Received: January 17, 2016
Accepted: February 8, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016017, DOI 10.4084/MJHID.2016.017
This article is available on PDF format at:

Marco Cerrano1, Elena Crisà1, Valentina Giai1, Mario Boccadoro1 and Dario Ferrero1
1 Hematology Division, Università degli Studi di Torino, Turin, Italy.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Anemia in the elderly is a common but
challenging clinical scenario. Here we describe the case of an older
woman who presented with anemia and elevated inflammation markers.
After a complete diagnostic work-up, a definite etiology of the anemia
could not be found so eventually a bone marrow biopsy was performed and
she was diagnosed with myelodysplastic syndrome. She responded well to
erythropoietin treatment but her inflammation markers remained elevated
thus a positron emission tomography was performed. It turned out that
the patient suffered from giant cell artheritis and her anemia
completely resolved after steroid treatment. Our case outlines that it
is necessary to pay particular attention to anemia of inflammation,
which could be due to several and often masked conditions.
Myelodysplatic syndromes should be considered when other causes have
been ruled out, but their diagnosis can be difficult and requires
expertise in the field. |
Introduction
Anemia in the elderly is a very common condition that contributes to morbidity and mortality and significantly impairs quality of life. In the Third National Health and Nutrition Examination Survey (NHANES III) study the incidence of anemia in men and women older than 65 was 11% and 10.2%, respectively,[1] thus representing a problem almost every physician deals with. Several causes often contribute to anemia in this age group, and it is not always possible to find a unifying diagnosis,[2] hence it frequently represents a challenging clinical scenario. Here we present the case of an elderly Italian woman investigated for persistent anemia and elevated inflammation markers.
Case Report
Case presentation:
A 68 year old woman was admitted to a primary care center for worsening
asthenia and fatigability. During the two months before admission, she
also referred weight loss, mild fever, and cough.
Clinical
history: She was diagnosed with ovarian cancer 13 years before, and she
was treated with surgery and a carboplatin-containing chemotherapy
regimen. No signs of disease recurrence were found afterwards. She
suffered from hypertension, well controlled with medical therapy,
arthrosis, and chronic constipation.
Initial work-up:
At admission clinical examination was unremarkable. Blood count showed
severe normocytic anemia (hemoglobin 7.5 g/dL, mean corpuscular volume
90 fL) and mild thrombocytosis (platelets 452000/µL) while reticulocyte
count, leucocyte count, and differential were normal. Iron or vitamin
deficiencies were ruled out, and lactate dehydrogenase, serum
creatinine, thyroid functioning and liver tests were normal. Reactive C
protein (RCP), erythrocyte sedimentation rate (ESR) and serum ferritin,
however, were significantly increased (18.2 mg/dL, 160 mm/h and 1136
ng/mL, respectively).
Differential diagnosis: Nutritional
deficiencies (i.e. lack of iron, vitamin B12 or folic acid), chronic
kidney disease, thalassemia trait and inflammation are the most
frequent causes of anemia in elderly patients and should be considered
first.[3] Hemolysis, radio- and chemo-therapeutic interventions,
hypothyroidism or hepatic insufficiency are other common causes that
should be ruled out. Anemia due to inflammation (AI) is maybe the most
complex one. As a matter of fact, several pathophysiological mechanisms
are involved such as disturbance of iron homeostasis, impaired
proliferation of erythroid progenitor cells and reduced erythropoietin
response. Furthermore, the causes of inflammation are often multiple
and not always the source is apparent (e.g. inflammatory bowel disease,
rheumatologic disorders, cancer, infections).[4]
Besides, AI
can mask iron deficiency because, in the presence of both these
conditions, the commonly used serum iron-status indicators (namely
iron, transferrin, transferrin saturation and ferritin) can be
difficult to interpret.[3] The discovery of hepcidin, the main
regulator of iron homeostasis, significantly improved our understanding
of the pathophysiology of AI and the measurement of serum hepcidin
level, which is down-regulated in case of iron deficiency and
up-regulated in presence of inflammation, soon could become a useful
tool in the diagnostic work-up of anemia in the elderly.[5]
Further examinations:
Since inflammation markers remained elevated in our patient, other
investigations were performed to rule out an infection, an autoimmune
disease or malignancy: microbiological tests were negative,
autoimmunity tests showed a low antinuclear antibody titer and a weak
rheumatoid factor positivity, chest, and abdomen computed tomography
and echocardiography were normal. Anemia persisted, no precise
inflammatory source could be found and a bone marrow (BM) trephine
biopsy was eventually performed and evaluated by the local pathologist.
It showed a hypercelullar bone marrow with trilinear dysplasia,
abnormal localization of immature precursors and 1.5% of blasts. She
was diagnosed with myelodysplastic syndrome (MDS)-unspecified, without
blast excess, likely secondary to chemotherapy exposure. Cytogenetic
analysis and BM aspiration were not performed.
Differential diagnosis:
A BM dysfunction, either primary, such as MDS, or secondary to
neoplastic or infectious agents, should be considered after ruling out
all other causes of anemia. MDS are suspected in elderly patients in
case of unexplained cytopenia, more commonly isolated macrocytic
anemia, and confirmed with a BM aspirate and biopsy.
Hematologic follow-up:
The patient was then discharged and referred to our center. When she
presented to our clinic, she was afebrile, reported fatigue and
weakness. Her blood count was stable with isolated anemia requiring
weekly transfusions and ESR and RCP remained elevated. In order to
confirm the diagnosis, we proposed her a new BM evaluation with
morphological and cytogenetic analysis but the patient refused it. As
per our policy, we tested the blood level of Wilms tumor gene
transcript (frequently elevated in acute myeloid leukemia and MDS),[6]
and it was in the normal range. Giving the diagnosis of low risk MDS
with isolated anemia, we checked serum erythropoietin level, that
resulted in the reference range but inadequate for the degree of anemia
(31.7 mUI/mL), therefore we started the patient on recombinant
erythropoietin, 40000 U weekly. Her anemia progressively improved and
she achieved an erythroid response[7] after 3 weeks of treatment,
becoming transfusion independent.
Final diagnosis:
So far it could have looked like a typical low-risk MDS responsive to
erythropoietin. However, the patient was still complaining of
arthralgias and ESR and RCP remained elevated. Moreover, she reported
the appearance of a livedo reticularis on her lower limbs. To better
clarify the case, given the persistently elevated inflammation markers
and the personal history of ovarian cancer, a total body positron
emission tomography (PET) was performed. Surprisingly, it revealed a
dishomogeneous and intense hyperfixation in the wall of medium and big
arterial vessels, consistent with a vascular inflammatory process (Figure 1).
We referred the patient to a rheumatologist, and she underwent a biopsy
of the temporal artery which revealed histology consistent with giant
cell arteritis (GCA). Erythropoietin was discontinued; she was started
on corticosteroid therapy and her anemia rapidly resolved. Seven years
after GCA diagnosis our patient is doing well, with normal blood counts
and without any further therapy.
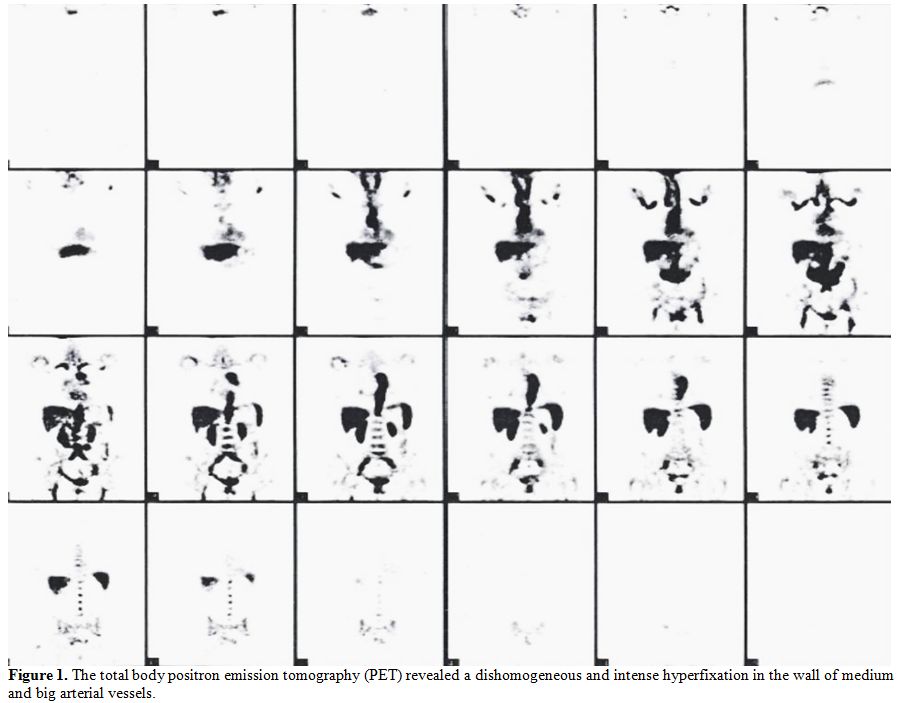 |
Figure 1. The total body positron emission tomography (PET) revealed a dishomogeneous and intense hyperfixation in the wall of medium and big arterial vessels. |
Discussion
In the case we are presenting, the initial work-up of our patient
demonstrated severe anemia associated with significantly elevated
inflammation markers, prompting the diagnosis of AI. However, even
though several examinations were performed, a clear etiology could not
be found and eventually the patient underwent a BM biopsy. The
diagnosis seemed to be MDS.
MDS usually present with anemia,
isolated or combined with neutropenia and thrombocytopenia,[8] but
anemia and thrombocytosis can seldom occur.[9,10] Anemia is usually
macrocytic (rarely it can be normocytic or even microcytic),[11]
reticulocyte count is (relatively) reduced, there can be signs of
dyserithropoiesis and serum ferritin level can be elevated. On
peripheral blood smear a dimorphic red-cell population that includes
oval macrocytes can be seen, often together with neutrophils or
platelets abnormalities (e.g. hypogranulated neutrophils or pseudo
Pelger–Huët cells).[8] MDS diagnosis is confirmed by performing a BM
examination. It is important to carry out both BM aspirate and trephine
biopsy as the first one is essential to evaluate cellular morphology
and to count the proportion of blasts while the latter allows for
determination of BM cellularity and architecture.[12] Indeed the
pathological hallmark of MDS is BM dysplasia that can only be assessed
by morphology, which represents the most important tool to establish a
definite diagnosis.[13] The 2008 World Health Organization
classification of MDS requires the demonstration of unequivocal
dysplasia in at least 10% of the cells of the erythroid, granulocytic
or megakaryocytic lineage.[14] However, nutritional deficiencies,
medications, toxins, growth factor therapy, inflammation or infections
can sometimes cause secondary dysplasia and thus they should be
excluded before a diagnosis of MDS is established. In the cases of MDS
with an excess of blasts or with more than 15% of ring sideroblasts in
the erythroid precursors the diagnosis is usually straightforward. In
the other cases, the morphologic evidence of dysplasia may not be
unequivocal, and the presence of a specific cytogenetic abnormality or
the immunophenotyping can be helpful in confirming the
diagnosis.[14,15] If unilinear dysplasia is the only proven sign of
myelodysplasia an observation period of 6 months and a second BM
investigation is recommended to establish a definite MDS diagnosis.[14]
The complexity of diagnosis of MDS can lead to mistakes and significant
discrepancies between peripheral and tertiary care centers have been
outlined.[16,17] Therefore, it appears important that an experienced
hematologist along with an hemopatologist establish the diagnosis of
MDS and a discussion with other colleagues experts in this field could
be useful when in doubt.
Our patient was eventually diagnosed with
GCA. GCA can present with typical symptoms such as a headache, jaw and
tongue claudication, scalp tenderness, visual disturbance and
manifestations of polymyalgia rheumatica[18] and the diagnosis still
relies on the 1990 American College of Rheumatology classification
criteria [3 of the following 5 criteria are required: 1) age 50 years
or older, 2) new-onset localized headache, 3) temporal artery
tenderness or decreased temporal artery pulse, 4) ESR of at least 50
mm/h, 5) abnormal artery biopsy specimen characterized by mononuclear
infiltration or granulomatous inflammation].[19] However, the diagnosis
of GCA can be difficult due to the variability of clinical
presentation, and the utility of these criteria in clinical practice
has been questioned.[20] Furthermore, the presentation of GCA can be
atypical and cases showing only anemia and raised inflammatory markers
have been reported.[21] A persistent dry cough can be a presenting
symptom too, either isolated or associated with typical
manifestations.[22] Our case was challenging because the patient
complained only of anemia related symptoms, arthralgias, and mild fever
and the elevated inflammation markers could not be easily interpreted.
It outlines that is it is necessary to be aware of the entire spectrum
of symptoms of GCA in order to consider it in the differential
diagnosis of anemia. Indeed, our patient initially complained of a
cough, but this symptom was not reported later. The use of PET can
certainly facilitate the diagnosis of GCA, but it is expensive, and it
exposes patients to a significant radiation risk.
An association
between autoimmune diseases, including vasculitis, and MDS has been
reported, especially in case of chronic myelo-monocytic leukemia and
high risk MDS.[23-25] However, in our patient anemia completely
resolved with steroid treatment and she is currently doing well seven
years after GCA diagnosis without any further therapy.
Conclusion
Authorship and Disclosures
MC reviewed the literature and wrote the paper. DF followed the patient and wrote the paper. EC and VG reviewed the paper. MB supervised the work and provided founds.
References

























[TOP]