Received: October 20, 2016
Accepted: November 4, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016062, DOI 10.4084/MJHID.2016.062
This article is available on PDF format at:

Francesca Pavanello*, Sara Steffanoni*, Michele Ghielmini and Emanuele Zucca

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract The natural history of follicular lymphoma is usually characterized by an indolent course with a high response rate to the first line therapy followed by recurrent relapses, with a time to next treatment becoming shorter after each subsequent treatment line. More than 80% of patients have advanced stage disease at diagnosis. The time of initiation and the nature of the treatment are mainly conditioned by symptoms, tumor burden, lymphoma grading, co-morbidities and patients' preference. A number of clinical and biological factors have been determined to be prognostic in this disease, but the majority of them could not show to be predictive of response to treatment, and therefore can’t be used to guide the treatment choice. CD20 expression is the only predictive factor recognized in the treatment of FL and justifies the use of “naked” or “conjugated” anti-CD20 monoclonal antibodies as a single agent or in combination with chemo- or targeted therapy. Nevertheless, as this marker is almost universally found in FL, it has little role in the choice of treatment. The outcome of patients with FL improved significantly in the last years, mainly due to the widespread use of rituximab, autologous and allogeneic transplantation in young and fit relapsed patients, the introduction of new drugs and the improvement in diagnostic accuracy and management of side effects. Agents as new monoclonal antibodies, immuno-modulating drugs, and target therapy have recently been developed and approved for the relapsed setting, while studies to evaluate their role in first line treatment are still ongoing. Here we report our considerations on first line treatment approach and on the potential factors which could help in the choice of therapy. |
Introduction
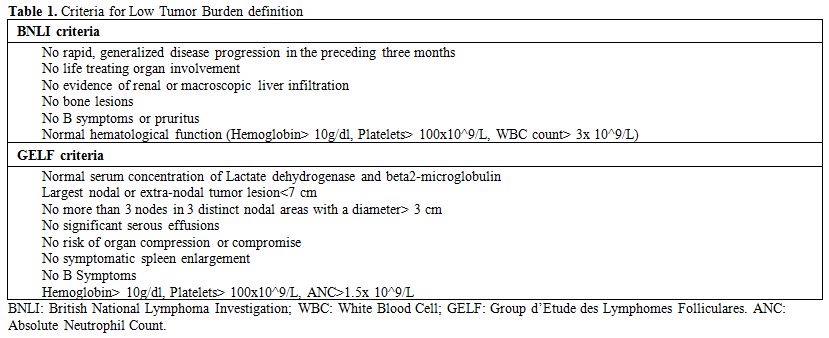 |
Table 1. Criteria for Low Tumor Burden definition |
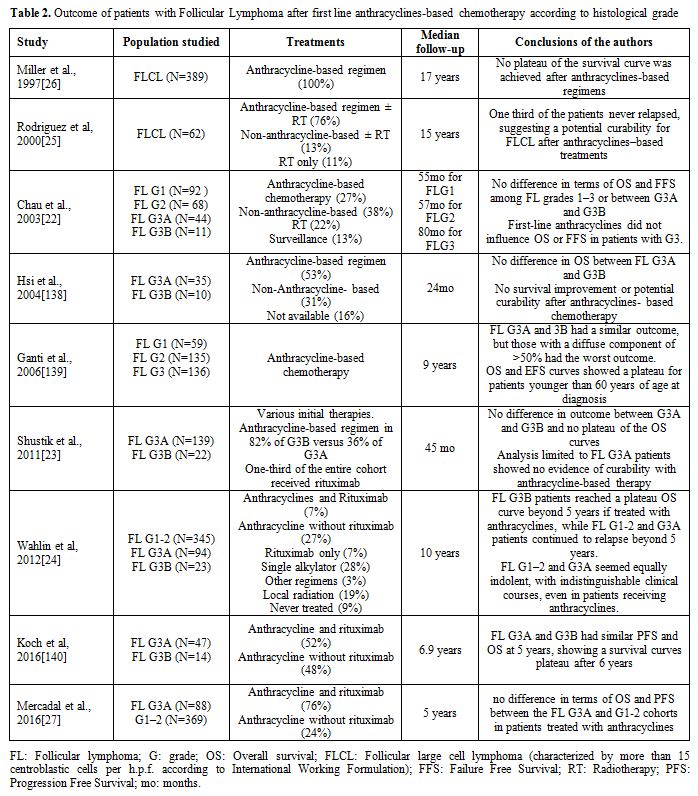 |
Table 2. Outcome of patients with Follicular Lymphoma after first line anthracyclines-based chemotherapy according to histological grade |
Treatment
References







































































































































[TOP]