also significant. In a study of 1991,[36] reporting 349 episodes of
sepsis in patients with asplenia, 57% of infections and 59% of deaths
were caused by Str. pneumoniae. Furthermore, 6% of infections were caused by H. influenzae, with a mortality rate of 32%; N. meningitidis
was the organism in 3.7% of cases in the same study.[38] Today, after
the introduction of vaccination, and oral penicillin antibiotics,
patients submitted to splenectomy can suffer from disparate strains of
bacterial infection, which are not strictly correlated with the splenic
function. In fact, particularly in the post-intervention phase, the
type of bacteria isolated in the blood is not so different from those
found in other abdominal interventions. So, gram- bacteria are
prevalent (51% in the Australian report).[6,8,38] At present in
vaccinated patients, the rate of sepsis by pneumococcus is very low. In
fact, encapsulated bacteria, such as pneumococcus, meningococcus, and H. influenzae, were rarely encountered in Australian and Danish cohort Series,[6,11,38] in whom vaccination was routinely adopted.However,
the infection from capsulated bacteria continue to be important because
vaccination does not cover all bacterial strains and assumes a
particular virulence in patients with absent or reduced splenic
function. OPSI, also today, has a mortality of 30-60%.[37] Sepses by
uncommon bacteria[39-40] as well by protozoa infections such as malaria
and babesiosis are also known to affect asplenic patients.[41-43]Why the asplenic patients are so sensitive to encapsulated organism? The
spleen was once considered unnecessary for life; however, it clearly
serves extremely important hematologic and immunologic functions.
Spleen function consists of several aspects, according to the three
anatomical splenic subunits: (a) the white pulp, containing B-cell
follicles, (b) the marginal zone (MZ), containing specialized
macrophages and memory B-cells, and (c) the red pulp, where
erythrocytes are filtered from the circulation by entrapment in the
splenic cords and subsequent phagocytosis, as well as by retention
through receptor–ligand interaction.[44]The
white pulp contains a large mass of lymphoid tissue and serves a vital
role in the recognition of antigens and production of antibodies. The
red pulp of the spleen consists of a tight meshwork of sinusoids, the
cords of Billroth, which primarily serve hematologic functions,
especially filtration of the blood. The milieu of the red pulp is
relatively acidic and hypoglycemic. Therefore aged or damaged red cells
not able to tolerate this harsh environment are ultimately removed by
splenic macrophages.[44] Particulate matter is also removed from red
cells as they pass through the splenic sinusoids, and so “polished” or
“conditioned” red cells, free of surface imperfections, come back to
the bloodstream. The red pulp also acts as a reservoir for
approximately one-third of the total platelet mass and a smaller
proportion of granulocytes. Both
lymphocytes and monocytes present in the spleen are important to assure
a complete immunological defense. MZ B cells have a unique ability to
produce natural antibodies and can initiate T-cell–independent immune
responses to infections or vaccination with capsular polysaccharide
antigens. In fact, the human immunoglobulin M memory B cells
controlling Str. pneumoniae
infections are generated in the spleen.[45-54] After splenectomy, the
levels of antibody are preserved but there is a loss of memory B cells
against pneumococcus and tetanus.[51] The fundamental rule of splenic
monocytes in the immunological defense from capsulated bacteria should
be always taken in consideration.[54-55]The
most conspicuous macrophage populations of the spleen are located in
the marginal zone and adorned with unique sets of pattern recognition
receptors. The MZ is a strategically positioned in the bloodstream and
contains both macrophages and memory B cell.[46] The macrophage subsets
present in the spleen marginal zone show various pathogen receptors on
in the recognition and elimination of certain pathogens, in particular,
encapsulated bacteria.[55,56] It is noteworthy that complement defects
induce streptococcal and meningococcal infections very similar to that
found in splenectomized subjects.[57] Complement system, such as C1q
and C3, and macrophages in the splenic marginal zone (sMZ) play pivotal
roles in the efficient uptake and processing of circulating apoptotic
cells. SIGN-R1, a C-type lectin that is highly expressed in a
subpopulation of MZ Macrophages, regulates the complement fixation
pathway by interacting with C1q, to fight blood-borne Streptococcus
pneumoniae.[57-59] SIGN-R1+ macrophages are critical for the uptake of
circulating apoptotic cells in the MZ and are essential for Str. pneumoniae clearance.[55-57]In
conclusion, the specific role in the removal of encapsulated bacteria
is related to marginal zone macrophages, which can detect and capture
encapsulated bacteria.[54-57] In addition, marginal zone cells respond
to capsule polysaccharide antigens by differentiating into
IgM-producing memory B cells or antigen presenting cell.[56-57]At
present splenectomy is performed both in subjects with and without a
previous pathology. Therefore, we firstly treat the infections of
healthy people splenectomized as a consequence of trauma, considering
them as a control group. Accordingly the literature[2-12] we make an
important distinction between the early post intervention infections
and the late infections. Afterward, we consider pathology by pathology
the different hematologic groups requiring splenectomy. In fact, the
previous pathology does influence the rate, the type and the severity
of the early as well the late infections.
Early Infections
Infections
related to splenectomy can occur early in direct association with
intervention (post-operative infectious complications) and late in
connection only with the reduced immunological defense induced by
splenectomy. Infective complications account for most of the
perioperative morbidity and include lower respiratory tract infections,
intra-abdominal collections, wound infection and non-specific
infections requiring antibiotics.[5-10] The Danish series[5] reports
3812 persons who underwent splenectomy from 1996 to 2005. The maximum
relative risk of infection and death was within the first 90 days of
intervention, attaining a RR of about 20 fold higher in all indication
groups than in the general population comparisons, whereas odds ratios
in comparison with appendicectomized patients ranged from 1.0 to 12.7.
The
distribution of microbial agents was similar between groups. Of note,
encapsulated bacteria, such as pneumococci, meningococci, and H. influenzae,
were rarely encountered in the splenectomized cohort, recently reported
in the west countries.[5,11] Similarly the adjusted relative risk (RR)
and 95% confidence interval (CI) of death among splenectomized patients
by indication, compared to the general population of Denmark, was the
highest in the first 90 days, attaining a RR of 33-fold. However,
although splenectomized patients have a high risk for infection, this
risk is different in the various subgroups, and some degree seems due
to underlying conditions and not to splenectomy alone. (Figure 3)
The risk of death within the first 90 days ranges from 2,5% in patients
splenectomized for ITP to 10% in patients with hemopoietic cancer or
trauma.[5] Older age can also be an important factor in increasing
infection morbidity and mortality in the post-intervention period in
elective splenectomy of hematologic patients.[6,12]
|
|
Figure
3. Relative risk of infections after splenectomy with different matchings. |
Heuer[59]
report the Germany experience of 1,630 patients with a splenic injury,
whose, 758 patients undergoing splenectomy compared with 872
non-splenectomized patients. 96 (18.3%) of the patients with
splenectomy and 102 (18.5%) without splenectomy had an apparent
infection after the operation. Additionally, there was no difference in
mortality (24.8% versus 22.2%) in both groups. Patients with minor
trauma take advantage from conservative treatment, at contrary patients
with major trauma take advantage from splenectomy. It is important to
note that the perioperative sepsis rate was the same in both groups.[59]
Bickenbach
et al.[9] report in 2013 the MD Anderson experience of 381 patients,
who underwent splenectomy for diagnosis or treatment of hematological
diseases. Overall 136 patients (35.7 per cent) experienced
complications. Independent predictors of any morbidity on multivariable
analysis were age more than 65 years, KPS score 60 or less, and
hemoglobin level 9 g/dl or lower. The complications in this series were
mainly infectious (41,9 %), and the majority of the deaths were
directly related to infections. The microorganisms involved in the
infections were not cited.
Barmparas et al.[34] compared 2 groups
of patients submitted to abdominal surgery including or not
splenectomy. In a series of 493 patients submitted to abdominal
surgery, 33 underwent to splenectomy too, the two groups were well
balanced for age. Patients undergoing splenectomy were more likely to
have sustained a traumatic injury (30% vs. 7%, p < 0.01). After
adjustment, splenectomy was associated with increased risk for
infectious complications (49% vs. 29%, Adjusted Odds Ratio (AOR) [95%
CI]: 2.7 [1.3, 5.6], p <0.01), including intra-abdominal abscess (9%
vs. 3%, AOR [95% CI]: 4.3 [1.1, 16.2], p < 0.03). On a subgroup
analysis, there were no differences between traumatic and elective
splenectomy with regards to overall infectious complications (50% vs.
46%, p = 0.84), although, abdominal abscess developed only in those who
had an elective splenectomy (0% vs. 12%, p =0.55). The authors
concluded that splenectomy increased the risk for postoperative
infectious complications. In fact, even when the intra-abdominal
diseases were eliminated, splenectomy increased the risk for early
overall infectious complications and postoperative intraperitoneal
abscess. However the increase the post-intervention infections could
not induce a significant increase in early mortality.[58,59] In adult
patients the early mortality raises with age6 particularly in patients
with hematologic neoplasms.[9]
The laparoscopic approach to
splenectomy is clearly superior to standard laparotomy in terms of
postoperative complications, including infections,[60] although the
rate of OPSI remains similar in early as well in late phase.[37,60] In
fact, most of these early post-splenectomy bacteremia was caused by Enterobacteriaceae, Pseudomonas spp and Staphylococcus spp, and occurred mostly in patients with gastrointestinal malignancies while Str. pneumoniae caused only a few.[6,7,8]
In
conclusion, it seems that the splenectomy does not significantly
influence the type of the early infection that is mostly related to
surgical trauma. Laparoscopic approach reducing surgical trauma reduces
infections rate and early mortality.
Late Infections
PATIENTS SPLENECTOMIZED AFTER TRAUMA WITHOUT A PREVIOUS PATHOLOGY.
Patients
without a previous pathology are splenectomized because of trauma, and
rarely for spontaneous rupture or after G-CSF. The difference in the
incidence of bacterial infections could depend on the age at
splenectomy. The sepsis incidence and mortality is higher in children
than in adult,[4,5,8] the recent Swedish experience8 confirm the previous
data of Bisharat et al.[4] The incidence of Sepsis, expressed as
standardized incidence ratios (SIR), varied with age and
follow-up, with the highest SIRs among children. When restricting to
those who were splenectomized at the age of 0 to 12 years, the SIR was
higher: 6.1 (95% CI, 3.3–10), in respect of total population, SIR of
3.1 (95% CI, 2.1–4.3).
However in the adults the older age is a negative factor both in term of morbidity and mortality.[5]
The
Swedish experience[8] takes into consideration only the splenectomized
patients after 180 days from intervention, (20,132 patients), excluding
them who either died or were censored within 180 days of first
discharge.[8] The cumulative incidence of first hospitalization for or
death from sepsis varied both by indication and calendar year of
splenectomy. The overall 30-day mortality after a hospitalization for
sepsis was 17% (372 deaths after 2243 hospitalizations) and ranged from
13% for patients splenectomized for trauma to 22% for those
splenectomized for a hematologic malignancy. In all, there were 2243
hospitalizations for sepsis, corresponding to an overall nearly
six-fold increased risk of sepsis (SIR 5.7; 95% CI, 5.6–6.0). The risk
of a new hospitalization for sepsis varied by indication, with the
lowest risk among the trauma patients (SIR 3.4; 95% CI, 3.0–3.8) and
highest among the hematologic malignancy patients (SIR 18; 95% CI,
16–19). SIRs varied with age and follow-up, with the highest SIRs among
young patients, and in the earliest follow-up periods after the
splenectomy. The incidence of sepsis was higher in the first 2 years,
but it remain higher also after ten years .[7,10]
Kristnsson and
others[10] have reported infectious and thrombo-hemorrhagic complications
in American veterans, cancer free, submitted to splenectomy for
different reasons. No differences were found in term of infections
between patients splenectomized for trauma and those splenectomized for
hematological nonmalignant diseases. In the late follow-up infections
from capsulated bacteria in patients splenectomized after trauma become
prevalent in the veteran American series[9] but not in the Danish
series,[5] in which the percentage of Str. pneumoniae infection is only
4%.
In the American series splenectomized patients had a
significantly increased risk of pneumococcal pneumonia (RR=2,06,
meningitis RR=2,44 and septicemia 3.44), however, the risk of death is
particularly increased only from septicemia and meningitis. In Denmark,
pneumococcal vaccination is recommended within 2 weeks before elective
splenectomy, or a soon as possible and within less than 2 weeks after
emergent splenectomy, but no vaccination for H. influenzae is
recommended. Neither of two studies would give sufficient data about
the vaccination, even if both stressed the importance of vaccination.
In any case from epidemiological data, it is evident that there is a
reduction of Str. pneumoniae infections since the vaccination is
beginning to be a routine procedure in most countries.[6,7,38]
PATIENTS SPLENECTOMIZED FOR HEMATOLOGIC DISEASES
Nonmalignant Diseases
Splenectomy
also represents at present a key treatment option for the
treatment of many benign hematological diseases, including immune
thrombocytopenia (ITP), Auto Immune Hemolytic Anemia (AIHA) and
hereditary disorders associated with ongoing hemolysis (Spherocytosis,
Thalassemia major and intermedia, Sickle cell anemia).[11,12] In fact,
the number of patients splenectomized for hematological non-malignant
diseases remains stable and at present represent the most frequent
indication for splenectomy in high-income countries.8 However, among
the hematological non-malignant diseases with a sound indication
to splenectomy, we must distinguish the acquired diseases, ITP, AIHA in
which the autoimmunity play a fundamental role, and the congenital
forms, such as Spherocytosis and Hemoglobin disorders.
Immune Thrombocytopenic Purpura (ITP).
Although new drugs such as Rituximab and Thrombopoietin analogs have
been introduced in the treatment of ITP resistant to steroids, the
splenectomy remains the gold standard for the therapy of resistant
patients.[12] At present ITP represent in many western series the larger
indication to splenectomy.[5,8,10] Splenectomy remains the only treatment
that appears to have a long lasting effect in patients with
ITP.[12,61-63] Response rates are around 70% in children with chronic ITP
and 60 % in adults. The guidelines show considerable differences in
recommendations for splenectomy.[12,61] The more recent ASH guidelines[61]
recommend delaying surgery to after 12 months vs. six months as
recommended in the past.
Infections remain the major
contraindication to splenectomy in ITP, particularly in children.[12,61],
However, it is important to consider that also the immunosuppressive
agent increase the incidence of infection. Therefore, the comparison
should be made between resistant patients treated with the splenectomy
or those treated with immunosuppressive agents (Figure 3).[65,67,68]
In
a large series, Boyle et Al.[35] report a cohort of 9976 patients with
ITP; all patients were 18 years of age or older and had a diagnosis of
ITP, as the main disease, from January 1990 to November 2009. 1762 of
them underwent splenectomy.
The cumulative incidence of sepsis
was 11.1% among the ITP patients who underwent splenectomy and 10.1%
among the patients who did not. Splenectomy was associated with a
higher adjusted risk of sepsis, both early (HR 3.3 [CI, 2.4-4.6]) and
late (HR 1.6 or 3.1, depending on comorbidities). He concludes that ITP
patients post-splenectomy are at increased risk for abdominal venous
thromboembolism (AbVTE), venous thromboembolism, (VTE), and sepsis.
Sepsis
developed in 1016 cases: 191 splenectomized cases (cumulative incidence
11.1%) and 825 nonsplenectomized cases (cumulative incidence 10.1%),
with a median follow-up of 56 months. The cumulative incidence of early
sepsis after splenectomy (<90 days) was 2.6% and of late sepsis
(>90 days) was 8.8%. Among the splenectomy cases, the median time
from splenectomy to hospitalization with sepsis was 35.5 months (range,
0-219). In the multivariable model for sepsis, splenectomy was a
significant predictor of both early and late sepsis, with a more than
threefold higher hazard ratio (HR) for early sepsis (HR 3.3 [CI,
2.4-4.6]). For late sepsis, there was an interaction between
splenectomy and number of comorbidities. Cases with none or one
comorbidity had an HR of 1.6 (CI, 1.3-2.0), and for cases with 2 or
more comorbidities, the HR was 3.1 (CI, 2.2-4.4). There was also an
interaction between age and number of comorbidities. In addition to
splenectomy, age >60 years, the presence of comorbidities, the
male sex, and the African ethnicity, were also significant
predictors of sepsis.[35]
In a retrospective analyze, Vianelli et
al.[63] reported 233 ITP adult patients , who underwent splenectomy
between 1959 and 2001 in 6 European hematologic institutions and who
have now a minimum follow-up of ten years from surgery. Of the 233
patients, 180 (77%) achieved a complete response and 26 (11%) response.
Sixty-eight of 206 (33%) responsive patients relapsed, mostly (75%)
within four years from the first response. In 92 patients (39.5%),
further treatment was required after splenectomy that was effective in
76 cases (83%). In 138 patients (59%), the response was maintained free
of any treatment at last contact. Overall, 73 patients (31%)
experienced at least one infectious complication, for a total of 159
events, most often pneumonia (40%). Forty-three of these patients (59%)
had received prophylactic vaccinations. Median time from splenectomy to
the first infection was 35 months (range 0-355). Infectious
complications were significantly more frequent in refractory patients
compared to stable responders (P=0.004) but were comparable (P>0.05)
in vaccinated and non-vaccinated patients. Two fatal infectious
episodes (sepsis and intestinal infection) occurred, after 176 and 318
months from splenectomy. Both patients were stable responders to
splenectomy and were 78 and 80 years old.
Today to avoid
splenectomy and the consequent major infection rate, alternative
treatments are performed in patient with resistant ITP. In the last few
years, rituximab has been indicated as the first line treatment of
resistant ITP patients.[64] However, Rituximab is not free of side
effects.[65] Recently a study[65] assessed the safety in 248 adult patients
with immune thrombocytopenia (ITP) treated with rituximab. In total,
173 patients received four infusions of 375 mg/m2 and 72 received 2
fixed 1-g infusions two weeks apart. The authors observed 11 cases of
infection in 7 patients (3%; 95% CI, 1-6) corresponding to an incidence
of 2.3 infections/100 patient years (95% CI, 1.2-4.1). Infections
occurred 2 to 18 months after the first rituximab infusion. Eight cases
are recovered, but three patients died of infection 12 to 14 months
after the first rituximab infusion. These patients were older than 70
years, 2 had severe comorbidities (diabetes and peritoneal carcinosis),
and they had received prolonged treatment with corticosteroids for
refractory ITP. Theses series of patients was not vaccinated, and the
cause of infections was due to capsulated bacteria in two cases and
both recovered.
At present the French guidelines[67,68] recommend
the vaccinations against Streptococcus pneumoniae, Haemophilus
influenzae b (Hib) and Neisseria meningitidis not only before
splenectomy but also before rituximab in patients aged less than
65. However, also in France this vaccination was made in a small
proportion of patients (32.4%, 18.9%, and 3.8%.respectively).
Furthermore, it is worth of noting that advanced age and comorbidities
are the major risk factors for infections.[69]
The splenectomy was
considered particularly dangerous in children in the past, the risk of
fatal post-splenectomy sepsis was found to be severe especially in
children less than five years and during the first year the following
splenectomy.[4,5,7] The mortality rate of children is higher than of
adults.[8] The mortality risk is estimated to be of 3% in children.[66] The
infectious risk in children and adults splenectomized for ITP is
similar to that of children splenectomized after trauma.[4,5,8,11] The
increased risk compared with the general population persists for
life.[12] It is evident vaccinations does not eliminate post-splenectomy
sepsis. However even if there are limited comparative data on the
efficacy of vaccinations against encapsulated bacteria, is evident by
the recent epidemiological data that vaccination reduces the incidence
of infections by capsulated bacteria.[70-73] In fact, in more recent
publications,[70-72] when vaccinations for pneumococcus and meningococcus
are more and more becoming frequent, the capsulated infections are
becoming rarer. The Intercontinental Childhood ITP Study (ICIS) Group
Registry[71] reported 134 children splenectomized in 57 institutions of
25 countries over a period of 225.2 patient-years. Of the 134 children
in the ICIS Splenectomy Registry, 65 underwent a laparoscopic
procedure, and perioperative bleeding occurred in eight patients, three
of whom had laparoscopic splenectomy; four patients received packed red
blood cells, postoperative fever was reported in 9.7% without signs of
infection. This group signaled seven episode of sepsis (0.031 sepsis
episodes per patient-year), without a fatal outcome. In this study 21
patients were not submitted to vaccination, however of the seven
episodes of sepsis only one was found in not vaccinated patients. The
bacteria isolated was not reported in this paper but in the discussion
was affirmed that “Sepsis caused by encapsulated bacteria was rarely
encountered in patients on this Registry” independently of vaccination.
Similarly, Aladjidi et al.[72] conducted retrospective analysis
in 16 French departments involving 78 children with ITP and
splenectomy. Sixty-two children had chronic ITP of more than 12
months; laparoscopic splenectomy was utilized in 81% of children. Four
patients experienced postoperative complications: two severe
hemorrhages, one mesenteric thrombosis, and one pulmonary atelectasis.
All four patients with complications had preparation by at least one
platelet- enhancing treatment. Severe infections were not reported.
The
choice of splenectomy in children has also been also advocated for cost
problem.[73] In an American monocentric series of 22 patients from 2002
through 2009, only one child experienced overwhelming post-splenectomy
infection after a dog bite.[73] The authors conclude that earlier
surgical consultation for children with chronic ITP may be justified
given the high success rate and low morbidity, particularly given the
significant complication rate and cost of continued medical treatment.[73]
In
conclusion in children splenectomized for ITP pre-vaccinated then risk
of late sepsis is present but low, the etiology of capsulated bacteria
is rare. Data to make a comparison in children and adults with
resistant ITP treated with rituximab are scarce, and inconclusive.[73-80]
Liang
and Al.[74] report, in 2012, 11 studies (190 patients) on ITP resistant
treated with Rituximab. 78 patients (41.%) experienced adverse events.
The most frequently described adverse events were mild allergic
reactions and immediate hypersensitivity reaction during rituximab
infusion. Four patients developed infections that could be associated
with rituximab, including two patients with varicella, one patient with
pneumonia, and another patient with life-threatening enterovirus
meningoencephalitis. An increased incidence of bacterial infection is
also reported in adults treated with rituximab for autoimmune diseases;
the presence of diabetes and contemporary use or/and prednisone is a
further risk factor.[80,81] Hypogammaglobulinemia has also been reported
among adults and children,[76] although the overall number is unclear and
appears to occur with repeated doses and in patients with underlying
immune dysfunction. Studies have shown impaired humoral responses to
vaccination after rituximab.[82] However, bacterial infections are
reduced in vaccinated patients, and conjugate vaccine should be
preferred.[81,83] In conclusion, children and adult with further risk
factors should be vaccinated before the treatment with rituximab. This
approach is particularly requested if splenectomy is to be considered
in the future of the patient.[83,84]
In a summary, post-splenectomy
infections rate is increased 2-6-fold for first 90 d, and 2.5 (CI, 2.2
to 2.8) more than 365 days after splenectomy in adults with ITP versus
indication-matched controls;[7,12] however splenectomy for chronic ITP
has a risk of infection not different from subject splenectomized for
trauma.[11] The treatment with rituximab presents a similar risk of
bacterial infections. In both conditions, patients should be vaccinated
versus Str. pneumoniae, N. meningitidis and H. influenza.[83-84]
Autoimmune Hemolytic Anemia (AIHA).
Patients with AIHA resistant to steroids can be treated with
splenectomy or rituximab.[85-87] Response rates to splenectomy and
rituximab seem equivalent even if no prospective study comparing the
success rates of both approaches is available.[85-87] So, the side
effects are very important in the decision on the choice. In the GIMEMA
study, thrombotic events were more frequent in patients who had
undergone splenectomy (24% vs 8.7%) and grade 3 pulmonary infections
were associated with splenectomy but not with the number of lines of
treatment or with the use of rituximab.[86] In a recent metanalysis[88]
including nineteen studies, among 38 adverse events in 364 patients
were reported 4 neutropenias, 18 severe infections, including 1 viral
infection, and one Pneumocystis jiroveci pneumonia. In conclusion at
present in AIHA, the rituximab is increasingly considered the preferred
therapy of steroid resistant AIHA.
Hemolytic Spherocytosis (HS).
According British guidelines splenectomy should be performed in
children with severe HS, considered in those who have moderate disease,
and should probably not be performed in those with mild disease.[89] The
two major adverse events are thrombosis and infections.[89,90]
Immunization and prophylactic antibiotics could eliminate the increased
risk of catastrophic sepsis due to pneumococcus, meningococcus, or
haemophilus, and there is evidence that immunization and early use of
antibiotics forever have reduced the frequency of positive blood
cultures for pneumococcus in children who have had a splenectomy.[89]
Certainly a good compliance of the patients or their relatives to
accomplish post-splenectomy infection prophylaxis is fundamental in
reducing bacterial infections.[89,91] Accordingly, in a recent report of
the American splenectomy in congenital hemolytic anemia registry[91]
among 40 children 2-17 years of age splenectomized for spherocytosis,
the infections are relatively low: the rate of early infection was of
2,5 %. Regarding the late adverse events, there were no infections or
thrombotic events, and one reoperation (3.1%) over 1 year of follow-up.
About 75% of the children were vaccinated, and 97 underwent antibiotics
prophylaxis. Furthermore, the most of the patients were submitted to
laparoscopic splenectomy.
Sickle Cell Anemia.
(SCA). SCA is a hereditary hemolytic anemia due to a homozygous
mutation in the gene for β globin, a subunit of adult hemoglobin A
(HbA), that results in red blood cell deformity.[92] It is characterized
by recurrent vaso-occlusive episodes, accelerated hemolysis, increased
susceptibility to infection, and chronic end-organ damage.[92-94] Acute
splenic sequestration crisis (ASSC) is a life-threatening complication
of sickle cell disease that occurs secondary to trapping of deformed
cells in the splenic vasculature. The result is rapid splenic
enlargement, a compensatory elevation of the reticulocyte count, a
decrease in hemoglobin level, and potential shock. The mortality for
the first episode of ASSC is high particularly in developing countries,
approximately 10%, and sequestration can recur in most of the
patients.[93-96]These crises can occur as early as the first year of life
and can be precipitated by infections.[95] The consequence of these
vaso-occlusive episodes can be the a functional splenectomy, which can
occur within the first year of life.[96] Bacterial infections are one of
the main causes of morbidity and mortality in SCD in patients living in
both developed or developing countries.[93-95,97-99] However, the type of
bacteria could be different.[97-100] So the utility of vaccination for
capsulated bacteria in developing countries has been questioned.[98] This
increased susceptibility is mainly a result of impaired splenic
function. However other factors, such as defects in complement
activation, micronutrient deficiencies, tissue ischemia and
inflammation also contribute.[94,96] Surgical splenectomy seems do not
increase the burden of infections while preventing, if complete,
further sequestrations and if partial, reducing the recurrence of acute
splenic sequestration crises.[100-102] However, there is a lack of
evidence that splenectomy improves survival and decreases morbidity in
people with SCA.[101,102]
Splenectomy has been considered for a long
time at high risk of infections, early and late, and of death in
children and adult with SCA,[3,4] particularly in children of 4 years or
below. Recently, after the introduction of conjugate vaccines[103,104]
and prophylactic antibiotics,[105] splenectomy is considered in developed
countries feasible at all age with a moderate risk,[31,106,107] which, in
any case, is superior to that of other non-malignant hematological
diseases.[92] Data from low-income countries are scarce; splenectomy is
considered only in urgency, and then a comparison cannot be done.[108]
Thalassemia (Tha).
Splenectomy is recommended in transfusion-dependent Thalassemia to
reduce excessive blood consumption and consequent severe iron
overload.[109,110] Moreover, a variety of complications such as
pulmonary hypertension, silent brain infarcts, venous thrombosis, and
sepsis are linked to splenectomy. In particular infections are becoming
the leading cause of death in western countries due, in part, to a
significant reduction in the number of fatalities from iron-induced
cardiac diseases.[109] Therefore, physicians should keep a guarded
approach towards splenectomy because of the its side effects.. At the
current time, according the Guidelines for the Management of
Transfusion Dependent Thalassaemia,[109] splenectomy is not recommended
standard procedure in transfusion-dependent thalassemia (TDT) subjects.
Splenectomy should generally be avoided in Non TDT patients younger
than 5 years. Splenectomy should be reserved for cases of:
1° Worsening anemia leading to poor growth and development
2° When transfusion therapy is not possible or iron chelation therapy is unavailable
3°
Hypersplenism leading to worsening anemia, leucopenia, or
thrombocytopenia and causing clinical problems such as recurrent
bacterial infections or bleeding
4° Splenomegaly accompanied by symptoms such as left upper quadrant pain or early satiety
5° Massive splenomegaly (largest dimension >20 cm) with concern about possible splenic rupture
In
the past reports a high rate of bacterial infections has been reported
in splenectomized patients with thalassemia[4,109,110] with the
prevalence of sepsis by capsulated bacteria. Nowadays after the
widespread adoption of vaccination, the rate of infection is reduced,
and most of sepsis is due to Gram- bacteria and Staphylococcus
aureus.[100,111]
Overwhelming post-splenectomy infection (OPSI) by
capsulated bacteria have been reported frequently in the past in
children (11,6%) with a death of (7,4% ) and also if less commonly in
adults, (7.4%) with a death of 3,2%.[4] A recent Indian study reports a
rate of bacterial infection of 17% through 5 years. However, it did not
document any OPSI.[111] It is noteworthy that in this set of patients
Malaria was the most frequent post-splenectomy infection Comparisons of
the infection rate between thalassemia patients splenectomized or not
are rare. A comparative study made in Taiwan[112] between splenectomized
and nonsplenectomized thalassemia patients has been reported in 2003.
In this study, the infections were more frequent in splenectomized
patients. Notwithstanding the episodic prophylactic vaccination, most
of the bacterial infections were Gram negative with a prevalence of
Klebsiella pneumoniae, which was the most common causative organism in
this patient population (10 of 20 isolates). Other pathogens, more
frequenly isolated, were Pseudomonas aeruginosa and Vibrio
vulnificus. Recently Chirico et al.[113] assesses the
relationship between infectious events and splenectomized status, HCV
infection and serum HMGB1 in 51 adult thalassemia patients. Thirty-six
of them (70%) had undergone splenectomy before enrollment. All the
patients were vaccinated for capsulated bacteria. During the
observational period, 15 patients (29%) reached a primary study
endpoint, represented by infectious diseases, requiring hospitalization
or parenteral antibiotic administration. Klebsiella infection was
documented in 4 cases. Univariate analysis showed that hemoglobin,
serum ferritin, splenectomized status and serum HMGB1 values were
significantly associated with a primary study endpoint. Results from
Cox regression analysis indicated that serum HMGB1, as well as serum
ferritin and splenectomized status, predicted a higher risk of
infectious disease.
In the last few years the infections by
Yersinia, frequently reported in the last decade of the twenty century
and associated with an iron overload in transfusion dependent
thalassemia,[114,115] are no more signaled. The reduced frequency of
capsulated bacterial infections can be attributed to vaccinations and
widespread utilization of antibiotic prophylaxis. Furthermore, the use
of iron chelator could favor the growth of Klebsiella.[116] The utility
of vaccination and/or preservation of splenic function is undoubtable
as demonstrated by an attractive study of Sheikha and coll.[117]
Two
populations of patients from Iraq and Saudi Arabia underwent
splenectomy for thalassemia in the same period. All patients from Saudi
Arabia were given a preoperative pneumococcal vaccine, polysaccharide
pneumococcal vaccine (PPV 23), and underwent total splenectomy after
about four weeks. Unfortunately, vaccination was not possible to Iraqi
patients, so to this group partial splenectomy was offered to many of
these patients as a protective measure against Streptococcus pneumoniae
infection. Results: A significant difference was found between the
total splenectomy fatalities in the two groups. There were five deaths
in the 30 enrolled Iraqi patients over four years. One death over a
12-year period was reported in the 22 patients from Saudi Arabia.
Partial splenectomy was associated with a dramatic reduction of
mortality in the Iraqi patients. None of the 12 patients died during a
follow-up period of 4 years. Conclusions: PPV 23 is a powerful
prophylactic tool against overwhelming post-splenectomy infection in
patients with thalassemia and should be used whenever available. In
poor or problematic countries with limited health resources, partial
rather than total splenectomy could offer an alternative measure to
avoid this fatal complication.
Nonmalignant Lymphoid Disorders
Common variable immunodeficiency disorders.
Splenectomy has been used in patients with common variable
immunodeficiency disorders (CVID), mainly in the context of refractory
autoimmune cytopenia and suspected lymphoma.[118] Splenectomy proved to
be an effective long-term treatment in 75% of CVID patients with
autoimmune cytopenia, even in some cases when rituximab had failed.
Splenectomy does not worsen mortality in CVID, and adequate
immunoglobulin replacement therapy appears to play a protective role in
overwhelming post-splenectomy infections. Nine episodes of OPSI
including eight cases of bacterial meningitis (two meningococcal, two
pneumococcal, one H. influenzae and three not stated) and one case of
pneumococcal sepsis were reported among 40 patients. IgG trough levels
were available for 36 of 40 patients (mean = 8•46 g/l). Six episodes of
OPSI occurred prior to Ig replacement therapy, as CVID was not yet
diagnosed; one patient made a personal choice not to commence
replacement therapy until a later date. Seven of the nine (77•8%)
episodes of OPSI occurred within three years of splenectomy, two
(22•2%) took place between 4–6 years and none beyond. The annual risk
of OPSI was calculated at 2.47% year.
Autoimmune lymphoproliferative syndrome.
A condition that has characteristics similar to asplenia is found in
Autoimmune lymphoproliferative syndrome (ALPS), a rare hereditary
disease, caused by impaired FAS-mediated apoptosis of
lymphocytes.[119-121] Autoimmune lymphoproliferative syndrome (ALPS)
presents in childhood with nonmalignant lymphadenopathy and
splenomegaly associated with a characteristic expansion of mature CD4
and CD8 negative or double negative T-cell receptor ab1 T
lymphocytes.[119-121] Elevated counts of circulating TCRab1
double-negative CD42CD82 T lymphocyte cells (DN-Ts) are hallmarks of
the disease. There is an infiltration of double-negative T-cell (DN-T)
in the MZ, which depletes B cells MZ in ALPS patients. These
observations suggest that accumulating DN-Ts, trapped within stromal
cell meshwork, interfere with correct localization of MZB cells.
An
elevated risk of infection was observed in patients with active disease
and was associated with a B-cell immunodeficiency characterized by low
serum IgM levels, poor production of IgM (but not IgG) anti–Str.
pneumoniae antibodies, low circulating SMB-cells counts, very low
circulating MZB, including memory B cells (CD27+/CD19+), MZ B cells
(CD27+IgD+/CD19+), and switched memory (SM) B cells (CD27IgD-/CD19+).[120,121]
This immunodeficiency strongly correlated with the
intensity of lymphoproliferation.[125] ALPS results in
anti-polysaccharide IgM antibody production–specific defect with an
increased rate of infections from capsulated bacteria. Patients often
present with chronic multilineage cytopenias. Cytopenias in these
patients can be the result of splenic sequestration as well as
autoimmune complications manifesting as autoimmune hemolytic anemia,
immune-mediated thrombocytopenia, and autoimmune neutropenia. [119,120]
The cytopenias suggested, in the past, to perform frequently
splenectomy.[119] After splenectomy, patients show a significant
reduction in anemia (P <0.0001), but neutropenia or thrombocytopenia
recur and persist. The rate of invasive bacterial infection in
splenectomized patients increases greatly attaining a rate of 30%. A
similar risk of severe, post-splenectomy sepsis in ALPS is reported by
Price et al.[120] and by Neven et al.[121] This risk is much higher
than the values of 2%, and 11.6% observed after post trauma splenectomy
and in splenectomized thalassemia patients, respectively. Asplenic ALPS
patients require vigilance for septicemia because of pneumococcal
bacteremia can be fatal. Asplenic ALPS patients can have fatal
opportunistic infections and frequently pneumococcal sepsis. All
asplenic ALPS patients should preferably remain on long-term antibiotic
prophylaxis against pneumococcus using penicillin V or
fluoroquinolones, such as levofloxacin. In addition to advising the
asplenic patients to wear Medic Alert bracelets, their parents and
guardians should be educated about the importance of seeking medical
care promptly for a significant febrile illness requiring intravenous
antibiotics.[120,121] Recommendations for asplenic ALPS patients
include life-long daily antibiotic prophylaxis as well as periodic
surveillance and reimmunization against pneumococci using a combination
of both 13-valent conjugate (Prevnar-13) and 23-valent
polysaccharide.[110-120] The most common bacteria causing septicemia
are in the order, Str. pneumoniae seen in 70% of patients, H. influenzae bacteremia, N. meningitides and Capnocytophaga cynodegmi.
Sepsis can develop notwithstanding antibiotic for prophylaxis and
immunization with Prevnar. Clearly overwhelming post-splenectomy sepsis
is a major cause of morbidity and mortality.[119-121] Therefore
nowadays, avoidance of splenectomy is recommended,[119-120] so, the
prognosis for ALPS-FAS is improving and depends, on steroid-sparing
management of cytopenias with mycophenolate mofetil or sirolimus, and
vigilance for lymphoma.[120,121]
Malignant Hematologic Diseases.
Patients splenectomized for malignant hematologic diseases had the
highest rates of complication both thrombo-hemorrhagic and
infectious.[7-10] In the lymphoproliferative diseases, the infectious
complications are prevalent;[1,4-8,122-124] on the contrary the
thrombo-hemorrhagic complications are prevalent in myeloid
neoplasms.[125-128] The condition of malignant hematologic disease per
se increases the incidence of bacterial infections.[5] Regarding Str. pneumoniae infection in the United States, the Advisory Committee on
Immunization Practices (ACIP) reports the data of the Central Disease
Control, (unpublished data, 2012).[129] An estimated 4,000 deaths occur
each year because of Str. pneumoniae,
primarily among adults. The incidence of invasive pneumococcal disease
(IPD) ranges from 3.8 per 100,000 among persons aged 18–34 years to
36.4 per 100,000 among those aged ≥65 years. Adults with certain
medical conditions also are at increased risk for IPD. For adults aged
18–64 years with hematologic cancer, the rate of IPD in 2010 was 186
per 100,000, and for persons with human immunodeficiency virus (HIV)
the rate was 173 per 100,000. The disease rates for adults in these
groups can be more than 20 times those for adults without high-risk
medical conditions.
Linfoproliferative Diseases. At
present, most of the patients with lymphoproliferative diseases
splenectomized are affected by non-Hodgkin Lymphoma (NHL). In the past
splenectomy has been utilized for staging Hodgkin Diseases (HD). In
splenectomized patients with HD an increase incidence of infection,
superior to that found in post-trauma splenectomy has been reported[4]
and vaccination with encapsulated bacteria vaccine is advisable.[122]
Splenectomy
in non-Hodgkin lymphoma (NHL), excluding marginal lymphoma, has not a
curative intent. It is performed for massive splenomegaly and/or
cytopenias to palliate symptoms or in an attempt to improve
hematological reserve, so allowing additional medical therapy, or for
diagnosis.[2,4-9] Although at present most of the patients (50-70 %)
splenectomized for hematologic malignant neoplasm in high-income
countries are affected by non-Hodgkin lymphoma,[7-10] the data of NHL
are not considered separately. There are not investigations separately
comparing the rate of infections of NHL patients splenectomized, but
the comparison is made between the rate of infections of splenectomized
hematological patients with and without a malignant pathology. (Table 1,2)
In
these circumstances, the rate of infections is always superior in
malignant diseases.[5-11] Thus, since the influence of the basal
pathology in determining the complications, to clarify the importance
of splenectomy; the comparison should be made with a matched group of
NHL patients. It is noteworthy that when the control group is
matched-indication the difference in infection risk between
splenectomized and not splenectomized is mild (Figure 3).[5]
Splenectomy remains the treatment of choice in marginal lymphoma of the spleen.[1,123,124]
One
significant concern with splenectomy is the risk of infection from
encapsulated organisms and then it is recommended immunization at least
two weeks prior to splenectomy. The 4% and 5% patients who underwent
splenectomy died from infectious complications in two large
series.[123,124] In a recent confrontation between patients treated
either with splenectomy or immunochemotherapy the adverse events
and, in particular, the infections were more frequent in the follow-up
of patients treated with immunochemotherapy.[124]
Myeloid Neoplasm.
Splenectomy rarely is indicated for myeloid neoplasms. Among them,
myelofibrosis and monocytic leukemia find more frequently indication
for splenectomy.[125,126]
Myeloproliferative Diseases (MPD).
Among the Myeloproliferative Diseases splenectomy at present is
performed for the most in Myelofibrosis because of a huge and/or
painful spleen and/or cytopenias.[125-128] Splenectomy is an effective
treatment for MPD-related splenic pain and/or cytopenias but is
associated with substantial operative morbidity and a mortality ranging
from 5 to 18%.[125-128] It is also associated with an increased risk of
blast phase transformation,[126-128] and according some studies[127] to
reduced survival.
The recent development of JAK2 inhibitors (e.g.
ruxolitinib) as an efficient and safe therapy for patients with MF
diminishes the role of splenectomy in everyday management of MF
patients.[128] The main complications are thrombo-hemorrhagic.
Infections have been reported as an important complication in the
perioperative period ranging from 8,5% to 23% in the different
series.[126-128] Rialon et al.[125] in a series including also patients
with MDS report a mortality rate of 18%, whose 13% was due to
infections.
Overwhelming Post-Splenectomy Infection (OPSI)
Although
OPSI is reducing after the introduction of vaccinations,[7,80,130,131]
and becoming rare when vaccination are correctly performed,[131] it
remains a possible dangerous event also in the post-anti-pneumococcal
vaccination era.[20,37,132] The OPSI can repeat in the same patient.
At present it is a minimal proportion of all type of infections in
splenectomized patients. In the series of Kyaw et al.[7] among the 350
(21.2%) patients with severe infection requiring hospitalization only
49 (3.0%) had at least 1 overwhelming infection. Of these, 30 (61.2%)
experienced only 1 overwhelming infection, 9 (18.4%) had 2 infections,
and 10 (20.4%) had 3 or more severe infections. The incidence of first
overwhelming infection was 0.89 per 100 person-years (95% CI,
0.76-1.17). A similar incidence or also lower is reported by
others.[131]OPSI is defined as fulminating sepsis, meningitides or pneumonia triggered mainly by Str. pneumoniae followed by H. influenzae
type B and N. meningitides. The risks of OPSI and associated death are
highest in the first year after splenectomy, at least among young
children, but remain elevated for more than 10 years and probably for
life.[37,38,129,130] OPSI is a medical emergency. Following brief
prodromal symptoms such as fever, shivering, myalgia, vomiting,
diarrhea, and headache, septic shock develops in just a few hours, with
anuria, hypotension, hypoglycemia. A disseminated intravascular
coagulation and massive adrenal gland hemorrhage
(Waterhouse-Friderichsen syndrome), progressing to multiorgan failure
and eventually death can also be present.[37] The mortality rate is
from 50 to 70%, and most death occurs within the first 24 hours; only
prompt diagnosis and immediate treatment can reduce mortality.[37,132]Splenectomized
children younger than 5 years of age have a greater overall risk of
overwhelming infection with an increased death compared with
adults.[4,5,20,37]Physicians
must be aware of the potential life-threatening infections in patients
who underwent splenectomy and patients should be educated for seeking
early care when fever develops.In
patients at risk and with indicative symptoms, prompt initiation of empirical antibiotics is essential.[37,132] Intravenous infusion of
third generation cephalosporin (cefotaxime 2 g every 8 h or ceftriaxone
2 g every 12h), combined with gentamicin (5–7 mg/kg every 24 h) or
ciprofloxacin (400 mg every 12 h) or vancomycin (1–1.5 g every 12
h). While waiting results of blood culture, bacteria can be visualized
by gram staining. An RT-PCR test for simultaneous identification
of 3 main encapsulated bacteria (Str pneumonia, H. influenzae type B and N. meningitidis)
is available.[20,37,132] Taking into account the possibility of
Gram-negative bacteria in the overwhelming sepsis patient could be
started on empirical therapy with carbopenemic antibiotics associated
with chinolones and/or vancomycin.
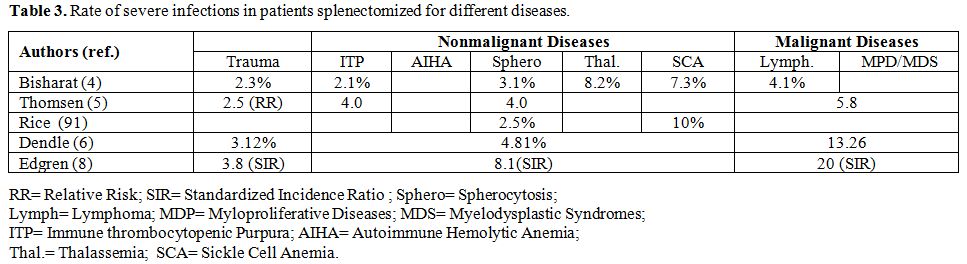 |
Table 3. Rate of severe infections in patients splenectomized for different diseases. |
Prevention of infections in patients with an absent or dysfunctional spleen
Education
of the patient and its relatives, Vaccination, and antibiotic
prophylaxis are the basis to prevent infection by capsulated bacteria
and the consequent OPSI.[37,129-132]The
patient should be aware of the risk and the necessity of vaccination,
which in any case does not preserve from all infections. He should have
a clear action for febrile illness, animal bite and planned oversea
travel.[131]Vaccination.
The mainstay of pneumococcal vaccination has, for many years, been the
polyvalent polysaccharide pneumococcal vaccine (PPV 23). The PPV23,
available since 1983, consist of the capsular polysaccharides of the 23
most prevalent pneumococcal serotypes. It has a coverage of 85–90% of
the invasive pneumococcal infections among children and adults.[133]
Its efficacy below five years of age is scarce. Although this
polysaccharide vaccine induces an immune response, it does not result
in the generation of memory B-cells and long-lived plasma cells. To
maintain sufficiently high antibody levels, re-immunization with PPV23
every five years is therefore recommended in hyposplenic and asplenic
patients.[133] Furthermore there is a reduced response to PPV23 in
splenectomized patients with hematological diseases.[134] Conjugate
vaccines consist of a polysaccharide covalently linked to a carrier
protein (conjugation), this linkage can significantly enhance
immunoprotection against the polysaccharide by inducing a
T-cell-dependent immune response. Conjugate vaccines are highly
immunogenic in infants as young as two months of age, provide higher
antibody titers and induce immunological memory.[129,135]The
first heptavalent pneumococcal polysaccharide-protein conjugate vaccine
(PCV7) was introduced in USA 2000 and Europe in 2006.[135-138] This
conjugate vaccine leads to T-cell dependent induction of antibodies and
immunological memory. The seven serotypes, included in the conjugate
vaccine, handle 64% of the invasive pneumococcal infections in young
children (<2 years) of the Netherlands.[135] The inclusion of
conjugated pneumococcal polysaccharide vaccines might be of additional
value in the vaccination schedule for asplenic patients because of
their high immunogenicity.[136-139] A strong serological response was
found in splenectomized patients within the first five years after
pneumococcal vaccination by PCV7. Nevertheless, post-vaccine
pneumococcal sepsis was still diagnosed in 3.3% of splenectomized
survivors. However, sepsis and death were found for the most in
patients with hematologic malignancies, frequently with severe
neutropenia.[140]Since
2010, two improved pneumococcal conjugate vaccines (PCVs) received
market authorization in many countries, including in the US and the
EU.[138] These vaccines cover the seven serotypes included in the PCV7
vaccine, and additional serotypes responsible for an increasing
proportion of IPD. Specifically, PCV10 (“SynflorixTM”, GSK) contains
additional antigens from serotypes 1, 5 and 7F.[133-135,138] The
manufacturer claims a high protective effect against diseases not only
due to pneumococcal serotypes but also against disease due to
non-typeable H. influenzae PCV13 (“Prevnar13TM”, Pfizer) contains
antigens from serotypes 1, 3, 5, 6A, 7F and 19A in addition to the
PCV7 serotypes.[138] The 13-valent pneumococcal conjugate vaccine
(PCV13) has replaced in the last few year the PCV7. As predicted, PCV13
is more immunogenic than PPV23 albeit with a more limited repertoire
and is highly effective in preventing invasive disease caused by the 13
serotypes included in the vaccine.[138,139]At
present, the PCV 13 has been added to PPV 23 in all guidelines of
high-income countries in children age.[129,140-142] According the UK
guidelines of 2011, at present, for older children and adults who may
or may not have received previous PCV there is insufficient evidence to
recommend a change in policy from PPV to PCV either for primary
immunization or for boosting. Similarly, in the United States,
guidelines from the Centers for Diseases Control and Prevention (CDC),
published in 1997 and updated in 2010, recommended the use of the
23-valent pneumococcal polysaccharide vaccine (PPV23) in adults with
anatomical or functional asplenia and revaccination after 5 years.[140]
Whether patients should be recommended pneumococcal polysaccharide
vaccine (PPV) or pneumococcal conjugate vaccine (PCV) and the possible
benefits of repeated vaccinations be the subject of a current debate in
Europe.[137-138] However, in USA, the high rate of invasive
pneumococcal diseases (IPD) found through 2010 among adults aged 18–64
years with hematologic cancer induced the Advisory Committee on
Immunization Practices (ACIP) on 20 June 2012 to extend routine use of
13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13, Wyeth
Pharmaceuticals, Inc., a subsidiary of Pfizer, Inc.) to adults aged ≥19
years with functional or anatomic asplenia, other than to other
immunocompromised conditions, cerebrospinal fluid (CSF) leaks, or
cochlear implants.[129] This decision was made by considering that 50%
of IPD cases among immunocompromised adults in 2010 were caused by
serotypes contained in PCV13; an additional 21% were caused by
serotypes only contained in PPSV23 (CDC, unpublished data, 2011).
Consequently the PCV13 should be administered to eligible adults in
addition to the 23-valent pneumococcal polysaccharide vaccine (PPSV23;
Pneumovax 23, Merck & Co. Inc.), the only vaccine currently
recommended for these groups of adults in most European guidelines. The
recent paper of Nived[139] demonstrate after PVC 13 vaccination high
levels of pneumococcal serotype-specific antibodies in the previous
PPV23 vaccinated group, demonstrating that PCV 13 can be used as a
booster dose in asplenic patients with previous PPV23 vaccination. High
levels of serotype-specific IgG concentration ≥0.35 mg/mL were observed
in previous PPV23 vaccinated but PCV-naïve asplenic patients for
serotypes 1, 3, 4, 5, 7F, 18C,19A, 19F, and 23F.[137] Safety and
immunogenicity of sequential administration have been demonstrated in
older people[144,145] and recently its safety and efficacy has been
confirmed by a large trial including nonhematological patients.[146]Polysaccharide vaccines and conjugate vaccines are both available against Haemophilus influenzae B and Neisseria meningitidis.[147-150]
Conjugate vaccines activate a superior immune response compared with
polysaccharide vaccines and shows efficacy in children 2-4 years old as
well in older adults. Thus, conjugate vaccines should be used
preferentially whenever possible not in substitution but also of
polysaccharide vaccine. A
quadrivalent meningococcal diphtheria toxoid conjugate vaccine
(Menactra®, Sanofi Pasteur) (MCV4) including serogroups A, C, Y, and W
was licensed for use in 2005 by the US FDA [4] and in 2007 licensure
was approved in Canada, and in the Arab Gulf countries.[147] This
vaccine should be utilized in Arab countries, where the serotype W is
particularly frequent.[147] It does not cover against the strain B,
which is the predominant cause of invasive meningococcal disease in
most of Europe and Australia countries, especially where serogroup C
vaccination is part of routine recommendations.[148] However, at
present is available also a vaccine against the strain B. The
multicomponent meningococcal B vaccine, 4CMenB (Bexsero, Novartis
Vaccines and Diagnostics), recently approved in Europe and Australia,
contains three surface-exposed recombinant proteins (fHbp, NadA, and
NHBA) and New Zealand strain outer membrane vesicles (NZ OMV) with PorA
1.4 antigenicity.[148]In
our opinion the modality of vaccination should follow the scheme
adopted by the Canadian Paediatric Society,[143] integrated by the
recent recommendation of ACIP (Table 4).[129]
In
programmed splenectomy, the vaccine should be administered two weeks
before intervention and in urgency two weeks afterward both in adults
and children. Both
the conjugated 13-valent conjugate pneumococcal vaccine and the
23-valent polysaccharide vaccine should be utilized in the prevention
of Streptococcus pneumoniae infection. In
pneumococcal vaccine-naïve persons: Adults aged ≥19 years with
immunocompromised conditions, functional or anatomic asplenia, CSF
leaks, or cochlear implants, and who have not previously received PCV13
or PPSV23, should be given a dose of PCV13 first, followed by a dose of
PPSV23 at least 8 weeks later. Subsequent doses of PPSV23 should follow
current PPSV23 recommendations for adults at high risk. Specifically, a
second PPSV23 dose is recommended five years after the first PPSV23
dose for persons aged 19–64 years with functional or anatomic asplenia
and for persons with immunocompromised conditions. Additionally, those
who received PPSV23 before age 65 years for any indication should be
given another dose of the vaccine at age 65 years, or later if at least
five years have elapsed since their previous PPSV23 dose. In
the previous vaccinated with PPSV23: Adults aged ≥19 years with
immunocompromised conditions, functional or anatomic asplenia, who
previously have received ≥1 doses of PPSV23 should be given a PCV13
dose ≥1 year after the last PPSV23 dose was received. For those who
require additional doses of PPSV23, the first such dose should be given
no sooner than eight weeks after PCV13 and at least five years after
the most recent dose of PPSV23. - In prevention of Haemophilus influenzae: type b conjugate vaccine; specific recommendations vary by age. - In prevention of Neisseria meningitidis:
conjugate quadrivalent meningococcal vaccine (MCV4) should be utilized.
Experience with the multi-component meningococcal B vaccine is scarce,
however epidemiological studies suggest its utilization in Europe and
Australia. Apart
from the scarce compliance,[149] some patients remain unvaccinated,
despite this double vaccination and a true vaccine failure also
contribute to pneumococcal infection. Failure
to mount an antibody response may be genetically determined but is also
more frequent in older patients and those splenectomized for
hematological malignancies.[150,151] The failure to respond to
immunization can be demonstrated by the absent rise in titer of the
anti-pneumococcal antibody.[133,134,136] A surge of non-vaccine
serotypes could be another cause of failure of vaccination as described
after the addition of pneumococcal protein conjugate vaccine
(PCV7).[152] Antibiotic prophylaxis:
Lifelong antibiotics are recommended for immunosuppressed patients, and
for at least two years after splenectomy for all other patients.
Further, patients are advised to keep an emergency supply of
antibiotics for the event of febrile illness. Oral penicillins remain
the prophylactic drugs of choice in areas with low pneumococcal
resistance. Specialist microbiological advice should be sought where
this is not the case or for travel abroad. In patients with confirmed
penicillin allergy, an appropriate macrolide may be substituted
depending on local epidemiology.
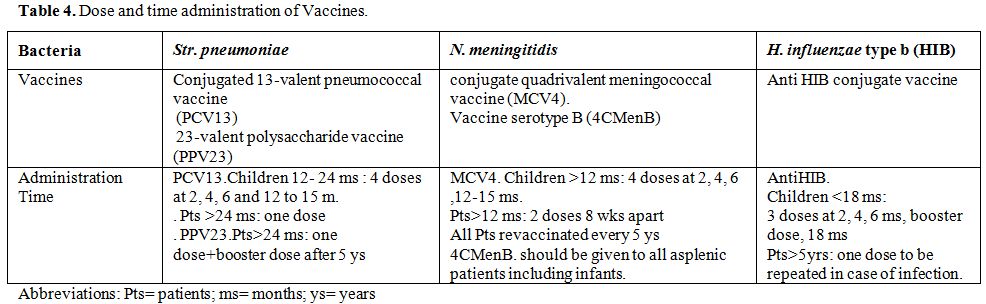 |
Table 4. Dose and time administration of Vaccines. |
Concluding Remarks
Splenectomy, even if the
incidence of OPSI is reducing in high-income countries for the
widespread pneumococcal vaccination, also represent today's an
important risk factor for infections. The introduction of conjugate
vaccines also in the older population could induce a further reduction
of sepsis from encapsulated bacteria. The case reported at the incipit
of this review presented meningitis with a culture of liquor positive
for Str. pneumoniae. The
addition of a conjugate vaccine could have increase the immunological
response reducing the risk of infection. In the high-income countries,
the antipneumococcal vaccination is adequate, at least in terms of
primary vaccination with Pneumovax, but conjugate vaccines have not
been introduced so far in most of the countries. In contrast,
vaccination against N. meningitidis serogroups A + C was insufficient
and introduction of vaccination against B serotype is warranted. There
is a need to improve the awareness among healthcare professionals of
the greatly increased risk of severe infection with encapsulated
bacteria post-splenectomy and how these infections, in particular,
overwhelming post-splenectomy infection, can be prevented. However, at
present gram- negative sepsis are prevalent. Further work is required
to characterize these infections and determine whether or not they were
related to asplenia.
OPSI continue to be described in 1-1.5 patients/year also in vaccinated patients, but Streptococcus pneumoniae,
which was in the past the major cause of morbidity and mortality among
such patients, has become infrequent as a cause of infections, at least
in European series. Poorly controlled iron overload can be the cause of
Gram-negative infections that are still frequently diagnosed in
post-splenectomy patients for congenital hemoglobin disorders. This
information needs to be taken into account when a splenectomized
patient presents with fever and/or sepsis. At the first indication of
systemic infection (high fever) all patients should have access to
primary care and start urgent treatment with appropriate antibiotics,
(in general treatment with a third generation cephalosporin, either
alone or in combination with other antibiotics that are active against
Gram-negative pathogens, should be promptly initiated in order to save
the patient’s life). In patients taking prophylaxis treatment should be
from an antibiotic class likely to be non-cross resistant. Choice of
antibiotic should be made concerning appropriate microbiological advice
and local protocols. The importance of the primitive disease remains
fundamental in determining the rate and the severity of infections and
the overall survival.
References
- Olszewski AJ, Ali S. Comparative outcomes of rituximab-based
systemic therapy and splenectomy in splenic marginal zone lymphoma. Ann
Hematol. 2014 Mar;93(3):449-58.
http://dx.doi.org/10.1007/s00277-013-1900-4

- Katz SC, Pachter HL. Indications for splenectomy. Am Surg. 2006;72:565-80. Review. PubMed PMID: 16875077

- Weledji
EP. Benefits and risks of splenectomy. Int J Surg. 2014;12(2):113-9.
Epub 2013 Dec 3. Review. PubMed PMID: 24316283.
http://dx.doi.org/10.1016/j.ijsu.2013.11.017

- Bisharat
N, Omari H, Lavi I, Raz R. Risk of infection and death among
post-splenectomy patients. J Infect. 2001 Oct;43(3):182-6. PubMed PMID:
11798256

- Thomsen RW, Schoonen WM, Farkas DK, Riis
A, Jacobsen J, Fryzek JP, Sørensen HT. Risk for hospital contact with
infection in patients with splenectomy: a population-based cohort
study. Ann Intern Med. 2009 Oct 20;151(8):546-55. PubMed PMID: 19841456

- Dendle C, Sundararajan V, Spelman T, Jolley D, Woolley
I. Splenectomy sequelae: an analysis of infectious outcomes among
adults in Victoria. Med J Aust. 2012 May 21;196(9):582-6. Erratum in:
Med J Aust. 2012 Jun 4;196(10):628.PubMed PMID: 2262115

- Kyaw
MH, Holmes EM, Toolis F, Wayne B, Chalmers J, Jones IG, Campbell
H.Evaluation of severe infection and survival after splenectomy. Am J
Med. 2006 Mar;119(3):276.e1-7. PubMed PMID: 16490477

- Edgren
G, Almqvist R, Hartman M, MD, Utter, MD G., Splenectomy and the Risk of
Sepsis: A Population-Based Cohort Study. Ann Surg 2014;260:1081–1087
http://dx.doi.org/10.1097/SLA.0000000000000439
PMid:24374533

- Bickenbach KA,
Gonen M, Labow DM, Strong V, Heaney ML, Zelenetz AD, Coit DG.
Indications and efficacy of splenectomy for haematological disorders.
Br J Surg. 2013 May;100(6):794-800. doi: 10.1002/bjs.9067.
http://dx.doi.org/10.1002/bjs.9067

- MagrodiaN,
Button AM, Spanheimer PM, Belding-Schmitt ME, Rosenstein LJ, Mezhir JJ.
Morbidity and mortality following elective splenectomy for benign and
malignant hematologic conditions: analysis of the American College
of Surgeons National Surgical Quality Improvement Program data. JAMA
Surg. 2014 Oct;149(10):1022-9.
http://dx.doi.org/10.1001/jamasurg.2014.285

- Kristinsson
SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after
splenectomy among 8,149 cancer-free American veterans: a cohort study
with up to 27 years follow-up. Haematologica. 2014 Feb;99(2):392-8.
http://dx.doi.org/10.3324/haematol.2013.092460

- Rodeghiero
F, Ruggeri M. Short- and long-term risks of splenectomy for benign
haematological disorders: should we revisit the indications? Br J
Haematol. 2012 Jul;158(1):16-29. Epub 2012 May 10.Review. PubMed PMID:
22571181 http://dx.doi.org/10.1111/j.1365-2141.2012.09146.x

- Rubin
LG, Schaffner W. Clinical practice. Care of the asplenic patient. N
Engl J Med. 2014 Jul 24;371(4):349-56. Review. PubMed PMID: 25054718
http://dx.doi.org/10.1056/NEJMcp1314291

- Aubrey-Bassler
FK, Sowers N. 613 cases of splenic rupture without risk factors or
previously diagnosed disease: a systematic review. BMC Emerg Med. 2012
Aug 14;12:11. Review. http://dx.doi.org/10.1186/1471-227X-12-11

- Rabie
ME, Hashemey AA, El Hakeem I, Al Hakamy MA, Obaid M, Al Skaini M,
Shabbir G, Al Sareii S, Hussain MN. Spontaneous rupture of malarial
spleen: report of two cases. Mediterr J Hematol Infect Dis. 2010 Dec
13;2(3):e2010036. http://dx.doi.org/10.4084/mjhid.2010.036

- Al-Salem
AH. Massive splenic infarction in children with sickle cell anemia and
the role of splenectomy. Pediatr Surg Int. 2013 Mar;29(3):281-5. Epub
2012 Nov 27 http://dx.doi.org/10.1007/s00383-012-3223-2

- kKubber
MM, Kroft LJ, de Groot B. Non-traumatic splenic rupture in a patient on
oral anticoagulation. Int J Emerg Med. 2013 May 21;6(1):16.
http://dx.doi.org/10.1186/1865-1380-6-16

- Maria V,
Saad AM, Fardellas I. Spontaneous spleen rupture in a teenager: an
uncommon cause of acute abdomen. Case Rep Med. 2013;2013:675372. Epub
2013 Apr 23. PubMed PMID: 23710190; PubMed Central PMCID: PMC3655517
http://dx.doi.org/10.1155/2013/675372

- Cesaro S,
Marson P, Gazzola MV, De Silvestro G, Destro R, Pillon M, Calore E,
Messina C, Zanesco L. The use of cytokine-stimulated healthy donors in
allogeneic stem cell transplantation. Haematologica. 2002 Aug;87(8
Suppl):35-41. Review. PubMed PMID: 12412388.

- Di
Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic
states. Lancet. 2011 Jul 2;378(9785):86-97. Epub 2011 Apr 5. Review.
http://dx.doi.org/10.1016/S0140-6736(10)61493-6

- Harbrecht
BG, Franklin GA, Miller FB, Richardson JD. Is splenectomy after trauma
an endangered species? Am Surg. 2008 May;74(5):410-2. PubMed
PMID:18481497.

- Fung HC, Nademanee AP. Approach to
Hodgkin's lymphoma in the new millennium. Hematol Oncol. 2002
Mar;20(1):1-15. Review. PubMed PMID: 11921013

- Mahévas
M, Fain O, Ebbo M, Roudot-Thoraval F, Limal N, Khellaf M, Schleinitz N,
Bierling P, Languille L, Godeau B, Michel M. The temporary use of
thrombopoietin-receptor agonists may induce a prolonged remission in
adult chronic immune thrombocytopenia. Results of a French
observational study. Br J Haematol. 2014 Jun;165(6):865-9

- Casaccia
M, Torelli P, Pasa A, Sormani MP, Rossi E; IRLSS Centers. Putative
predictive parameters for the outcome of laparoscopic splenectomy: a
multicenter analysis performed on the Italian Registry of Laparoscopic
Surgery of the Spleen. Ann Surg. 2010 Feb;251(2):287-91. PubMed
PMID:20010087 http://dx.doi.org/10.1097/SLA.0b013e3181bfda59

- Sheikha
AK, Salih ZT, Kasnazan KH, Khoshnaw MK, Al-Maliki T, Al-Azraqi TA,
Zafer MH. Prevention of overwhelming postsplenectomy infection in
thalassemia patients by partial rather than total splenectomy. Can J
Surg. 2007 Oct;50(5):382-6.

- National Hospital Discharge
Survey. Atlanta: Centers for Disease Control and Prevention
(http://www.cdc.gov/nchs/nhds/nhds_tables.htm#detailed).
- Sickle
Cell Association of America. Research and screening
(http://www.sicklecelldisease.org/index.cfm?page=research-screening).
- Khamechian
T, Alizargar J, Farzanegan M. Pattern of splenectomy indications
inkashan shahid-beheshti hospital: a 5-year study. Arch Trauma Res.
2013;1(4):180-3. Epub 2013 Feb 1. PubMed PMID: 24396775;
http://dx.doi.org/10.5812/atr.8258

- Deodhar M, Kakkar N. An
audit of splenectomies in a teaching hospital in North India. Are
postsplenectomy guidelines being complied with? J Clin Pathol. 2004
Apr;57(4):407-10. PubMed PMID: 15047746; PubMed Central PMCID:
PMC1770256

- Machado NO, Grant CS, Alkindi S, Daar S, Al-Kindy N,
Al Lamki Z, Ganguly SS. Splenectomy for haematological disorders: a
single center study in 150 patients from Oman. Int J Surg. 2009
Oct;7(5):476-81. http://dx.doi.org/10.1016/j.ijsu.2009.08.004.Epub 2009
Aug 18. PubMed PMID: 19695352

- Alufohai E, Odusanya OO. Splenectomy in a rural surgical practice. Niger J Clin Pract. 2006 Jun;9(1):81-3.

- Wang
X, Li Y, Crook N, Peng B, Niu T. Laparoscopic splenectomy: a surgeon's
experience of 302 patients with analysis of postoperative
complications. Surg Endosc. 2013 Oct;27(10):3564-71.
http://dx.doi.org/10.1007/s00464-013-2978-4

- Hayashi H,
Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia
due to liver cirrhosis: a review. World J Gastroenterol. 2014 Mar
14;20(10):2595-605. Review. PubMed PMID: 24627595; PubMed Central
PMCID: PMC3949268 http://dx.doi.org/10.3748/wjg.v20.i10.2595

- Barmparas
G, Lamb AW, Lee D, Nguyen B, Eng J, Bloom MB, Ley EJ. Postoperative
infection risk after splenectomy: A prospective cohort study. Int J
Surg. 2015 Mar 14;17:10-14. doi: 10.1016/j.ijsu.2015.03.007. [Epub
ahead of print] PubMed http://dx.doi.org/10.1016/j.ijsu.2015.03.007

- Boyle
S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous
thromboembolism and sepsis in patients with immune thrombocytopenia.
Blood. 2013 Jun 6;121(23):4782-90. Epub 2013 May 1. PubMed PMID:
23637127; PubMed Central PMCID: PMC3674676
http://dx.doi.org/10.1182/blood-2012-12-467068

- Holdsworth
RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality
rate: actual versus perceived risks. Br J Surg 1991;78:1031–8.
http://dx.doi.org/10.1002/bjs.1800780904 PMid:1933181

- Sinwar PD. Overwhelming
post splenectomy infection syndrome - review study. Int J Surg. 2014
Dec;12(12):1314-6. doi: 10.1016/j.ijsu.2014.11.005. Epub 2014 Nov 7.
PubMed PMID: 25463041
http://dx.doi.org/10.1016/j.ijsu.2014.11.005

- Ejstrud P,
Kristensen B, Hansen JB, Madsen KM, Schønheyder HC, Sørensen HT. Risk
and patterns of bacteraemia after splenectomy: a population-based
study.Scand J Infect Dis. 2000;32(5):521-5. PubMed PMID: 11055658.

- Yu
RK, Shepherd LE, Rapson DA. Capnocytophaga canimorsus, a potential
emerging microorganism in splenectomized patients. Br J Haematol. 2000
Jun;109(4):679. PubMed PMID: 10929015.

- Sica S, Di Mario A,
Salutari P, d'Onofrio G, Antinori A, Chiusolo P, Leone G. Morganella
morganii pericarditis after resolvent splenectomy for immune
pancytopenia following allogeneic bone marrow transplantation for acute
lymphoblastic leukemia. Clin Infect Dis. 1995 Oct;21(4):1052-3. PubMed
PMID: 8645811.

- Demar M, Legrand E, Hommel D, Esterre P, Carme B.
Plasmodium falciparum malaria in splenectomized patients: two case
reports in French Guiana and a literature review. Am J Trop Med Hyg.
2004 Sep;71(3):290-3. Review. PubMed PMID: 15381808.

- Rosner F,
Zarrabi MH, Benach JL, et al. Babesiosis in splenectomized adults.
Review of 22 reported cases. Am J Med. 1984; 76(4):696–701. [PubMed:
6424470]

- Stowell CP, Gelfand JA, Shepard JA, et al. Case records
of the Massachusetts General Hospital. Case 17-2007. A 25-year-old
woman with relapsing fevers and recent onset of dyspnea. N Engl J Med.
2007; 356(22):2313–9. [PubMed: 17538091]

- Mebius RE, Kraal G.
Structure and function of the spleen. Nat Rev Immunol.
2005;5(8):606-616 http://dx.doi.org/10.1038/nri1669 PMid:16056254

- Zandvoort A, Timens W. The
dual function of the splenic marginal zone:essential for initiation of
anti-TI-2 responses but also vital in the general first-line defense
against blood-borne antigens. Clin Exp Immunol. 2002 Oct;130(1):4-11.
Review. PubMed PMID: 12296846; PubMed Central PMCID: PMC1906503

- Kruetzmann
S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells
controlling Streptococcus pneumoniae infections are generated in the
spleen. J Exp Med. 2003;197(7):939-945.
http://dx.doi.org/10.1084/jem.20022020 PMid:12682112 PMCid:PMC2193885

- Weller
S, Braun MC, Tan BK, et al. Human blood IgM "memory" B cells are
circulating splenic marginal zone B cells harboring a prediversified
immunoglobulin repertoire. Blood. 2004;104(12):3647-3654.
http://dx.doi.org/10.1182/blood-2004-01-0346 PMid:15191950 PMCid:PMC2590648

- Carsetti
R, Pantosti A, Quinti I. Impairment of the antipolysaccharide response
in splenectomized patients is due to the lack of immunoglobulin M
memory B cells. J Infect Dis. 2006;193(8):1189-1190.
http://dx.doi.org/10.1086/501375 PMid:16544264

- Moens L, Wuyts M, Meyts I,
De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill
distinct roles in the anti-polysaccharide and antiprotein immune
response. J Immunol. 2008; 181(8):5306-5312.
http://dx.doi.org/10.4049/jimmunol.181.8.5306
PMid:18832686

- Wasserstrom H, Bussel J,
Lim LC, Cunningham-Rundles C. Memory B cells and pneumococcal antibody
after splenectomy. J Immunol. 2008;181(5):3684-3689.
http://dx.doi.org/10.4049/jimmunol.181.5.3684 PMid:18714044
PMCid:PMC2919219

- Rosado MM, Gesualdo F, Marcellini V, Di
Sabatino A, Corazza GR, Smacchia MP,Nobili B, Baronci C, Russo L, Rossi
F, Vito RD, Nicolosi L, Inserra A, Locatelli F, Tozzi AE, Carsetti R.
Preserved antibody levels and loss of memory B cells against
pneumococcus and tetanus after splenectomy: tailoring better
vaccination strategies. Eur J Immunol. 20 Oct;43(10):2659-70. Epub 2013
Jul 25. PubMed PMID: 23813052.
http://dx.doi.org/10.1002/eji.201343577

- Koppel EA, Saeland
E, de Cooker DJ, van Kooyk Y, Geijtenbeek TB: DC-SIGN specifically
recognizes Streptococcus pneumonia serotypes 3 and 14. Immunobiology
2005; 210: 203–210

- Bronte V, Pittet MJ. The spleen in local and
systemic regulation of immunity. Immunity. 2013;39(5):806-818.
http://dx.doi.org/10.1016/j.immuni.2013.10.010

- den Haan
JM, Kraal G. Innate immune functions of macrophage subpopulations in
the spleen. J Innate Immun. 2012;4(5-6):437-45. Epub 2012 Feb 7.
Review. PubMed PMID: 22327291.
http://dx.doi.org/10.1159/000335216

- Ram S, Lewis LA, Rice
PA. Infections of people with complement deficiencies and patients who
have undergone splenectomy. Clin Microbiol Rev. 2010 Oct;23(4):740-80.
doi: 10.1128/CMR.00048-09. Review. PubMed PMID: 20930072; PubMed
Central PMCID: PMC2952982. http://dx.doi.org/10.1128/CMR.00048-09

- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM,
Steinman RM, Park CG. A dominant complement fixation pathway for
pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q.
Cell. 2006 Apr 7;125(1):47-58. PubMed PMID: 16615889

- Prabagar
MG, Do Y, Ryu S, Park JY, Choi HJ, Choi WS, Yun TJ, Moon J, Choi IS, Ko
K, Ko K, Young Shin C, Cheong C, Kang YS. SIGN-R1, a C-type lectin,
enhances apoptotic cell clearance through the complement deposition
pathway by interacting with C1q in the spleen. Cell Death Differ. 2013
Apr;20(4):535-45. doi:10.1038/cdd.2012.160. Epub 2012 Dec 14. PubMed
PMID: 23238564; PubMed Central PMCID: PMC3595488.
http://dx.doi.org/10.1038/cdd.2012.160

- Heuer M, Taeger G,
Kaiser GM, Nast-Kolb D, Kühne CA, Ruchholtz S, Lefering R, Paul A,
Lendemans S; Trauma Registry of DGU. No further incidence of sepsis
after splenectomy for severe trauma: a multi-institutional experience
of the trauma registry of the DGU with 1,630 patients. Eur J Med Res.
2010 Jun 28;15(6):258-65. PubMed PMID: 20696635; PubMed Central PMCID:
PMC3351995.

- Wiseman J, Brown CV, Weng J, Salim A, Rhee P,
Demetriades D. Splenectomy for trauma increases the rate of early
postoperative infections. Am Surg. 2006 Oct;72(10):947-50. PubMed PMID:
17058742

- Boni L, Benevento A, Rovera F, Dionigi G, Di Giuseppe
M, Bertoglio C, Dionigi R. Infective complications in laparoscopic
surgery. Surg Infect (Larchmt). 2006;7 Suppl 2:S109-11. Review. PubMed
PMID: 16895490.

- Neunert CE. Current management of immune
thrombocytopenia. Hematology Am Soc Hematol Educ Program.
2013;2013:276-82. doi: 10.1182/asheducatio 2013.1.276. Review. PubMed
PMID: 24319191

- Leone G, Larocca LM, Scribano D, Storti S, Caputo
G, Landolfi R. T lymphocyte subsets and platelet-associated IgG in
idiopathic thrombocytopenic purpura: effect of splenectomy. Acta
Haematol. 1986;75(2):83-8. PubMed PMID: 3090827

- Vianelli N,
Palandri F, Polverelli N, Stasi R, Joelsson J, Johansson E, Ruggeri M,
Zaja F, Cantoni S, Catucci AE, Candoni A, Morra E, Björkholm M,
Baccarani M, Rodeghiero F. Splenectomy as a curative treatment for
immune thrombocytopenia: a retrospective analysis of 233 patients with
a minimum follow up of 10 years. Haematologica. 2013 Jun;98(6):875-80.
http://dx.doi.org/10.3324/haematol.2012.075648

- Cooper N,
Evangelista ML, Amadori S, Stasi R. Should rituximab be used before or
after splenectomy in patients with immune thrombocytopenic purpura?
Curr Opin Hematol. 2007 Nov;14(6):642-6. Review. PubMed PMID: 17898569.

- Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard JF,
Cheze S,Graveleau J, Slama B, Audia S, Ebbo M, Le Guenno G, Cliquennois
M, Salles G,Bonmati C, Teillet F, Galicier L, Hot A, Lambotte O,
Lefrère F, Sacko S, KengueDK, Bierling P, Roudot-Thoraval F, Michel M,
Godeau B. Safety and efficacy of rituximab in adult immune
thrombocytopenia: results from a prospective registryincluding 248
patients. Blood. 2014 Nov 20;124(22):3228-36. Epub 2014 Oct 7. PubMed
PMID: 25293768. http://dx.doi.org/10.1182/blood-2014-06-582346

- Fianchi
L, Rossi E, Murri R, De Stefano V, Pagano L, Leone G. Severe infectious
complications in a patient treated with rituximab for idiopathic
thrombocytopenic purpura. Ann Hematol. 2007 Mar;86(3):225-6. Epub 2006
Oct 10. PubMed PMID: 17031685.

- Moulis G, Lapeyre-Mestre M, Mahévas
M, Montastruc JL, Sailler L. Need for an improved vaccination rate in
primary immune thrombocytopenia patients exposed to rituximab or
splenectomy. A nationwide population-based study in France. Am J
Hematol. 2015 Apr;90(4):301-5. doi: 10.1002/ajh.23930. Epub 2015 Mar 2.
PubMed PMID: 25557586. http://dx.doi.org/10.1002/ajh.23930

- Moulis
G, Sailler L, Sommet A, Lapeyre-Mestre M, Derumeaux H, Adoue D.
Rituximab versus splenectomy in persistent or chronic adult primary
immune thrombocytopenia: an adjusted comparison of mortality and
morbidity. Am J Hematol. 2014 Jan;89(1):41-6. Epub 2013 Sep 30. PubMed
PMID: 24038066. http://dx.doi.org/10.1002/ajh.23580

- Gonzalez-Porras
JR, Escalante F, Pardal E, Sierra M, Garcia-Frade LJ, Redondo S, Arefi
M, Aguilar C, Ortega F, de Cabo E, Fisac RM, Sanz O, Esteban C, Alberca
I, Sanchez-Barba M, Santos MT, Fernandez A, Gonzalez-Lopez TJ; Grupo de
Trombosis y Hemostasia de Castilla y León. Safety and efficacy of
splenectomy in over 65-yrs-old patients with immune thrombocytopenia.
Eur J Haematol. 2013 Sep;91(3):236-41. Epub 2013 Jul 7. PubMed PMID:
23679653. http://dx.doi.org/10.1111/ejh.12146

- Aronis S,
Platokouki H, Avgeri M, et al. Retrospective evaluation of long-term
efficacy and safety of splenectomy in chronic idiopathic
thrombocytopenic purpura in children. Acta Paediatr. 2004;93:638–42.
http://dx.doi.org/10.1111/j.1651-2227.2004.tb02989.x PMid:15174787

- Kuhne T, Blanchette V,
Buchanan GR, et al. Splenectomy in children with idiopathic
thrombocytopenic purpura: a prospective study of 134 children from the
intercontinental childhood ITP Study Group. Pediatr Blood Cancer.
2007;49:829–34. http://dx.doi.org/10.1002/pbc.21108 PMid:17171689

- Aladjidi N, SantiagoR,
Pondarre´ C, et al. Revisiting splenectomy in childhood chronic immune
thrombo- cytopenia at the era of new therapies:the French experience. J
Blood Disorders Transf. 2012; S:3: 003

- Gwilliam NR, Lazar DA,
Brandt ML, Mahoney DH Jr, Wesson DE, Mazziotti MV, Nuchtern JG, Lee TC.
An analysis of outcomes and treatment costs for children undergoing
splenectomy for chronic immune thrombocytopenia purpura. J Pediatr
Surg. 2012 Aug;47(8):1537-41. Review. PubMed
http://dx.doi.org/10.1016/j.jpedsurg.2012.02.013

- Liang Y,
Zhang L, Gao J, Hu D, Ai Y. Rituximab for children with immune
thrombocytopenia: a systematic review. PLoS One. 2012;7(5):e36698. Epub
2012 May 30. Review.
http://dx.doi.org/10.1371/journal.pone.0036698

- Zhou H, Xu
M, Qin P, Zhang HY, Yuan CL, Zhao HG, Cui ZG, Meng YS, Wang L, Zhou F,
Wang X, Li DQ, Bi KH, Zhu CS, Guo CS, Chu XX, Wu QC, Liu XG, Dong XY,
Li J, Peng J, Hou M. A multicenter randomized open-label study of
rituximab plus rhTPO vs rituximab in corticosteroid-resistant or
relapsed ITP. Blood. 2015 Mar 5;125(10):1541-7. Epub 2015 Jan 9. PubMed
PMID: 25575541; PubMed Central PMCID: PMC4351949.
http://dx.doi.org/10.1182/blood-2014-06-581868

- Ansari Sh,
Rostami T, Yousefian S, Kiumarsi A, Miri-Aliabad G, Ramim T. Rituximab
efficacy in the treatment of children with chronic immune
thrombocytopenic purpura. Pediatr Hematol Oncol. 2014 Sep;31(6):555-62.
Epub 2014 Jul 9. PubMed PMID: 25007304.
http://dx.doi.org/10.3109/08880018.2014.930766

- Cooper N. A
review of the management of childhood immune thrombocytopenia: how can
we provide an evidence-based approach? Br J Haematol. 2014
Jun;165(6):756-67. Epub 2014 Apr 25. Review. PubMed PMID: 24761791.
http://dx.doi.org/10.1111/bjh.12889

- Matsubara K, Takahashi
Y, Hayakawa A, Tanaka F, Nakadate H, Sakai M, Maeda N, Oka T, Ishii E,
Bessho F, Morimoto T, Goto H, Hashii Y, Hatakeyama N, Shirahata A,
Imaizumi M. Long-term follow-up of children with refractory immune
thrombocytopenia treated with rituximab. Int J Hematol. 2014
Apr;99(4):429-36. Epub 2014 Mar 8. PubMed PMID: 24609717.
http://dx.doi.org/10.1007/s12185-014-1541-y

- Moulis G,
Sailler L, Sommet A, Lapeyre-Mestre M, Derumeaux H, Adoue D. Rituximab
versus splenectomy in persistent or chronic adult primary immune
thrombocytopenia: an adjusted comparison of mortality and morbidity. Am
J Hematol. 2014 Jan;89(1):41-6. Epub 2013 Sep 30. PubMed PMID:
24038066. http://dx.doi.org/10.1002/ajh.23580

- Patel VL,
Mahévas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, Kanter J,
Neufeld E, Taube T, Ramenghi U, Shenoy S, Ward MJ, Mihatov N, Patel VL,
Bierling P, Lesser M, Cooper N, Bussel JB. Outcomes 5 years after
response to rituximab therapy in children and adults with immune
thrombocytopenia. Blood. 2012 Jun 21;119(25):5989-95. Epub 2012 May 7.
PubMed PMID: 22566601;
http://dx.doi.org/10.1182/blood-2011-11-393975

- Heusele M,
Clerson P, Guery B, Lambert M, Launay D, Lefevre G, Morell-Dubois S,
Maillard H, Le Gouellec N, Hatron PY, Hachulla E. Risk factors for
severe bacterial infections in patients with systemic autoimmune
diseases receiving rituximab. Clin Rheumatol. 2014 Jun;33(6):799-805.
http://dx.doi.org/10.1007/s10067-014-2509-2

- Crnkic
Kapetanovic M, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab
and abatacept but not tocilizumab impair antibody response to
pneumococcal conjugate vaccine in patients with rheumatoid arthritis.
Arthritis Res Ther. 2013 Oct 30;15(5):R171. PubMed PMID: 24286269;
PubMed Central PMCID: PMC3978887.
http://dx.doi.org/10.1186/ar4358

- Svensson M, Dahlin U,
Kimby E. Better response with conjugate vaccine than with polysaccaride
vaccine 12 months after rituximab treatment in lymphoma patients. Br J
Haematol. 2012 Feb;156(3):407-9. Epub 2011 Sep 19. PubMed PMID:
21923649. http://dx.doi.org10.1111/j.1365-2141.2011.08867.x

- Moulis
G, Lapeyre-Mestre M, Mahévas M, Montastruc JL, Sailler L. Need for an
improved vaccination rate in primary immune thrombocytopenia patients
exposed to rituximab or splenectomy. A nationwide population-based
study in France. Am J Hematol. 2014 Dec 30. [Epub ahead of print] http://dx.doi.org/10.1002/ajh.23930

- Zanella
A, Barcellini W. Treatment of autoimmune hemolytic anemias.
Haematologica. 2014 Oct;99(10):1547-54. doi:
10.3324/haematol.2014.114561. Review. PubMed PMID: 25271314; PubMed
Central PMCID: PMC4181250.
http://dx.doi.org/10.3324/haematol.2014.114561

- Barcellini
W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors
of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of
308 patients. Blood. 2014; 124(19):2930-2936
http://dx.doi.org/10.1182/blood-2014-06-583021
PMid:25232059

- Dierickx D, Kentos A,
Delannoy A. The role of rituximab in adults with warm antibody
autoimmune hemolytic anemia. Blood. 2015 May 21;125(21):3223-9.
http://dx.doi.org/10.1182/blood-2015-01-588392

- Reynaud Q,
Durieu I, Dutertre M, Ledochowski S, Durupt S, Michallet AS,
Vital-Durand D, Lega JC. Efficacy and safety of rituximab in
auto-immune hemolytic anemia: A meta-analysis of 21 studies. Autoimmun
Rev. 2015 Apr;14(4):304-13. doi: 10.1016/j.

- Bolton-Maggs,
P.H.B., Stevens, R.F., Dodd, N.J., Lamont, G., Tittensor, P. &
King, M.J. (2004) Guidelines for the diagnosis and management of
hereditary spherocytosis. British Journal of Haematology, 126, 455–474
http://dx.doi.org/10.1111/j.1365-2141.2004.05052.x
PMid:15287938

- Schilling RF. Risks and
benefits of splenectomy versus no splenectomy for hereditary
spherocytosis--a personal view. Br J Haematol. 2009 Jun;145(6):728-32.
Epub 2009 Apr 15. Review. PubMed PMID:19388926.
http://dx.doi.org/10.1111/j.1365-2141.2009.07694.x

- Rice
HE, Englum BR, Rothman J, Leonard S, Reiter A, Thornburg C, Brindle
M,Wright N, Heeney MM, Smithers C, Brown RL, Kalfa T, Langer JC, Cada
M, Oldham KT,Scott JP, St Peter S, Sharma M, Davidoff AM, Nottage K,
Bernabe K, Wilson DB,Dutta S, Glader B, Crary SE, Dassinger MS, Dunbar
L, Islam S, Kumar M, RescorlaF, Bruch S, Campbell A, Austin M, Sidonio
R, Blakely ML; Splenectomy in Congenital Hemolytic Anemia (SICHA)
Consortium. Clinical outcomes of splenectomy in children: report of the
splenectomy in congenital hemolytic anemia registry.Am J Hematol. 2015
Mar;90(3):187-92. Epub 2014 Nov 24. PubMed PMID: 25382665; PubMed
Central PMCID: PMC4333061. http://dx.doi.org/10.1002/ajh.23888

- De
Franceschi L. Pathophisiology of sickle cell disease and new drugs for
the treatment. Mediterr J Hematol Infect Dis. 2009 Dec
20;1(1):e2009024. http://dx.doi.org/10.4084/MJHID.2009.024

- Booth
C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J
Infect Dis. 2010 Jan;14(1):e2-e12. Epub 2009 Jun 3. Review. PubMed
PMID: 19497774. http://dx.doi.org/10.1016/j.ijid.2009.03.010

- Sobota
A, Sabharwal V, Fonebi G, Steinberg M. How we prevent and manage
infection in sickle cell disease. Br J Haematol. 2015 May 27. [Epub
ahead of print] PubMed PMID: 26018640.
http://dx.doi.org/10.1111/bjh.13526

- Ahmed SG. The
role of infection in the pathogenesis of vaso-occlusive crisis in
patients with sickle cell disease. Mediterr J Hematol Infect
Dis.2011;3(1):e2011028. Epub 2011 Jul 8. PubMed PMID:21869914; PubMed
Central PMCID: PMC3152450.
http://dx.doi.org/10.4084/mjhid.2011.028

- Brousse V, Buffet
P, Rees D. The spleen and sickle cell disease: the sick(led)spleen. Br
J Haematol. 2014 Jul;166(2):165-76. Epub 2014 May 26. Review. PubMed
PMID: 24862308. http://dx.doi.org/10.1111/bjh.12950

- Adamkiewicz
TV, Sarnaik S, Buchanan GR, Iyer RV, Miller ST, Pegelow CH, Rogers ZR,
Vichinsky E, Elliott J, Facklam RR, O'Brien KL, Schwartz B, Van Beneden
CA, Cannon MJ, Eckman JR, Keyserling H, Sullivan K, Wong WY, Wang WC.
Invasive pneumococcal infections in children with sickle cell disease
in the era of penicillin prophylaxis, antibiotic resistance, and
23-valent pneumococcal polysaccharide vaccination. J Pediatr. 2003
Oct;143(4):438-44. Erratum in: J Pediatr. 2004 Mar;144(3):412. PubMed
PMID: 14571216.

- Kizito ME, Mworozi E, Ndugwa C, Serjeant GR.
Bacteraemia in homozygous sickle cell disease in Africa: is
pneumococcal prophylaxis justified? Arch Dis Child. 2007
Jan;92(1):21-3. Epub 2006 Mar 10. PubMed PMID: 16531454; PubMed Central
PMCID: PMC2083172.

- Makani J, Mgaya J, Balandya E, Msami K, Soka
D, Cox SE, Komba AN, Rwezaula S, Meda E, Muturi D, Kitundu J, Fegan G,
Kirkham FJ, Newton CR, Snow RW, Lowe B. Bacteraemia in sickle cell
anaemia is associated with low haemoglobin: a report5of 890 admissions
to a tertiary hospital in Tanzania. Br J Haematol. 2015 Jun 17. [Epub
ahead of print] PubMed PMID: 26084722.
http://dx.doi.org/10.1111/bjh.13553

- Sakran W, Levin C,
Kenes Y, Colodner R, Koren A. Clinical spectrum of serious bacterial
infections among splenectomized patients with hemoglobinopathies in
Israel: a 37-year follow-up study. Infection. 2012 Feb;40(1):35-9. Epub
2011 Aug 25. PubMed PMID: 21866338.
http://dx.doi.org/10.1007/s15010-011-0178-5

- Al-Salem AH.
Splenic complications of sickle cell anemia and the role of
splenectomy. ISRN Hematol. 2011;2011:864257. doi: 10.5402/2011/864257.
Epub 2010 Oct 31. PubMed PMID: 22084706; PubMed Central PMCID:
PMC3200071. http://dx.doi.org/10.5402/2011/864257

- Owusu-Ofori
S, Hirst C. Splenectomy versus conservative management for acute
sequestration crises in people with sickle cell disease. Cochrane
Database SystRev. 2013 May 31;5:CD003425. doi:10. PubMed PMID:
23728644. http://dx.doi.org/10.1002/14651858.CD003425.pub2

- Adamkiewicz
TV, Silk BJ, Howgate J, Baughman W, Strayhorn G, Sullivan K,Farley MM.
Effectiveness of the 7-valent pneumococcal conjugate vaccine inchildren
with sickle cell disease in the first decade of life. Pediatrics. 2008
Mar;121(3):562-9. PubMed PMID: 18310206.
http://dx.doi.org/10.1542/peds.2007-0018

- Adamkiewicz TV,
Silk BJ, Howgate J, Baughman W, Strayhorn G, Sullivan K, Farley MM.
Effectiveness of the 7-valent pneumococcal conjugate vaccine in
children with sickle cell disease in the first decade of life.
Pediatrics. 2008 Mar;121(3):562-9. PubMed PMID: 18310206.
http://dx.doi.org/10.1542/peds.2007-0018

- Hirst C,
Owusu-Ofori S. Prophylactic antibiotics for preventing
pneumococcalinfection in children with sickle cell disease. Cochrane
Database Syst Rev. 2014 Nov 6;11:CD003427. Review. PubMed PMID:
25375222. http://dx.doi.org/10.1002/14651858.CD003427.pub3

- Lesher
AP, Kalpatthi R, Glenn JB, Jackson SM, Hebra A. Outcome of splenectomy
in children younger than 4 years with sickle cell disease. J Pediatr
Surg. 2009 Jun;44(6):1134-8; discussion 1138. PubMed PMID: 19524729.
http://dx.doi.org/10.1016/j.jpedsurg.2009.02.016

- Mouttalib
S, Rice HE, Snyder D, Levens JS, Reiter A, Soler P, Rothman
JA,Thornburg CD. Evaluation of partial and total splenectomy in
children with sickle cell disease using an Internet-based registry.
Pediatr Blood Cancer. 2012 Jul15;59(1):100-4. Epub 2012 Jan 11. PubMed
PMID: 22238140; PubMed Central PMCID: PMC3330148.
http://dx.doi.org/10.1002/pbc.24057

- Afuwape O, Ogole G,
Ayandipo O. Splenectomy in a Nigerian Teaching Hospital: Acomparison of
sonographic correlation with intra-operative findings in trauma. J
Emerg Trauma Shock. 2013 Jul;6(3):186-8. PubMed PMID: 23960375; PubMed
Central PMCID: PMC3746440.
http://dx.doi.org/10.4103/0974-2700.115336

- Cappellini MD,
Cohen A, Porter J, Taher A, Viprakasit V, editors. Guidelines for the
Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 3rd
edition. Nicosia (CY): Thalassaemia International Federation; 2014.
Available from http://www.ncbi.nlm.nih.gov/books/NBK269382/ PubMed
PMID: 25610943.

- Ricerca BM, Di Girolamo A, Rund D. Infections in
thalassemia and hemoglobinopathies: focus on therapy-related
complications. Mediterr J Hemato Infect Dis. 2009 Dec 28;1(1):e2009028.
PubMed PMID: 21415996; PubMed Central PMCID: PMC3033166
http://dx.doi.org/10.4084/MJHID.2009.028

- Merchant RH, Shah
AR, Ahmad J, Karnik A, Rai N. Post Splenectomy Outcome in
ß-Thalassemia. Indian J Pediatr. 2015 Jun 23. [Epub ahead of print]
PubMed PMID: 26099360.

- Wang SC, Lin KH, Chern JP, Lu MY, Jou ST,
Lin DT, Lin KS. Severe bacterial infection in transfusion-dependent
patients with thalassemia major. Clin Infect Dis. 2003 Oct
1;37(7):984-8. Epub 2003 Sep 5. PubMed PMID: 13130412

- Chirico
V, Lacquaniti A, Piraino B, Cutrupi M, Cuppari C, Grasso L, Rigoli L,
David A, Arrigo T, Salpietro C. Thalassaemia major and infectious risk:
High Mobility Group Box-1 represents a novel diagnostic and prognostic
biomarker. Br J Haematol. 2015 Jun 8. [Epub ahead of print] PubMed
PMID: 26058743 http://dx.doi.org/10.1111/bjh.13530

- Cherchi
GB, Pacifico L, Cossellu S, et al. Prospective study of Yersinia
enterocolitica infection in thalassemic patients. Pediatr Infect Dis J
1995; 14:579–84.
http://dx.doi.org/10.1097/00006454-199507000-00005
PMid:7567285

- Adamkiewicz TV, Berkovitch
M, Krishnan C, Polsinelli C, Kermack D, Olivieri NF. Infection due to
Yersinia enterocolitica in a series of patients with b-thalassemia:
incidence and predisposing factors. Clin Infect Dis 1998; 27:1362–
http://dx.doi.org/10.1086/515025 PMid:9868642

- Chan GC, Chan S, Ho
PL, Ha SY. Effects of chelators (deferoxamine, deferiprone and
deferasirox) on the growth of Klebsiella pneumoniae and Aeromonas
hydrophila isolated from transfusion-dependent thalassemia patients.
Hemoglobin. 2009;33(5):352-60. PubMed PMID: 19814682.
http://dx.doi.org/10.3109/03630260903211888

- Sheikha AK,
Salih ZT, Kasnazan KH, Khoshnaw MK, Al-Maliki T, Al-Azraqi TA, Zafer
MH. Prevention of overwhelming postsplenectomy infection in thalassemia
patients by partial rather than total splenectomy. Can J Surg. 2007
Oct;50(5):382-6. PubMed PMID: 18031639; PubMed Central PMCID:
PMC2386178.

- Wong GK, Goldacker S, Winterhalter C, Grimbacher B,
Chapel H, Lucas M,Alecsandru D, McEwen D, Quinti I, Martini H, Milito
C, Schmidt RE, Ernst D,Espanol T, Vidaller A, Carbone J, Fernandez-Cruz
E, Lougaris V, Plebani A,Kutukculer N, Gonzalez-Granado LI, Contreras
R, Kiani-Alikhan S, Ibrahim MA,Litzman J, Jones Gaspar HB, Hammarstrom
L, Baumann U, Warnatz K, Huissoon AP; Clinical Working Party of the
European Society for Immunodeficiencies (ESID). Outcomes of splenectomy
in patients with common variable immunodeficiency (CVID):a survey of 45
patients. Clin Exp Immunol. 2013 Apr;172(1):63-72. Erratum in: Clin Exp
Immunol. 2013 Jul;173(1):161. Milito, C [added]. PubMed PMID: 23480186;
PubMed Central PMCID: PMC3719932.
http://dx.doi.org/10.1111/cei.12039

- Rao VK, Oliveira JB.
How I treat autoimmune lymphoproliferative syndrome. Blood. 2011 Nov
24;118(22):5741-51. doi: 10.1182/blood-2011-07-325217. Epub 2011 Sep 1.
Review. PubMed PMID: 21885601; PubMed Central PMCID: PMC3228494.
http://dx.doi.org/10.1182/blood-2011-07-325217

- Price S,
Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, Perkins K, Hornung RL,
Folio L, Rosenberg PS, Puck JM, Hsu AP, Lo B, Pittaluga S, Jaffe ES,
Fleisher TA, Rao VK, Lenardo MJ. Natural history of autoimmune
lymphoproliferative syndrome associated with FAS gene mutations. Blood.
2014 Mar 27;123(13):1989-99. Epub 2014 Jan 7. PubMed PMID: 24398331;
PubMed Central PMCID: PMC3968385.
http://dx.doi.org/10.1182/blood-2013-10-535393

- Neven B,
Bruneau J, Stolzenberg MC, Meyts I, Magerus-Chatinet A, Moens L,
Lanzarotti N, Weller S, Amiranoff D, Florkin B, Bader-Meunier B,
Leverger G,Ferster A, Chantrain C, Blanche S, Picard C, Molina TJ,
Brousse N, Durandy A, Rizzi M, Bossuyt X, Fischer A, Rieux-Laucat F.
Defective anti-polysaccharide response and splenic marginal zone
disorganization in ALPS patients. Blood. 2014 Sep 4;124(10):1597-609.
Epub 2014 Jun 26.PubMed PMID: 24970930.
http://dx.doi.org/10.1182/blood-2014-02-553834

- Andersson
A, Enblad G, Gustavsson A, Erlanson M, Hagberg H, Molin D, Tavelin B,
Melin B. Long term risk of infections in Hodgkin lymphoma long-term
survivors. Br J Haematol. 2011 Sep;154(5):661-3. Epub 2011 Apr 7.
PubMed PMID: 21470197.
http://dx.doi.org/10.1111/j.1365-2141.2011.08638.x

- Lenglet
J, Traullé C, Mounier N, Benet C, Munoz-Bongrand N, Amorin S, Noguera
ME, Traverse-Glehen A, Ffrench M, Baseggio L, Felman P, Callet-Bauchu
E, Brice P,Berger F, Salles G, Brière J, Coiffier B, Thieblemont C.
Long-term follow-up analysis of 100 patients with splenic marginal zone
lymphoma treated with splenectomy as first-line treatment. Leuk
Lymphoma. 2014 Aug;55(8):1854-60. Epub 2014 May 6. PubMed PMID:
24206091. http://dx.doi.org/10.3109/10428194.2013.861067

- Xing
KH, Kahlon A, Skinnider BF, Connors JM, Gascoyne RD, Sehn LH, Savage
KJ, Slack GW, Shenkier TN, Klasa R, Gerrie AS, Villa D. Outcomes in
splenic marginal zone lymphoma: analysis of 107 patients treated in
British Columbia. Br J Haematol. 2015 May;169(4):520-7. Epub 2015 Apr
8. PubMed PMID: 25854936. http://dx.doi.org/10.1111/bjh.13320

- Rialon
KL, Speicher PJ, Ceppa EP, Rendell VR, Vaslef SN, Beaven A, Tyler
DS,Blazer DG 3rd. Outcomes following splenectomy in patients with
myeloid neoplasms. J Surg Oncol. 2015 Mar 15;111(4):389-95. Epub 2014
Dec 9 http://dx.doi.org/10.1002/jso.23846

- Barosi G,
Ambrosetti A, Buratti A, Finelli C, Liberato NL, Quaglini S, Ricetti
MM, Visani G, Tura S, Ascari E. Splenectomy for patients with
myelofibrosis with myeloid metaplasia: pretreatment variables and
outcome prediction. Leukemia. 1993 Feb;7(2):200-6. PubMed PMID: 8426474

- Tefferi
A, Mesa RA, Nagorney DM, Schroeder G, Silverstein MN. Splenectomy
inmyelofibrosis with myeloid metaplasia: a single-institution
experience with 22 patients. Blood. 2000 Apr 1;95(7):2226-33. PubMed
PMID: 10733489

- Santos FP, Tam CS, Kantarjian H, Cortes J,
Thomas D, Pollock R Verstovsek S. Splenectomy in patients with
myeloproliferative neoplasms efficacy,complications and impact on
survival and transformation. Leuk Lymphoma. 2014 Jan;55(1):121-7.
http://dx.doi.org/10.3109/10428194.2013.794269

- Centers for
Disease Control and Prevention (CDC). Use of 13-valentpneumococcal
conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for
adults with immunocompromising conditions: recommendations of the
Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal
Wkly Rep. 2012 Oct 12;61(40):816-9. PubMed PMID: 23051612.

- Mourtzoukou
EG, Pappas G, Peppas G, Falagas ME. Vaccination of asplenic or
hyposplenic adults. Br J Surg 2008;95:273–80,
http://dx.doi.org/10.1002/bjs.6106

- Wang J, Jones P, Cheng
AC, Leder K. Adherence to infection prevention measures in a statewide
spleen registry. Med J Aust. 2014 May 19;200(9):538-40

- Serio B,
Pezzullo L, Giudice V, Fontana R, Annunziata S, Ferrara I, Rosamilio R,
De Luca C, Rocco M, Montuori N, Selleri C. OPSI threat in
hematological patients. Transl Med Uni Sa. 2013 May 6;6:2-10. eCollection
2013. PubMed PMID:24251241; PubMed Central PMCID: PMC3829791.

- Shapiro
ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al.
The protective efficacy of polyvalent pneumococcal polysaccharide
vaccine. N Engl J Med 1991;325:1453–60,
http://dx.doi.org/10.1056/NEJM199111213252101

- Cherif H,
Landgren O, Konradsen HB, Kalin M, Bjorkholm M. Poor antibody response
to pneumococcal polysaccharide vaccination suggests increased
susceptibility to pneumococcal infection in splenectomized patients
with hematological diseases. Vaccine 2006;24:75–81.
http://dx.doi.org/10.1016/j.vaccine.2005.07.054
PMid:16107293

- Meerveld-Eggink A, de
Weerdt O, van Velzen-Blad H, Biesma DH, Rijkers GT. Response to
conjugate pneumococcal and Haemophilus influenzae type b vaccines in
asplenic patients. Vaccine. 2011 Jan 17;29(4):675-80. Epub 2010 Nov 27.
PubMed PMID: 21115060
http://dx.doi.org/10.1016/j.vaccine.2010.11.034

- American
Academy of Pediatrics. Committee on Infectious Diseases. Policy
statement: Recommendations for the prevention of pneumococcal
infections, including the use of pneumococcal conjugate , pneumococcal
polysaccharidevaccine, and antibiotic prophylaxis. Pediatrics
2000;106:362–366.

- Forstner C, Plefka S, Tobudic S, Winkler HM,
Burgmann K, Burgmann H. Effectiveness and immunogenicity of
pneumococcal vaccination in splenectomized and functionally asplenic
patients. Vaccine. 2012 Aug 10;30(37):5449-52. Epub 2012 Jun 27. PubMed
PMID: 22749594. http://dx.doi.org/10.1016/j.vaccine.2012.06.048

- Wu
DB, Chaiyakunapruk N, Chong HY, Beutels P. Choosing between 7-, 10- and
13-valent pneumococcal conjugate vaccines in childhood: a review of
economic evaluations (2006-2014). Vaccine. 2015 Mar 30;33(14):1633-58.
Epub 2015 Feb 11. Review. PubMed PMID: 25681663.
http://dx.doi.org/10.1016/j.vaccine.2015.01.081

- Nived P,
Jørgensen CS, Settergren B. Vaccination status and immune response to
13-valent pneumococcal conjugate vaccine in asplenic individuals.
Vaccine. 2015 Mar 30;33(14):1688-94. Epub 2015 Feb 21. PubMed PMID:
25707692 http://dx.doi.org/10.1016/j.vaccine.2015.02.026

- Centers
for Disease Control and Prevention (CDC), Advisory Committeeon
Immunization Practices. Updated recommendations for prevention
ofinvasive pneumococcal disease among adults using the 23-valent
pneu-mococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly
Rep 2010;59:1102–6.

- Davies JM, Lewis MP, Wimperis J, Rafi I,
Ladhani S, Bolton-Maggs PH; British Committee for Standards in
Haematology. Review of guidelines for the prevention and treatment of
infection in patients with an absent or dysfunctional spleen: prepared
on behalf of the British Committee for Standards in Haematology by
aworking party of the Haemato-Oncology task force. Br J Haematol.
2011Nov;155(3):308-17. Review. PubMed
http://dx.doi.org/10.1111/j.1365-2141.2011.08843.x

- Spelman
D, Buttery J, Daley A, Isaacs D, Jennens I, Kakakios A, Lawrence
R,Roberts S, Torda A, Watson DA, Woolley I, Anderson T, Street A;
Australasian Society for Infectious Diseases. Guidelines for the
prevention of sepsis inasplenic and hyposplenic patients. Intern Med J.
2008 May;38(5):349-56. Epub 2008 Feb 14. Review. PubMed PMID:18284463.
http://dx.doi.org/10.1111/j.1445-5994.2007.01579.x

- Salvadori
MI, Price VE; Canadian Paediatric Society, Infectious Diseases and
Immunization Committee. Preventing and treating infections in children
with asplenia or hyposplenia. Paediatr Child Health. 2014
May;19(5):271-8. English, French. PubMed PMID: 24855431; PubMed Central
PMCID: PMC4029242.

- Greenberg RN, Gurtman A, Frenck RW, et al.
Sequential administration of 13-valent pneumococcal conjugate vaccine
and 23-valent pneumococcal polysaccharide vaccine in pneumococcal
vaccinena.ve adults 60-64 years of age. Vaccine 2014; 32: 2364-74.
http://dx.doi.org/10.1016/j.vaccine.2014.02.002
PMid:24606865

- Jackson LA, Gurtman A,
Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal
conjugate vaccine in adults 70 years of age and older previously
vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine
2013; 31:3585-93. http://dx.doi.org/10.1016/j.vaccine.2013.05.010
PMid:23688527

- Bonten MJ, Huijts SM,
Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van
Deursen AM, Sanders EA, Verheij TJ, Patton M, McDonough A,
Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G,
Schmoele-Thoma B, Scott DA, Jansen KU, Lobatto R, Oosterman B, Visser
N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE.
Polysaccharide conjugate vaccine against pneumococcal pneumonia in
adults. N Engl J Med. 2015 Mar 19;372(12):1114-25.
http://dx.doi.org/10.1056/NEJMoa1408544

- Smith
MJ. Meningococcal tetravalent conjugate vaccine. Expert Opin Biol Ther.
2008 Dec;8(12):1941-6. doi: 10.1517/14712590802538455 . Review. PubMed
PMID18990080.

- O'Ryan
M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component
meningococcal serogroup B vaccine (4CMenB): the clinical development
program. Drugs. 2014 Jan;74(1):15-30. doi:10.1007/s40265-013-0155-7. Review

- Dotel R, Gosbell
IB, Hofmeyr A. Compliance with Australian splenectomy guidelines in
patients undergoing post-traumatic splenectomy at a tertiary centre.
Med J Aust. 2015 Mar 16;202(5):III-V. PubMed PMID: 25758689.

- Meerveld-Eggink
A, de Weerdt O, van Velzen-Blad H, Biesma DH, Rijkers GT. Response to
conjugate pneumococcal and Haemophilus influenzae type b vaccines in
asplenic patients. Vaccine. 2011 Jan 17;29(4):675-80. Epub 2010 Nov 27.
PubMed PMID: 21115060.
http://dx.doi.org/10.1016/j.vaccine.2010.11.034

- Meerveld-Eggink
A, de Weerdt O, de Voer RM, Berbers GA, van Velzen-Blad H, Vlaminckx
BJ, Biesma DH, Rijkers GT. Impaired antibody response to conjugated
meningococcal serogroup C vaccine in asplenic patients. Eur J Clin
Microbiol Infect Dis. 2011 May;30(5):611-8. Epub 2010 Dec 24. PubMed
PMID: 21184126 http://dx.doi.org/10.1007/s10096-010-1129-2

- McCavit
TL, Xuan L, Zhang S, Flores G, Quinn CT. Hospitalization for invasive
pneumococcal disease in a national sample of children with sickle cell
disease before and after PCV7 licensure. Pediatr Blood Cancer. 2012
Jun;58(6):945-9. Epub 2011 Jul 25. PubMed PMID: 21793185; PubMed
Central PMCID: PMC4248562 http://dx.doi.org/10.1002/pbc.23259

[TOP]



























































































































































