Mohamed Yassin1, Ashraf Soliman2, Vincenzo De Sanctis3, Abdelqadir Nashwan4, Sandra Abusamaan5, Abbas Moustafa5, Samah Kohla6 and Dina Soliman6
1 Department of Hematology, Hamad Medical Center, Doha.
2 Department of Pediatric, University of Alexandria, Egypt.
3 Department of Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
4 Department of Nursing HMC, Doha, Qatar.
5 Department of Radiology, Doha, Qatar.
6 Department of Laboratory Medicine, Doha, Qatar.
Corresponding
author: Vincenzo De Sanctis MD,
Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, 44100
Ferrara, Italy; Tel. +39 0532 770243. E-mail:
vdesanctis@libero.it
Published: June 20, 2017
Received: December 21, 2016
Accepted: May 18, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017037 DOI
10.4084/MJHID.2017.037
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction: Sickle
cell disease (SCD) is one of the leading causes of morbidity and
mortality worldwide, causing damage and dysfunction in multiple organs.
The complications of this disease are numerous, affect every organ
and/or tissue in the body and vary considerably among patients over the
time challenging its management.
The aim of our study:
To determine the iron status of 17 patients with
non-transfusion-dependent sickle cell disease ( NT-SCD) patients and
six patients with transfusion dependent sickle cell disease (TD- SCD)
using both serum ferritin level (SF) and Ferriscan® evaluation of liver
iron content (LIC). We correlated the values of LIC with SF levels and
some hepatic enzymes (alanine transaminase-ALT, aspartate
aminotransferase -AST, alkaline phosphatase -ALP and albumin).
Results:
17 adults with NT-SCD (n = 17, age: 32±15 years) were studied. Seven of
NT-SCD had SF > 500 μg/L, 4 out of the seven had high liver iron
measured by FerriScan® (> 30 mg/g/ tissue dry weight - dw). Two
patients had high LIC despite a concomitant SF concentration < 500
μg/L. Two patients had high SF (1.117 μg/L and 675 μg/L) while their
LIC was normal (< 30 mg/g/dw). Five patients had elevated ALT and/or
AST) concentrations. In TD-SCD (n = 6, age = 25 ± 11 years), 2 patients
had SF <500 μg/L, one of them had high LIC (127 mg/g/DW). Liver
enzymes were high in two patients. SF concentration correlated
significantly with LIC (r = 0.85, p < 0.001). Neither SF level nor
LIC was correlated significantly with hepatic enzyme levels.
Conclusions:
A significant number of our patients with NT-SCD had high LIC, high SF
and elevated liver enzymes (ALT and AST). Despite some limitations of
our study, due to the limited number of NT-SCD patients, these findings
have important clinical implications. Therefore, we recommend measuring
SF and LIC in NT-SCD patients to apply preventive measures with iron
chelation therapy in patients with high LIC.
|
Introduction
Some
haemoglobinopathies, such as thalassaemia major (TM), are severe enough
to require life-long blood transfusions whereas patients with
non-transfusion-dependant thalassaemia (NT-T) and sickle cell disease
(SCD) will need either intermittent, regular or no transfusions,
dependent on disease severity and disease-related complications.
Regular blood transfusions result in the gradual accumulation of iron,
initially in the liver, and then throughout the body including the
heart and endocrine organs. In contrast, subjects with
non-transfusion-dependent sickle cell disease (NT-SCD) may be
relatively protected from iron-mediated liver, cardiac and endocrine
gland toxicity.[1-3]
Making a clinical diagnosis
of iron overload is difficult because patients do not usually develop
clinical symptoms until the advanced stages of the disease.
The
biochemical markers of the iron metabolism disorders include an
elevated concentration of iron and serum ferritin (SF) and transferrin
saturation in plasma. However, these parameters are not always specific
for body iron load.[4] Furthermore, SF can be unreliable in SCD due to the inflammatory nature of the condition, even in the steady state.[5]
In
a cross-sectional study of 27 children (10.9 ± 3.3 years) with SCD who
had received chronic transfusion therapy without chelation, transfusion
volume provided more insight on liver iron content (LIC) than serum
iron markers.[6]
In another study of 20 patients
with SCD undergoing chronic transfusion therapy with iron chelation,
LIC showed a positive correlation with the duration of transfusion and
liver fibrosis but not with serum markers.[7]
The
gold standard for assessing liver iron stores, in the absence of
cirrhosis, is the LIC, determined by liver biopsy and quantitation with
atomic absorption spectrophotometry. The normal LIC is between 0.4 and
2.2 mg/g of dry liver weight. Based on data from hereditary
hemochromatosis, < 7 mg/g is not associated with obvious hepatic
pathology while >15 mg/g is consistently associated with liver
fibrosis.[8]
The use of biopsy-measured LIC is
limited by the small but finite risk of complications of liver biopsy,
lack of reproducibility of quantitative assays, and sampling error.[9]
Magnetic
resonance imaging (MRI) is a non-invasive method that detects iron
overload and allows to monitor treatment after diagnosis, avoiding
repeated biopsies. In fact, iron ions have the paramagnetic properties,
and its accumulation in the tissues causes local distortion in the
magnetic fields, with a consequent loss of signal intensity in the
affected organs that is proportional to the amount of iron deposited.[10]
A standardized and validated MRI method is now registered in Europe and
the United States (Ferriscan®), with a reproducible relationship
between the value (R2) by MRI and LIC by biopsy over a clinically
useful range in which locally acquired data are analyzed at a central
facility. This is potentially available in any hospital with an MRI
scanner and with minimal training of local staff.[10,11]
SCD
patients, despite their transfusion-independence, can accumulate iron
due to increased intestinal absorption. Since the guidelines for the
use of chelation therapy in SCD with iron overload are based on the
same principles as those for TM to avoid serious clinical sequelae,[12]
we measured LIC using FerriScan® in two groups of SCD patients with
transfusion dependent (TD- SCD) and non-transfusion dependent (NT-SCD)
in order to assess which parameters most effectively predicted iron
loading in the liver.
Patients and Methods
Eleven
adult patients with NT-SCD who did not receive any blood transfusion
for at least five years and 6 NT-SCD patients with a clinical history
of occasional blood transfusions (less than six units of blood), for
sickling episodes during early childhood period, were studied.
Twenty-six
percent of patients were female. None of them had been splenectomized.
Their hemoglobin (Hb) level varied from 7 to 10.5 g/dl. Hepatitis
screening for HBV, HCV, and HIV was negative in all patients. Patients
were tested for hemochromatosis genes C282Y, and H63 D and both
mutations were negative. Our ND-SCD were slightly older than TD-SCD
patients.
Six patients with TD-SCD (on regular blood transfusion
and iron chelation) were studied as controls. They were all on top-up
transfusion, and none of them was on transfusion-exchange program. They
used to be chelated with oral deferasirox (30 mg/kg/day) for the past
four years and previously received subcutaneous daily desferrioxamine
therapy. Their compliance to chelation before oral therapy was variable.
An
extensive medical history, including transfusion and chelation therapy,
and a physical examination was performed for each patient. Their Hb
electrophoresis diagnosis of SCD was confirmed. All other
hemoglobinopathies were excluded. All SCD patients had a HbSS genotype.
Lab investigations included measurement of their serum concentrations
of iron, total iron binding capacity (TIBC), serum ferritin, alkaline
phosphatase (ALP), alanine transferase (ALT), aspartate transferase
(AST) and albumin concentrations. Liver iron content (LIC) was measured
using Ferriscan®.[10,11]
SF was measured by
immune-enzymatic and electrochemiluminescence immunoassays. The
manufacturer's normal reference range values were 30-350 μg/L in males
and 15-150 μg/L in females.
LIC values were expressed as mg/g dry
weight (DW). LIC (mg Fe/gr dw) were classified into: normal (LIC
<3); mild (LIC > 3 and < 7), moderate (LIC > 7 and < 14)
and severe overload (LIC > 14).[13]
All SCD
patients had cardiac MRI T2* for evaluation of their cardiac iron
overload using a 1.5 T scanner (GE Signa/Excite HD, Milwaukee, WI,
USA). A conservative cut-off value of heart T2* > 20 ms was
considered normal.[14]
Ethical approval for the
study was obtained by Ethical Committee of Hamad General Hospital which
were in accordance, by the Declaration of Helsinki (http://www.wma.net). All procedures were carried out with the adequate understanding and consent of patients.
Pearson's
and Spearman's correlation tests were used to studying correlations
between variables with parametric and non-parametric distributions
respectively. p < 0.05 was considered significant.
Results
17
adults with NT-SCD (n = 17, age: 32 ±15 years) were studied. Seven of
NT-SCD had SF > 500 μg/L. Four out of 7 had high LIC measured by
FerriScan® (> 30 mg/g/DW). Two of them had history of receiving two
blood transfusions during their childhood. Two NT-SCD patients had high
LIC despite a concomitant SF < 500 μg/L. Two patients had high SF
(1.117 μg/L and 675 μg/L) while their LIC was normal (< 30 mg/g/DW).
Five patients had elevated ALT and/or AST concentrations. Out of the 17
patients with NT-SCD, 1 had mild (LIC > 3 and < 7), 13 had
moderate (LIC > 7 and < 14) and 3 had severe iron overload (LIC
> 14).
The NT-SCD group consisted of 11 non-transfused, and six
occasionally transfused patients. There was no significant difference
between the two groups since the occasional transfusion group received
less than six units of blood which appears to be insufficient in
producing a significant iron overload.
The six patients with
TD-SCD (age: 25 ± 11 years), on regular blood transfusion and iron
chelation with deferasirox (30 mg/g body weight, Exjade®) had SF
<500 μg/L, and one had increased LIC (127 mg/g/DW. Liver enzymes
were high in 2 patients.
SF concentrations and LIC were significantly higher in TD-SCD versus NT-SCD patients (Table 1).
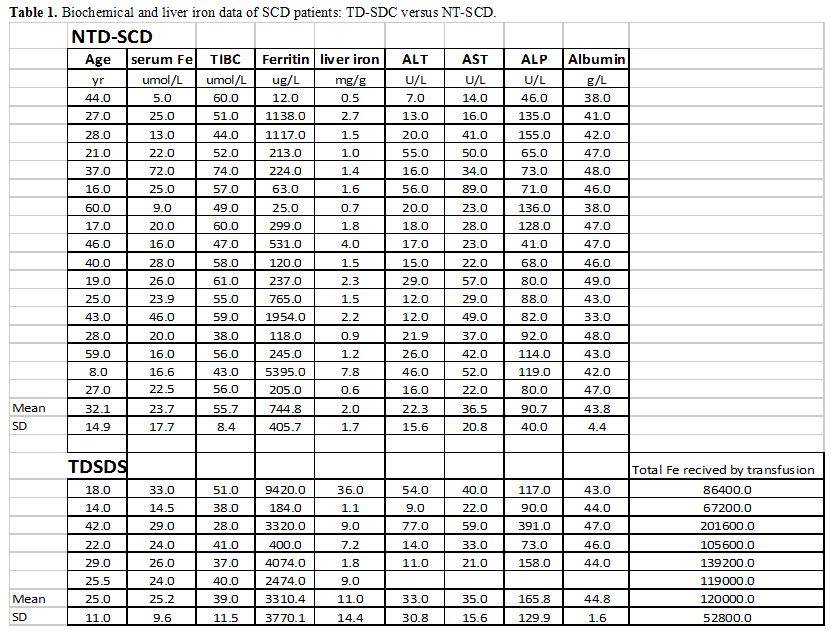 |
Table 1. Biochemical and liver iron data of SCD patients: TD-SDC versus NT-SCD. |
In
all studied patients (NT-SCD and TD-SCD) the SF concentrations were
correlated significantly with LIC, measured by FerriScan (r = 0.85, p
< 0.001) (Figure 1). LIC was
also significantly correlated with ALT concentrations (r= 0.464, p =
0.02) SF levels did not correlate significantly with serum ALT, AST or
ALP. Serum iron concentration and TIBC did not correlate with SF, LIC
or ALT and AST concentrations.
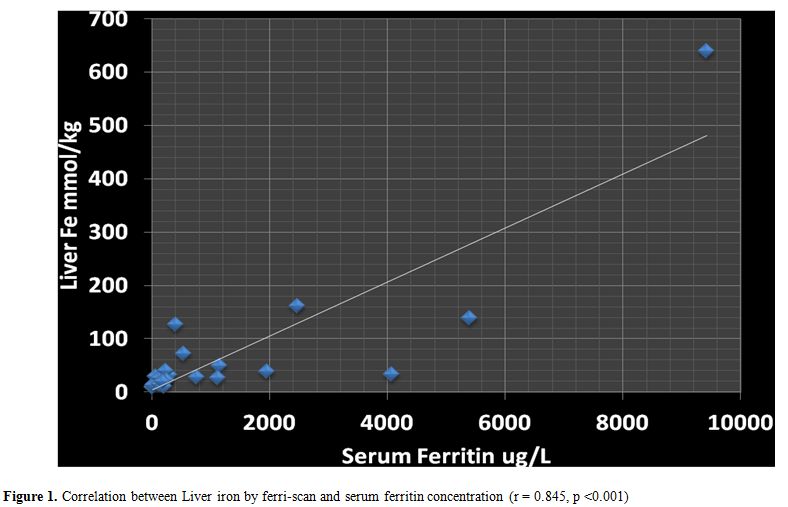 |
Figure 1. Correlation between Liver iron by ferri-scan and serum ferritin concentration (r = 0.845, p <0.001) |
In
the TD-SCD patient group, neither LIC nor serum ferritin was correlated
significantly with total elemental iron received by transfusions (r =
0.2 and 0.02; p > 0.05).
None of the NT-SCD or TD-SCD patients had significant cardiac iron overload.
Multiple
regression analysis including all studied factors (serum iron, iron
binding capacity, ALT, AST, albumin) revealed that LIC was the only
factor contributing significantly to serum ferritin level (coefficient
= 14.5; t stat = 5.7; p = 0.00003)
Discussion
Sickle
cell disease is an important cause of morbidity and mortality
worldwide, causing damage and dysfunction in multiple organs. The
complications of this disease are numerous, affect every organ and/or
tissue in the body and vary considerably among patients over the time
challenging its management. Greater focus on the long-term hepatic
consequences of iron overload is recommended in SCD.
Our study
confirms an increased (LIC >30 mg/g dry tissue) and high SF in a
considerable number of patients with NT-SCD with no or insignificant
previous blood transfusions. Some of the NT-SCD had a high LIC despite
an SF < 500 μg/L.
In SCD the liver can be affected by several
complications due to the disease itself and its treatment. Hepatic
siderosis is a growing area of concern and research.[15]
As red cell transfusions become routine for more indications, the
inevitable result is the accumulation of liver iron. Over many years,
hepatic dysfunction, insufficiency, fibrosis, and cirrhosis may lead to
morbidity. Chelation with deferoxamine, deferasirox, or deferiprone has
been used to reduce total body iron.[15,16]
Scarce information is available in the literature regarding patients receiving sporadically blood transfusions. Drasar et al.[17]
observed that even sporadically transfused patients can become heavily
iron overloaded, on par with those on transfusion programs.
Our
findings confirm these data and indicate that some adults with NT-SCD
have a considerable hepatic iron overload that may adversely affect
their hepatic function. The positive correlation between LIC and serum
concentration of ALT support the concept of the deleterious effect of
iron overload on hepatocytes.[3,17,18] It should be mentioned, however, that our NT-SDS were slightly older than TD-SCD patients.
In
our patients (NT-SCD and TD-SCD), SF serum ferritin levels were
correlated significantly with LIC measured by Ferriscan validating the
use of SF as a screening test for assessing iron overload in SCD
patients (Figure 1). However,
the finding of some cases with high LIC despite SF < 500 μg/L
necessitates measuring LIC with these non-invasive methods when hepatic
symptoms or signs (e.g, hepatomegaly, liver tenderness, elevated
hepatic enzymes appear, even in the absence of high serum ferritin).
Excess
iron may result from the parenteral administration (blood transfusion)
or increased intestinal absorption. The iron absorbed by the small
intestine (duodenum and proximal jejunum) binds to transferrin–a
transport protein in the blood. Once iron is bound to transferrin, it
is selectively deposited in hepatocytes, red blood cells or, to a
lesser extent, in other iron containing tissues, like muscle.[18,19]
This explains significant iron overload in TD-SDS especially those with
poor compliance to iron chelation. However, in patients with NT-SDS
iron overload appears to be primary due chronic hemolysis.
Ferriscan
is one of the available systems to evaluate LIC. MRI is noninvasive and
has been shown to provide accurate results compared to the gold
standard. It is widely available across the world and several different
models for calculating LIC using MRI, both T2 relaxometry and signal
intensity ratio (SIR) methods, are being used with satisfactory
results.[20]
Our data suggest that monitoring
for iron overload and its complications, using non-invasive methods, is
important, even though these are less frequent in SCD compared to
thalassaemia major patients (TM). Chelation treatment could be
reconsidered earlier in this cohort of patients with high LIC.
Guidelines for starting chelation therapy in SCD patients are based on
the same principles as those for TM (SF is > 1000 μg/L or LIC is
> 7 mg/g dry weight or > 20 top-up units of transfusion).[21]
Conclusions
In
both NT- SCD and TD- SCD monitoring liver iron status by measuring SF
and LIC, using Ferriscan® method, can diagnose early hepatic iron
overload. This helps to decide about starting and tailoring iron
chelation accordingly to reduce risk of developing hepatopathy in these
patients.
References
- Porter J , Garbowski M. Consequences and management
of iron overload in sickle cell disease. ASH Education Book December 6,
2013 vol. 2013 no. 1 447-456. http://asheducationbook.hematologylibrary.org/content/2013/1/447.full

- Mohanty
D, Mukherjee MB, Colah RB, Wadia M, Ghosh K, Chottray GP, Jain D,
Italia Y, Ashokan K, Kaul R, Shukla DK, Muthuswamy V. Iron deficiency
anaemia in sickle cell disorders in India. Indian J Med Res 2008;
127:366-369. PMid:18577791

- Walter PB, Harmatz P, Vichinsky E. Iron metabolism and iron chelation in sickle cell disease. Acta Haematol 2009;122:174-183. https://doi.org/10.1159/000243802 PMid:19907155

- Nielsen
P, Engelhardt R, Düllmann J, Fischer R. Non-Invasive Liver Iron
Quantification by SQUID-Biosusceptometry and Serum Ferritin Iron as New
Diagnostic Parameters in Hereditary Hemochromatosis. Blood Cells Mol
Dis 2002;29:451-458. https://doi.org/10.1006/bcmd.2002.0583 PMid:12547235

- Adamkiewicz
TV, Abboud MR, Paley C, Olivieri N, Kirby-Allen M, Vichinsky E, Casella
JF, Alvarez OA, Barredo JC, Lee MT, Iyer RV, Kutlar A, McKie KM, McKie
V, Odo N, Gee B, Kwiatkowski JL, Woods GM, Coates T, Wang W, Adams RJ.
Serum ferritin level changes in children with sickle cell disease on
chronic blood transfusion are nonlinear and are associated with iron
load and liver injury. Blood 2009;114:4632-4638. https://doi.org/10.1182/blood-2009-02-203323 PMid:19721013 PMCid:PMC2780299

- Brown
K, Subramony C, May W, Megason G, Liu H, Bishop P, Walker T, Nowicki
MJ. Hepatic iron overload in children with sickle cell anemia on
chronic transfusion therapy. J Pediatr Hematol Oncol 2009;31:309-312. https://doi.org/10.1097/MPH.0b013e3181a1c143 PMid:19415007

- Harmatz
P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D,
Neumayr L, Vichinsky E. Severity of iron overload in patients with
sickle cell disease receiving chronic red blood cell transfusion
therapy. Blood 2000;96:76-79. PMid:10891433

- Bassett
ML, Halliday JW, Powell LW. Value of hepatic iron measurements in early
hemochromatosis and determination of the critical iron level associated
with fibrosis. Hepatology 1986;6:24-29. https://doi.org/10.1002/hep.1840060106 PMid:3943787

- Angelucci
E, Baronciani D, Lucarelli G, Baldassarri M, Galimberti M, Giardini C,
Martinelli F, Polchi P, Polizzi V, Ripalti M, Needle liver biopsy in
thalassaemia: analyses of diagnostic accuracy and safety in 1184
consecutive biopsies. Br J Haematol 1995;89:757-761. https://doi.org/10.1111/j.1365-2141.1995.tb08412.x PMid:7772512

- St
Pierre TG, Clark PR, Chua-Anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK,
Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging
of liver iron concentrations using proton magnetic resonance. Blood
2005;105:855-861. https://doi.org/10.1182/blood-2004-01-0177 PMid:15256427

- Hankins
JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS,
Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the
liver in patients with iron overload. Blood 2009;113:4853-4855. https://doi.org/10.1182/blood-2008-12-191643 PMid:19264677 PMCid:PMC2686136

- Inati A. Recent advances in improving the management of sickle cell disease. Blood Rev 2009;23 (Suppl 1):S9-13. https://doi.org/10.1016/S0268-960X(09)70004-9

- Casale
M, Meloni A, Filosa A, Cuccia L, Caruso V, Palazzi G, Gamberini MR,
Pitrolo L, Putti MC, D'Ascola DG, Casini T, Quarta A, Maggio A, Neri
MG, Positano V, Salvatori C, Toia P, Valeri G, Midiri M, Pepe A.
Multiparametric Cardiac Magnetic Resonance Survey in Children With
Thalassemia Major: A Multicenter Study. Circ Cardiovasc Imaging. 2015
Aug;8(8):e003230. https://doi.org/10.1161/CIRCIMAGING.115.003230 PMid:26253625

- Positano
V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, Forni
G, Borgna-Pignatti C, Lai ME, Midiri M, Maggio A, Lombardi M, Landini
L. Standardized T2* map of normal human heart in vivo to correct T2*
segmental artefacts. NMR Biomed 2007;20:578-590. https://doi.org/10.1002/nbm.1121 PMid:17205488

- Vichinsky
E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie
L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused
patients with SCD or beta thalassemia.Am J Hematol. 2005;80:70-74. https://doi.org/10.1002/ajh.20402 PMid:16138345

- Ballas
SK, Kesen MR, Goldberg MF, Lutty GA, Dampier C, Osunkwo I, Wang WC,
Hoppe C, Hagar W, Darbari DS, Malik P. Beyond the definitions of the
phenotypic complications of sickle cell disease: an update on
management. Scientific World Journal. 2012; 2012:949535 https://doi.org/10.1100/2012/949535 PMid:22924029 PMCid:PMC3415156

- Drasar
E, Vasavda N, Igbineweka N, Awogbade M, Allman M, Thein SL. Serum
ferritin and total units transfused for assessing iron overload in
adults with sickle cell disease. Br J Haematol 2012;157:645-647. https://doi.org/10.1111/j.1365-2141.2012.09060.x PMid:22332939

- Spina
JC, Alvarez del Rivero MA, Kidd C, Pietrani M, Savluky L, García Mónaco
RD. Noninvasive assessment of hepatic iron overload in patients with
hemochromatosis Rev Argent Radiol 2013; 77:139-146.

- Lucania
G, Vitrano A, Filosa A,Maggio A.Chelation treatment in
sickle-cell-anaemia: much ado about nothing? Br J Haematol.2011;
154:545-555. https://doi.org/10.1111/j.1365-2141.2011.08769.x PMid:21707578

- Alústiza
JM, Emparanza JI, Castiella A, Casado A, Garrido A, Aldazábal P, San
Vicente M, Garcia N, Asensio AB, Banales J, Salvador E, Moyua A,
Arozena X, Zarco M, Jauregui L, Vicente O. Measurement of liver iron
concentration by MRI is reproducible. Biomed Res Int. 2015;2015:294024.
https://doi.org/10.1155/2015/294024

- Siegelman
ES, Mitchell DG, Semelka RC. Abdominal iron deposition: metabolism, MR
findings, and clinical importance. Radiology. 1996;199:13-22. https://doi.org/10.1148/radiology.199.1.8633135 PMid:8633135

[TOP]
























