Received: March 20, 2017
Accepted: May 10, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017041 DOI 10.4084/MJHID.2017.041
This article is available on PDF format at:

Kinjalka Ghosh1, M.G. Muddeshwar1, Manoj Lokhande1 and Kanjaksha Ghosh2

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract
Background: We evaluated albumin cobalt binding (ACB) assay also known as Ischaemia
Modified Albumin (IMA) assay as a prognostic marker for severe malaria in a
medical college setting. Methods:
Consecutive adult patients admitted with both vivax and falciparum malaria were
evaluated with ACB assay at the time of admission. Detailed work up and
individual patient directed management were instituted in addition to immediate
artemisin based antimalarial therapy. Results:
100 consecutive patients (50 with vivax and 50 with falciparum malaria) were
evaluated. The reference range for ACB assay was established using 50 adult
healthy (25 male and 25 female) individuals. 16 out of 50 p. Falciparum-Infected
developed complicated malaria. None of the P Vivax patients developed
complicated malaria. All malaria infected patients had high ACB levels (P<0.0001).
There was a stepwise increase in ACB levels from healthy volunteers to
different categories of malaria (P<0.0001) without any overlap. Conclusion:
ACB has the potential to be used as a robust simple and inexpensive prognostic
marker for organ dysfunction in severe malaria even if an evaluation at
multiple sites with a bigger number of patients should be initiated for final
recommendation. |
Introduction
Material and Methods
Results
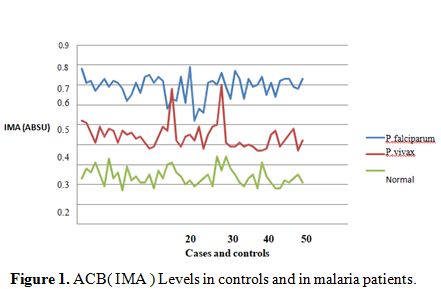 |
Figure 1. ACB( IMA ) Levels in controls and in malaria patients. |
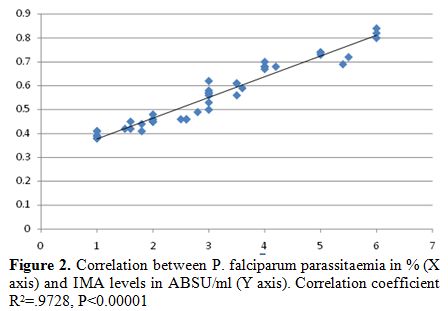 |
Figure 2. Correlation between P. falciparum parassitaemia in % (X axis) and IMA levels in ABSU/ml (Y axis). Correlation coefficient R2=.9728, P<0.00001 |
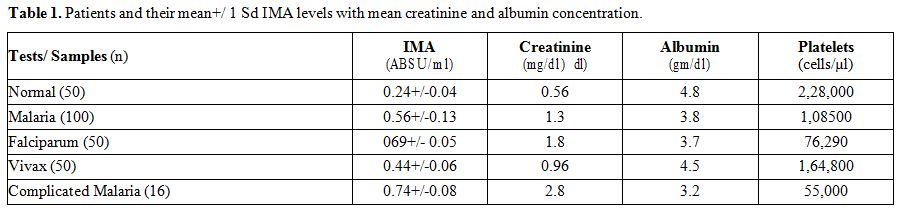 |
Table 1. Patients and their mean+/ 1 Sd IMA levels with mean creatinine and albumin concentration. |
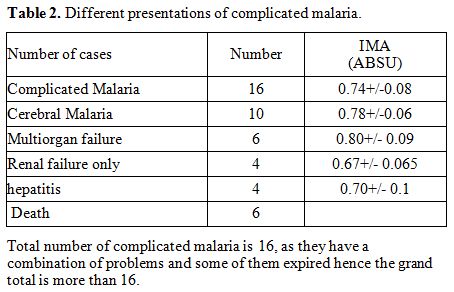 |
Table 2. Different presentations of complicated malaria. |
Discussion
Acknowledgement
Authors gratefully acknowledge the reagents and input provided by the scientists of National Institute of immunohaematology, Mumbai in writing this paper and Dean IGMC, Nagpur for permitting us to publish the paper.References










[TOP]