Biobele Brown1, Hannah Dada-Adegbola2, Catherine Trippe3 and Olufunmilayo Olopade4
1 Department of Paediatrics, University College Hospital/College of Medicine, University of Ibadan, Ibadan, Nigeria.
2 Department of Medical Microbiology, University College Hospital/College of Medicine, University of Ibadan, Ibadan, Nigeria.
3 University of Chicago Pritzker School of Medicine, Chicago, IL, USA.
4 Section of Hematology and Oncology, Department of Medicine, University of Chicago, Chicago, IL, USA.
Corresponding
author: Dr. B. J. Brown. Department of Paediatrics, University College Hospital, Ibadan, Nigeria. Tel: +234 8051875510. E-mail:
biosbrown@yahoo.com
Published: June 20, 2017
Received: February 26, 2017
Accepted: May 26, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017039 DOI
10.4084/MJHID.2017.039
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background & Objectives: As
a result of immune defects in Sickle cell disease (SCD), affected
individuals are prone to infection from encapsulated bacterial
pathogens like Streptococcus Pneumoniae.
Studies on the etiological agents of bacteremia in children with SCD in
Nigeria are few and have revealed a spectrum of organisms that is
different from those recorded in other parts of the world.
Aim and Objectives:
The objectives of this study were to determine the prevalence of
bacteremia, etiological agents and antibiotic susceptibility pattern in
febrile children with SCD attending the University College Hospital
(UCH), Ibadan, Nigeria.
Methods:
The study was cross-sectional and took place at the Department of
Pediatrics of the UCH, Ibadan. Children with SCD, ages 0-17 years
presenting with axillary temperature ≥ 38°C were enrolled after
obtaining informed consent. History was obtained and complete
physical examination performed after which blood was collected for
culture and antibacterial susceptibility tests.
Results:
A total of 116 children were studied of which 69 (59.5%) were males,
111 (95.7%) were of the Hemoglobin SS phenotype and 5 (4.3%) of the
Hemoglobin SC phenotype. Bacteremia was present in 16 (13.8%) of the
116 children. Gram negative bacteria constituted 10 (62.5%) of all
isolates, while the predominant isolates were Klebsiella pneumoniae 4, (25%) and Staphylococcus aureus, 4 (25%). Over 80% of the isolates were susceptible to Ceftriaxone, Amikacin and Meropenem.
Conclusions: Klebsiella pneumoniae and Staphylococcus aureus are the predominant causes of bacteremia in children with SCD in Ibadan, contrary to findings in western countries.
|
Introduction
Sickle
cell disease (SCD) is a genetic disorder of the hemoglobin in red
cells. Globally, about 5% of the world’s population carries genes
responsible for hemoglobinopathies. Each year, about 300 000 infants
are born with major hemoglobin disorders including more than 200 000
cases of sickle-cell anemia in Africa.[1] In Nigeria, by far the most
populous country in West Africa, 24% of the population carries the
mutant gene, and the prevalence of sickle-cell anemia is about 20 per
1000 births. This means that in Nigeria alone, about 150 000 children
are born annually with sickle-cell anemia.[1]
As a result of immune defects in SCD, affected individuals are prone to infection from encapsulated bacterial pathogens such as Streptococcus pneumoniae, Haemophilus influenzae,
Salmonellae and parasitic infections such as malaria, resulting in
significant morbidity and mortality.[2] Few studies have reported the
prevalence of bacteremia in children with sickle cell disorders in
Nigeria. Okuonghae et al.[3] found bacteremia in 32.5 per cent of
febrile children with sickle cell anemia in Benin. A recent
retrospective study on hospitalized children with SCD in Ibadan
revealed that 32.2 percent of them were managed for septicaemia.[4]
However, some of the cases could not be confirmed by blood culture due
to inability of their parents to pay for the tests. Therefore, there
remains a need to confirm the prevalence of bacteremia in children with
SCD prospectively to highlight the burden.
Bacterial infections have been shown to be the major cause of death in children with sickle cell disease with Streptococcus pneumoniae
being the commonest etiological agent.[5] Consequently, neonatal
diagnosis of sickle cell disorders and introduction of prophylaxis
against Pneumococcus using Penicillin and vaccines have resulted in
reduction in infection related deaths and improved survival of children
with sickle cell disease in the United States.[6] Studies on the
etiological agents of bacteremia in SCD children in Nigeria are few and
have revealed a spectrum of etiological agents that is different from
previously recorded in other parts of the world. Studies on etiological
agents of bacteremia in Nigeria have revealed mainly Gram negative
bacteria such as Klebsiella spp and Salmonella spp and in one setting Staphylococcus aureus as the major organisms responsible.[3,7,8] In contrast, a study from rural Kenya revealed Streptococcus pneumoniae as
the predominant agent responsible for septicemia in children with
sickle cell disease.[9] One possible explanation to the different
patterns of bacterial isolates in studies on infections in Nigeria in
contrast to previous documentations in other parts of the world may be
due to frequent use of antibiotics before presentation in hospital in
Nigeria which could affect the result of bacterial cultures.[3,8]
Rarity in isolation of Streptococcus pneumoniae
has made it difficult making evidence based prevention strategies
against Pneumococcal infections in Nigeria.[2] In spite of this, some
health centers in the country have implemented routine prophylaxis with
penicillin and Pneumococcal vaccines are offered to children whose
parents can afford the cost.[10] The site of the current study
routinely offers pneumococcal (Pneumococcal conjugate vaccine-13
[PCV13] and Pneumococcal Polysaccharide vaccine-23 [PPSV23]) and Haemophilus influenza
type b (Hib) vaccines to children with SCD. Never-the-less, the need to
identify the common etiological agents of bacteremia in this post
pneumococcal vaccination era remains pertinent in order to inform
appropriate antimicrobial choices for treatment. This study was
therefore carried out to determine the prevalence of bacteremia among
febrile children with sickle cell disorders, the etiological agents and
their antibiotic susceptibility patterns at the University College
Hospital (UCH), Ibadan. This study utilized the automated bacterial
culture systems (BACTECTM) which contain resins that are capable of
neutralizing a wide variety of antibiotics thus allowing higher
isolation rates in patients who might have commenced antibiotics before
presentation;[11] this has been a major advantage over the conventional
blood culture systems.
Materials and Methods
The
study was cross sectional in design and took place at the children’s
outpatient and children’s emergency ward of the University College
Hospital, Ibadan. Patient enrolment took place over a period of 15
months beginning from May, 2013 during which 580 children with HbSS and
HbSC were followed up at the health facility. All children with sickle
cell disease (Hb SS or HbSC) presenting with fever (axillary
temperature ≥ 38°C) were enrolled after obtaining informed consent from
their parents or guardians. History was be obtained and complete
physical examination performed on each child after which blood was
collected aseptically by venipuncture The needle was changed to a new
one before introducing the blood collected into the broth in BACTEC
bottle ped plusTM. Samples were
processed at the Medical Microbiology laboratory of UCH for culture
using BACTEC 9050 (Becton, Dickinson Diagnostic Systems, Sparks, USA)
blood culture systems.[11] All the bacterial isolates were subjected,
initially to direct biochemical tests for preliminary bacterial
identification and antimicrobial susceptibility thereby allowing
affected children to be commenced on appropriate antibiotic therapy.
Further identification of bacterial isolates to the level of species
was carried out by testing for enzyme systems that are characteristic
of each species using the 24E Oxoid Microbact™ identification system.
The antibacterial susceptibility tests were carried out using commonly
employed classes of antibiotics such as penicillins, cephalosporins,
aminoglycosides, quinolones, imipenem and others.[12] All microbiology
tests were carried out with the standard practice as approved by
Clinical and Laboratory Standard Institute (CLSI) for culture,
identification and antibiotic susceptibility of bacterial isolates.[13]
The disc diffusion method as described by Bauer and Kirby was employed
for susceptibility testing and the report was stated as sensitive or
resistant after measuring the zone of inhibition and comparing it with
standard chart.[14] Control organisms included for GPC was Staphylococcus aureus NCTC 6571 and for Gram negative bacteria (GNB), Escherichia coli NCTC 35218 and Acinetobacter baumanni NCTC
7363. Quality control in terms of adherence to standard operating
procedure (SOP) was built into each step in the isolation and
identification of the bacterial also; the use of known positive and
negative isolates was employed for the biochemical tests. Other
relevant investigations aimed at identifying the possible foci of
bacteremia including chest x-rays, culture of urine and other
appropriate body fluids were done.
Blood specimen was
obtained from finger prick, for preparing thick and thin films which
were, air dried and stained with Giemsa and examined microscopically
for malaria parasites. Results of blood culture and microscopy for
malaria parasites were given to the physicians to aid the care of the
children who were treated according to departmental guidelines.
Ethical Considerations:
Ethical approval was obtained from the University of Ibadan/ University
College Hospital Ethics Committee. Informed consent was obtained from
the parents/guardians and assent from the children who were of
understanding.
Data Capture and Analysis:
Demographic and clinical data were obtained by one of the investigators
aided by a research assistant. Data was entered on to a case record
form and subsequently into a microcomputer using SPSS version 20.0.
Means (and standard deviations) and medians were computed for
continuous variables and comparisons made using either the T-test or
Mann-Whitney U test as applicable. Categorical variables were presented
as frequencies and percentages and association tested using Chi-square
or Fisher’s exact test as applicable. Risk factors were also assessed
by computation of odd ratios and 95% confidence intervals. The
susceptibility of the isolates to antibiotics in the panel was
presented as frequencies. Statistical significance was set at p <
0.05.
Results
A
total of 116 children were studied comprising 69 males (59.5%) and 47
females (40.5%). Out of these, 111 (95.7%) were of the Hemoglobin SS
(Hb SS) phenotype and the remaining 5 (4.3%) of the Hemoglobin SC (Hb
SC) phenotype. The 116 febrile children admitted out of the 580
children with Hb SS and HbSC followed up in the health facility in the
15 month study period yielded an incidence of 20 admissions per 100
person years.
The ages of the study patients ranged from 0.6 to
17.0 years with a mean (standard deviation) of 6.7 (4.2) years and
median of 6 years. One hundred and seven (92.2%) of the study
population were admitted into the hospital while the remaining 9 (7.8%)
children were treated on outpatient basis. None of the study patients
had been splenectomized. Blood culture was positive in 16 (13.8%)
of the 116 children. In the 15 month study period, 580 children aged
0-17 years with Hb SS and Hb SC were seen in the health facility;
therefore the 16 cases of bacteremia translate to an incidence of 2.2
per 100 patient years. The Gram Negative Bacteria (GNB) constituted 10
(62.5%) of all the isolates, while the common bacterial isolates were Klebsiella pneumoniae 4 (25%) and Staphylococcus aureus 4 (25%) as shown in Table 1. One case of Streptococcus Pneumoniae
was isolated in the study and this was in a 10 year old child. This
single case out of 420 children followed up at the health facility in
the 0-10 year age group (in the 15 month study period) translates to
0.19 infections per 100 patient years.
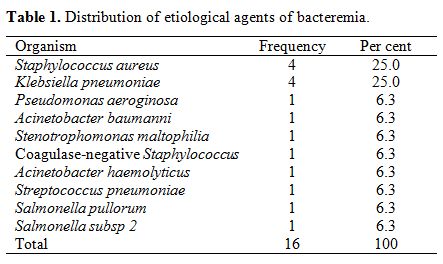 |
Table 1. Distribution of etiological agents of bacteremia. |
Ten
of the 16 cases of bacteremia were not associated with any focus of
infection but the remaining 6 were. Those associated with foci of
infection included 3 cases of Klebsiella pneumoniae infection associated with osteomyelitis, 1 case of Pseudomonas aeruginosa infection associated with cholecystitis and 2 cases of Staphylococcus aureus infections associated with Pneumonia and septic arthritis respectively.
Bacteremia
was present in 11(15.9%) of the 69 boys compared with 5 (10.6%) of the
47 girls (Chi-square = 661, P = 0.416). The mean (SD) age of children
with bacteremia was 6.4 (4.9) years, the median was 4.5 years and the
mode was 4.0 years. There was bacteremia in 8 (18.2%) of the 44
children aged less than 5 years compared with 8 (11.1%) of the 72
children aged 5 years and above (Fisher exact test p= 0.406). Thus,
there was no association between bacteremia, gender and age.
The
duration of fever in the study patients ranged from 1 to 10 days. The
median duration of fever was 3 days in both the group with bacteremia
and that without bacteremia (Mann-Whitney U test, p=0.712). The
hematocrit of the children on admission ranged from 8 to 34 per cent
with a mean (standard deviation= [SD]) of 21.6(5.3) percent. The mean
(SD) hematocrit in the 16 patients with bacteremia was 20.4 (5.2)
percent compared to 21.8 (5.3) percent in the 100 patients without
bacteremia (Independent samples T test t = -0.981, p = 0.338).
Fourteen
(12.1%) of the studied population had malaria parasitemia, with no
bacteremia, while bacteremia was found in 16 (15.7%) of the 102 malaria
negative patients. (Fisher’s exact test, p =0.212). All 14 children
with malaria parasitemia were given antimalarial drugs. A history of
prior antibiotic use was elicited in 16 (13.8%) of the studied
population, but this was not significantly associated with a reduced
likelihood of a positive blood culture (Table 2).
Out of the 16
children who used antibiotics prior to presentation in hospital, 5 each
had used Amoxicillin, and cefuroxime, 2 had used cefixime and 1each had
used Amoxicillin-Clavulanate and Ampicillin. One patient had
Amoxicillin-Clavulanate and Chloramphenicol serially and another
had, cefuroxime and Chloramphenicol.
With regards to
vaccine usage in the 116 children, 19 (16.4%) had received Hib vaccine,
10 (8.6%) had received PCV13 and 13 (11.2%) had received PPSV 23
vaccine and overall 20 (17.2%) had received at least one of any of the
vaccines. None of the children who received Pneumococcal conjugate
vaccine 13 (PCV 13), Pneumococcal Polysaccharide vaccine 23 (PPSV23)
and Haemophilus influenzae type b (Hib) vaccine had a positive blood culture. Failure to use PCV 13, PPSV23 and Haemophilus influenzae type b Hib vaccines was each associated with a slightly increased risk of bacteremia but p-values were all greater than 0.05 (Table 2).
Analysis
of the risk of various types of sickle cell crises with bacteremia
revealed no significantly increased risk of any form of crises with
bacteremia (Table 3).
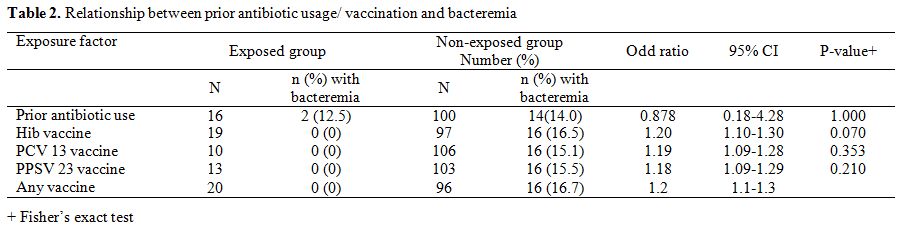 |
Table 2.
Relationship between prior antibiotic usage/ vaccination and bacteremia. |
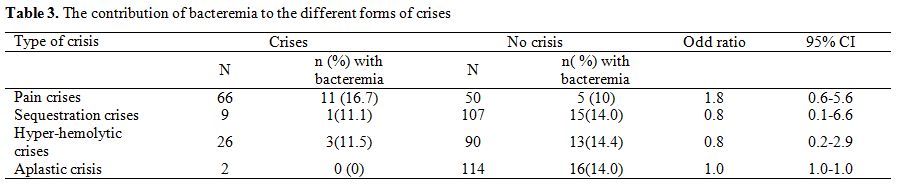 |
Table 3. The contribution of bacteremia to the different forms of crises. |
Table 3
also shows the contribution of bacteremia to the different forms of
crises viz: 16.7 percent of pain crises, 11.5 percent to
hyper-hemolytic crises and 11.1 percent of sequestration crises.
The
antimicrobial susceptibility of the isolates revealed highest
susceptibility to Meropenem followed by Ceftriaxone, Amikacin and
Ciprofloxacin and very low susceptibility to
Amoxicillin-clavulanic acid and ceftazidime (Table 4).
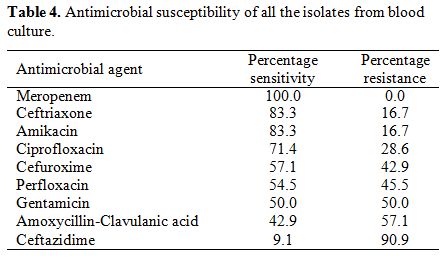 |
Table 4. Antimicrobial susceptibility of all the isolates from blood culture. |
Severe
anemia defined as a hematocrit of less than 15 percent was present in 3
(18.8%) of the 16 children with bacteremia compared with 13 (13.0%) out
of the 100 without bacteremia (Fisher’s exact test p =0.452). Nineteen
(16.4%) of the 116 children were transfused with blood, including 16
with severe anemia. Three (18.8%) of the 16 bacteremic children where
transfused with blood compared to 16 (16.0%) of the 100 non-bacteremic
children (Fisher’s exact test p= 0.725). Discussion
This
study has determined the incidence of bacteremia in febrile children
with sickle cell disease. The 13.8% incidence observed in this study is
higher than 3.4% reported in a retrospective study in London and 1.1%
in another retrospective study in the United States of America.[15,16]
It is however lower than 32.5% reported in Benin City, Nigeria,
about two decades earlier.[3] Our finding suggests that bacteremia is
more common in children with SCD in Nigeria than in developed
countries. It is tempting to assume that the lower incidence of
bacteremia in London and United States may be due to routine penicillin
prophylaxis and vaccinations in those settings.[15,16] However, the
majority of the etiological agents of bacteremia in the present study
were Gram negative organisms especially Klebsiella pneumoniae which corroborates two previous studies in Nigeria.[3,17] Another major etiological agent in the study was Staphylococcus aureus
which constituted the majority of GPC isolates. Since infections by
these agents are not at present vaccine-preventable, the disparity in
vaccinations between developed countries and Nigeria may not account
for the relatively high incidence of bacteremia in our setting.
However, patients living in developed countries are most likely better
nourished with better immunity and cleaner environments and with less
exposure to infection than those in developing countries like Nigeria.
This may explain the increased prevalence of bacteremia in this study
compared to those in developed countries.
It is important to note that only 20 (17.2%) children had any of the specific vaccines for prevention of Pneumococcal and Haemophilus influenza type b infections. Only one isolate of Streptococcus pneumoniae was found and it was in an unvaccinated child. Haemophilus influenzae type b was not isolated in the study. The predominant isolates were Klebsiella pneumoniae and Staphylococcus aureus which
is in keeping with previous studies and further confirms that both
organisms play significant roles in bacteremia among febrile
individuals with sickle cell disease in Nigeria.[3,17] The rarity of Streptococcus pneumoniae
infection in this study is contrary to findings in developed countries
before the institution of routine penicillin prophylaxis and
vaccination and also discordant with what was reported in Kenya.[5,9]
The 0.19 infection per 100 person-years incidence of invasive
pneumococcal disease (IPD) in children aged 10 years or less in the
present study is low compared to 1.7 infections per 100 person -years
in a US based study on children of a similar age group.[18] The low
incidence may be contributed to by the small proportion of children
aged 2 years or less in the cohort since most cases, sometime, as much
as 79% of IPDs may occur in the first 2 years of life[19] but only 15 %
of children followed up in our facility are in that age group due to
late diagnosis. The low incidence is however in agreement with findings
in previous studies in Nigeria and a study in Uganda.[3,8,20] A study
in Tanzania also revealed a predominance of Staphylococcus aureus and rarity of Streptococcus pneumoniae.[21]
Reasons that have been attributed to the low incidence of Pneumococcal
infections in some African countries include greater difficulty in
isolating fastidious organisms like Pneumococcus compared with
organisms like Staphylococcus aureus
and the possibility of unregulated antibiotic usage in these
countries.[9,22] It is possible that use of antibiotics purchased
across the counter for febrile illnesses eliminate some organisms
including Pneumococci such that affected children rarely present in
hospital. Consequently, only infections not cleared by such antibiotics
present in the hospital. Although only 13.8 percent of the study
population had used antibiotics, the drugs mainly used were penicillins
or penicillin derivatives and cephalosporins to which Streptococcus pneumoniae
is usually susceptible. It is however not clear if this degree of
pre-hospital antibiotic usage reflects that of the rest of the sickle
cell disease population and also if it is sufficient to account for the
low rate of isolation of Streptococcus pneumoniae. In spite of the various aforementioned postulations, the consistent rarity of Streptococcus pneumoniae and predominance of Klebsiella and/or Staphylococcus aureus in multiple African countries, may be true representations of common organisms in tropical African countries.
Since
the utility of Pneumococcal vaccines in the study population was low,
vaccine usage may not completely account for the low rate of
Pneumococcal infection. The slightly increased risk of bacteremia in
children who did not use any of the stipulated vaccines needs to be
interpreted with caution. Although the 95% confidence intervals did not
include the null value (1.0), the p values were all greater than 0.05
and the amount of increased risk reflected by the odd ratios were
minimal. This implies that protection from infections caused by
organisms (Streptococcus Pneumoniae and Haemophilus influenza
type b) covered by the stipulated vaccines has little if any
contribution to the relative reduction (albeit statistically
insignificant) of overall incidence of bacteremia in the vaccinated
group. This highlights the insignificant contribution of Streptococcus Pneumoniae and Haemophilus influenza type b infection to bacteremia in the study population.
Although
this study was not focused primarily on osteomyelitis, the association
with 3 cases of Klebsiella infection suggests an important role of this
organism in bone infections in sickle cell anemia in this setting and
therefore the need to bear this in mind when prescribing antibiotics
especially when first line drugs have failed.
In spite of
conflicting data on the incidence of pneumococcal infections in Africa
and consequent doubts on the need for routine vaccinations, some
countries in the continent have initiated routine pneumococcal
vaccinations against the backdrop of evidence of its benefits observed
in other parts of the world.[23] Emphasis should therefore be on the
knowledge of prevalent isolates of bacteremia and their antimicrobial
susceptibility patterns to guide the choice of first line antibiotics
in febrile children with sickle cell disease. A previous study on
adults with SCD in the same hospital as the present study reported a
similar pattern of etiological agents but reported Ceftazidime to be
the most effective antibacterial agent to which 93% of GNB and 82.5% of
Gram positive bacteria were susceptible,[17] while the current study
revealed a very low susceptibility to Ceftazidime. The reduced
susceptibility to ceftazidime is in keeping with development of
resistance over the last 20 years. The top four antibiotics to which
the isolates were most susceptible in this study were Meropenem,
Ceftriaxone, Amikacin and Ciprofloxacin. A hundred percent
susceptibility observed with Meropenem showed the need to restrict the
use of Carbapenems in order to reduce the development of multidrug
resistance.[24] We therefore recommend the use of Ceftriaxone and
Amikacin as first line antibiotics after collection of blood culture
specimen in this setting. Meropenem may therefore be a reserve drug
that is employed in multi-drug resistant cases and strictly after a
susceptibility report to justify its administration. This should also
be incorporated into the antibiotic policy of the hospital in keeping
with antibiotic drug stewardship guidelines.
In the present study,
there was no significant difference in the hematocrit on admission of
children with bacteremia compared with those without bacteremia. This
is at variance with findings by Makani et al.[21] in Tanzania where
children with bacteremia were more likely to have a lower hemoglobin
concentration compared with those without bacteremia. The reason for
this difference may be due to differences in the cohorts in the two
studies. Whilst the present study was only on febrile children who are
likely to have infections, that from Tanzania was from all SCD
admissions irrespective of the diagnosis which is therefore likely to
include non-infective cases. The co-morbidities in the non-bacteremic
cases in both studies are therefore likely to be different.
The
findings in this study of infections associated with crisis are keeping
with the recognized role of infections as precipitants of crisis in
SCD. During infection, changes occur at a cellular level, which
predispose to crises. Levels of circulating leukocytes and inflammatory
cytokines increase, with elevated expression of adhesion molecules on
both the vascular endothelium and leukocytes themselves. Leucocytes
attracted to sites of inflammation also produce cytotoxic proteins such
as proteases, collagenase, and elastase and generate reactive O2
radicals, which cause oxidative damage. This promotes further
endothelial activation and cell adhesion.[25] Cell adhesion
subsequently leads to microvascular occlusion and sickling. In
addition, fever with insensible water loss, reduced oral fluid intake,
diarrhea, and vomiting in infections may contribute to dehydration
which increases the risk of sickling.[26]
Conclusions
This
study has revealed a 13.8 percent incidence of bacteremia in febrile
children with sickle cell disease, a figure that appears higher than
observed in developed countries. The study has also highlighted the
rarity of Streptococcus pneumoniae
in African children in line with a number of studies in Africa, adding
to the debate of the need for Pneumococcal vaccines in children with
sickle cell disease in such settings. Incidentally, following the
implementation of routine Pneumococcal vaccinations in some African
countries, the actual role played by Pneumococcal infection may never
be known. Nevertheless, the study has revealed that like some other
African countries, the major causes of bacteremia are Klebsiella pneumoniae and Staphylococcus aureus.
The results of antibiotic susceptibility to the common organisms would
serve as a guide to first line antimicrobial prescription in suspected
cases of bacteremia.
Acknowledgement
This
project was funded by the University College Hospital, Ibadan, Research
Grant for the year 2013. The mentorship of Professor Olufunmilayo
Olopade through whom a medical student from the University of Chicago
participated in the research is also acknowledged. We are also
grateful to the resident doctors in the Department of Paediatrics who
assisted the dedicated project research assistant in collection of the
data.
References
- World Health Organization (2006). Sickle cell anaemia. WHO Fifty-Ninth World Assembly A59/9.
- Obaro
S. Pneumococcal infections and sickle cell disease in Africa: does
absence of evidence imply evidence of absence? Arch Dis Child. 2009;
94:713-716. https://doi.org/10.1136/adc.2008.154815 PMid:19414433

- Okuonghae
HO, Nwankwo MU, Offor EC. Pattern of bacteraemia in febrile children
with sickle cell anaemia. Ann Trop Paediatr. 1993; 13:55-64. https://doi.org/10.1080/02724936.1993.11747625 PMid:7681646

- Brown
BJ, Jacob NE, Lagunju I A, Jarrett OO. Morbidity and mortality pattern
in hospitalized children with sickle cell disorders at the University
College Hospital, Ibadan, Nigeria. Nig J Paediatr. 2013; 40: 34-39.

- Leiken
SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in
children and adolescents with sickle cell disease. Pediatrics 1989;
84:500-8.

- Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood 2004; 103: 4023-4027. https://doi.org/10.1182/blood-2003-11-3758 PMid:14764527 PMCid:PMC1828870

- Akinyanju O, Johnson AO. Acute illness in Nigerian children with sickle cell anaemia. Ann Trop Paediatr. 1987; 7: 181-6. https://doi.org/10.1080/02724936.1987.11748503 PMid:2445266

- Akuse
RM. Variation in the pattern of bacterial infection in patients with
sickle cell disease requiring admission. J Trop Paediatr 1996; 42:
318-323. https://doi.org/10.1093/tropej/42.6.318

- Williams
TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, Mwarumba S,
Makani J, Komba A, Ndiritu MN, Sharif SK, Marsh K, Berkley JA, Scott
JA. Bacteraemia in Kenyan children with sickle-cell anaemia: a
retrospective cohort and case-control study. Lancet 2009 ;374:1364-70. https://doi.org/10.1016/S0140-6736(09)61374-X

- Galadanci
N, Wudil BJ, Balogun TM, Ogunrinde GO, Akinsulie A, Hasan-Hanga F,
Mohammed AS, Kehinde MO, Olaniyi JA, Diaku-Akinwumi IN, Brown BJ,
Adeleke S, Nnodu OE, Emodi I, Ahmed S, Osegbue AO, Akinola N, Opara HI,
Adegoke SA, Aneke J, Adekile AD. Current sickle cell disease management
practices in Nigeria. Int Health. 2014;6:23-8.
https://doi.org/10.1093/inthealth/iht022 PMid:24114193

- Kirn
TJ, Weinstein MP. Update on blood culture: how to obtain, process,
report and interpret. Clin Microbiol Infect 2013; 19:513-520.
https://doi.org/10.1111/1469-0691.12180 PMid:23490046

- Cheesbrough,
M. Biochemical tests to identify bacteria. In: District Laboratory
Practice in Tropical Countries (Part 2). Low priced edn. Cambridge
University Press. U.K: 2002. pp 62- 70.
- Clinical and
Laboratory Standard Institute (CLSI) for culture, identification and
antibiotic susceptibility of bacterial isolates. http://www.clsi.org
- Bauer
AW, Kirby WMM, Sherris JC, Turck M. Antibiotics Susceptibility testing
by a standardized single disc method. AMJ Clin Pathol 1966; 45: 493.
PMid:5325707

- Bansil NH, Kim TY, Tieu L,
Barcega B. Incidence of Serious Bacterial Infections in Febrile
Children With Sickle Cell Disease. Clin Pediatr (Phila). 2013;52:661-6.
https://doi.org/10.1177/0009922813488645 PMid:23661790

- Morrissey
BJ, Bycroft TP, Almossawi O, Wilkey OB, Daniels JG. Incidence and
Predictors of Bacterial infection in Febrile Children with Sickle Cell
Disease. Hemoglobin. 2015;39:316-9. PMid:26207314

- Aken’ova
YA, Bakare RA, Okunade MA. Septicaemia in sickle cell anaemia patients:
the Ibadan experience. Cent Afri J Med 1998; 44:102-4.
PMid:9810403

- Adamkiewicz T V., Silk BJ,
Howgate J, Baughman W, Strayhorn G, Sullivan K, Farley MM.
Effectiveness of the 7-Valent Pneumococcal Conjugate Vaccine in
Children With Sickle Cell Disease in the First Decade of Life.
Pediatrics 2008; 121:562-9. https://doi.org/10.1542/peds.2007-0018
PMid:18310206

- Zangwill KM, Vadheim CM,
Vannier AM, Hemenway LS, Greenberg DP, Ward JI. 1996. Epidemiology of
invasive pneumococcal disease in southern California: implications for
the design and conduct of a pneumococcal conjugate vaccine efficacy
trial. J. Infect. Dis. 1996; 174:752-759.
https://doi.org/10.1093/infdis/174.4.752

- Kizito ME,
Mworozi E, Ndugwa C, Serjeant GR. Bacteraemia in homozygous sickle cell
disease in Africa: is pneumococcal prophylaxis justified? Arch Dis
Child. 2007;92:21-3. https://doi.org/10.1136/adc.2005.088807 PMid:16531454 PMCid:PMC2083172

- Makani
J, Mgaya J, Balandya E, Msami K, Soka D, Cox SE, Komba AN, Rwezaula S,
Meda E, Muturi D, Kitundu J, Fegan G, Kirkham FJ, Newton CR, Snow RW,
Lowe B. Bacteraemia in sickle cell anaemia is associated with low
haemoglobin: a report of 890 admissions to a tertiary hospital in
Tanzania. Br J Haematol. 2015;171:273 https://doi.org/10.1111/bjh.13553
PMid:26084722 PMCid:PMC4744759

- Kateete DP, Kajumbula H,
Kaddu-Mulindwa DH, Ssevviri AK. Nasopharyngeal carriage rate of
Streptococcus pneumoniae in Ugandan children with sickle cell disease.
BMC Res Notes 2012;5:28. https://doi.org/10.1186/1756-0500-5-28
PMid:22243524 PMCid:PMC3283489

- Centers for Disease
Control and Prevention. Progress in Introduction of Pneumococcal
Conjugate Vaccine — Worldwide, 2000-2012. Morb Mortal Wkly Rep.
2013;62(16):306-11.

- Sistanizad M, Kouchek M, Miri M,
Goharani R, Solouki M, Ayazkhoo L, Foroumand M, Mokhtari M. Carbapenem
Restriction and its Effect on Bacterial Resistance in an Intensive Care
unit of a Teaching Hospital. Iran J Pharm Res IJPR. 2013;12:503-9.
PMid:24250656

- Frenette, P.S. and Atweh,
G.F. Sickle cell disease: old discoveries, new concepts and future
promise. J Clin Investig. 2007; 117: 850-858.
https://doi.org/10.1172/JCI30920 PMid:17404610 PMCid:PMC1838946

- Booth
C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J
Infect Dis 2010; 14:e2-e12. https://doi.org/10.1016/j.ijid.2009.03.010
PMid:19497774

[TOP]




























