Sarita Rani Jaiswal1,2, Satyanker Gupta2, Rekha Saji Kumar3, Amit Sherawat2, Ashok Rajoreya, Saroj K Dash3, Gitali Bhagwati3 and Suparno Chakrabarti1,2.
1 Manashi Chakrabarti Foundation, Kolkata, Dharamshila Narayana Superspeciality Hospital, New Delhi.
2 Department of Blood and Marrow Transplantation, Dharamshila Narayana Superspeciality Hospital, New Delhi.
3 Department of Microbiology, Dharamshila Narayana Superspeciality Hospital, New Delhi.
Corresponding
author: Dr. Suparno Chakrabarti. Department of Blood and Marrow
Transplantation & Hematology, Dharamshila Hospital and Research
Centre, Vasundhara Enclave, New Delhi-110096, India. E-mail:
supchak@gmail.com
Published: May 1, 2018
Received: March 2, 2018
Accepted: March 30, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018025 DOI
10.4084/MJHID.2018.025
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Gut colonisation with carbapenem-resistant enterobacteriaceae (CRE) is
a risk factor for CRE bacteremia and patients with haematological
malignancies (HM) are at the highest risk of mortality.
Methods:
We conducted a prospective surveillance study of gut colonisation with
CRE and its impact on the outcome of 225 consecutive patients of HM
over 28 months.
Results:
The median age of the cohort was 46 years, the majority with acute
leukaemia. 48 (21%) patients were colonised with CRE on admission
(CAD). Another 46 patients were colonised with CRE in the hospital
(CIH). The risk factors for CAD and CIH were a diagnosis of acute
leukaemia and duration of hospital stay respectively. CRE accounted for
77% of infection-related mortality (IRM). The incidence of CRE
bacteremia in CRE positive patients was 18% (17/94), and mortality in
those with CRE bacteremia was 100%. IRM was 35.3% in CIH group compared
to 10.5% in the CAD group (p=0.0001). IRM was highest in those with
acute myeloid leukaemia (AML) and CIH (54.9% p=0.0001). On multivariate
analysis, CIH was the most important risk factor for IRM (HR-7.2).
Conclusion:
Our data demonstrate that a substantial proportion of patients with HM
are colonised with CRE without prior hospitalisation, but those with
nosocomial colonisation have the highest risk of mortality,
particularly in those with AML.
|
Introduction
The
smallest of the organisms have always evolved mechanisms of survival
amidst all odds. This concept is exemplified by the way pathogenic
bacteria have developed resistance to each generation of antibiotics,
which humans have designed to combat them. Gram-negative
Enterobacteriaceae (GNE) have been most prolific in this regard.,[1]
Development of carbapenems was hoped to provide a lasting solution to
the menace of antibiotics resistance. However, true to its survival
algorithm, GNE developed several pathways of resistance to carbapenems
within a decade of their arrival.
Carbapenem resistance is due to
either carbapenem-hydrolysing enzymes, which is the most common
mechanism or changes in the outer membrane porins combined with
overproduction of AmpC β lactamases.[2] The increasing
incidence of infection by carbapenem-resistant enterobacteriaceae (CRE)
is a significant public health challenge worldwide, especially in the
developing countries.[3-5] It has acutely exposed the limitations of our antibiotics armamentarium.[6]
Patients with haematological malignancies (HM) and the recipients of
hematopoietic stem cell transplantation (HSCT) are particularly
vulnerable to infections with CRE. Although precise data is scant,
mortality associated with CRE is 60-100% in such patients.[2,7]
The Centers for Disease Control and Prevention (CDC) has reported
increased CRE infections in parts of the United States and Europeans
countries.[2,8] India and other developing countries are worst affected by this emerging population of multidrug-resistant bacteria.[9] Despite the looming threat of a global epidemic, few studies[10,11] have evaluated the incidence and impact of CRE in the most vulnerable population of patients, i.e., those with HM.
We
conducted a prospective longitudinal study over 28 months to evaluate
the prevalence of colonisation with CRE in patients with haematological
malignancies and its impact on the outcome of the patients undergoing
treatment for these disorders.
Materials and Methods
This
was a prospective observational study of gut colonisation with CRE in
225 consecutive patients with newly diagnosed HM admitted to our
institution from October 2013 to January 2016, who underwent active
treatment. Patients previously treated for the same condition or those
with relapsed disease were not included in the study. The study was
approved by the Institutional Review Board, and informed consent was
obtained from patients.
Surveillance for CRE.
Rectal swabs of all patients were collected in an aseptic manner at the
bedside, during the first day of admission and repeated subsequently on
a weekly basis for a continuous hospital stay or in subsequent entries.
The duration of surveillance continued through the entire period of
active treatment. However, efforts were made to collect samples on a
weekly basis for the first four weeks on all patients whose therapies
were scheduled at 3-4-week intervals.
After collection, the
samples were immediately transported to the microbiology department,
and subsequently cultured. Records of Identification and antibiotic
sensitivity pattern of microorganism were maintained for all the
patients. Enterobacteriaceae was identified based on standard
laboratory protocols. All clinical specimens were inoculated on
MacConkey agar and blood agar for isolation of gram-negative bacteria.
After 18-24 hrs of incubation, the Mac-Conkey agar plates were examined
for both lactose-fermenting (pink) colonies as well as non-lactose
fermenting (pale) colonies. More than one colony morphology may
represent distinct species. Wherever there was a difference in the
colony morphology, colonies of each were sub-cultured in nutrient agar
media (non-selective media). Isolates were subjected to a series of
biochemical tests for identification, both manually or using automated
identification system, Vitek2® (BioMérieux, France), if necessary.
These colonies were identified up to species level using standard
protocol.[12] Susceptibility testing was performed by
disc diffusion (Kirby-Bauer) method following CLSI guidelines version
2016. Isolates showing positive disc screen test with ertapenem (10µg)
and meropenem (10µg) or imipenem (10µg) were suspected as possible CRE,
and they were further subjected to Modified Hodge Test (MHT) for
detecting carbapenemases with ZnSO4 supplementation of culture media to
increase the detection rate of NDM1.[13,14] Reference
strains used as controls were E. coli ATCC 25922, Klebsiella pneumonia
700603 and Pseudomonas aeruginosa 27853. CRE was defined as
non-susceptibility to anyone out of the three antibiotics tested. Since
breakpoints of colistin and tigecycline were not mentioned for
Enterobacteriaceae in CLSI guidelines, EUCAST guideline was followed.
Aminoglycosides used were Amikacin and Gentamycin
Monitoring and management of patients with CRE colonization.
Patients with a positive rectal swab screening on the first sample,
without any sign or symptoms of infection, were defined as Colonised at
Admission (CAD). Horizontal transmission during the current
hospitalisation was hypothesised for CRE positive patients who had a
negative screening at admission and were labelled as Colonized in
Hospital (CIH).
CRE-positive patients were put under barrier
nursing care precautions as per CDC guidelines. Patients were kept in
isolation rooms whenever available or cohorted in double-occupancy
rooms. Dedicated nurse and housekeeping staff were assigned to CRE
positive patients in single or cohort allocation at each shift. The
patients themselves were advised for regular sitz bath and cleaning
with chlorhexidine-based cleansing solutions
CRE infections and therapy.
All patients received levofloxacin as antibacterial prophylaxis on
admission unless they were initiated on empirical or definitive
antibiotics for febrile or infective episodes. Paired blood and urine
samples were sent for culture before starting of empirical antibiotics
for patients developing clinical pictures suggesting an infection. All
patients were assessed on the basis of age, comorbidities, performance
status, duration and severity of neutropenia, previous infections and
exposure to broad-spectrum antibiotics (i.e., beta-lactams, quinolones,
and aminoglycosides), and duration of central venous catheter
placement. Patients with known CRE colonisation were started on a high
dose of anti-pseudomonas carbapenems along with aminoglycosides.
Antibiotics were escalated as per sensitivity report and the clinical
status of patients. However, those with CRE colonisation had colistin
and tigecycline added if there were signs of progression of sepsis or
if there was a lack of response within 24-48 hours.
Statistics.
Binary variables were compared between the two groups using chi-square
test, and the continuous variables were analysed using independent
sample t-test considering the Levenes test for equality of variances.
Probabilities of survival were estimated using the Kaplan-Meier
product-limit method. CRE – related mortality (CRE-RM) was defined as
death attributable to microbiologically documented bacteremia caused by
CRE, in the absence of other confounding factors. Infection-related
mortality (IRM) was defined as death due to infectious causes verified
on culture of blood or sterile body fluids, in the absence of other
confounding factors. The cumulative incidence rates of IRM and CRE-RM
were computed to take account of the presence of competing risks such
as disease-progression or relapse. Multivariate analysis was carried
out using Cox Regression analysis. The data were censored if a patient
was treated with hematopoietic stem cell transplantation (HSCT) at the
time of admission for the same. An outcome was determined to be
significantly different if the observed P value was <0.05. All
analyses were performed using statistical software IBM SPSS Statistics
Version 22.
Results
Patient Characteristics (Table 1).
A total of 2263 samples from 225 patients with HM were evaluated. We
further analysed them in two cohorts as per their rectal swab
surveillance results as CRE positive and CRE negative. CRE positive
subgroup was also categorised as colonised at admission (CAD) and
colonised in the hospital (CIH) as described above.
The details of patients are mentioned in the Table 1.
The median age of the entire study group was 46 years with a male
predominance (61%). Acute leukaemia (45%) accounted for the majority,
followed by lymphoma (33.8%), myeloma (8.9%) and the rest. The median
duration of follow-up was 16 months (range 12 days-26 months). All
patients were newly diagnosed at our institution had active disease at
presentation. Patients with prior treatment and those with relapsed
diseases were not included in the study.
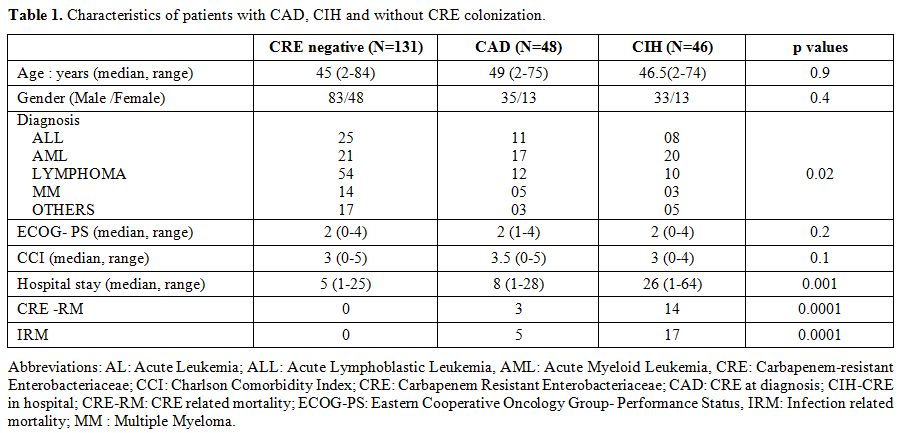 |
Table 1. Characteristics of patients with CAD, CIH and without CRE colonization. |
Colonisation with CRE and Risk Factors.
Out of 225 patients, 48 (21%) patients were colonised with CRE at
admission. Another 46 patients with the prolonged hospital stay or on
subsequent treatment had a positive CRE on surveillance, accounting for
26% of patients with CIH. The median time to acquisition of CRE amongst
the CIH group was 3 weeks (range 2-13). Amongst the CRE positive
cohort, the majority (n=56, 59.7%) were diagnosed with acute leukaemia
and 37 (66%) of those had acute myeloid leukaemia (AML). The median
duration of continuous hospital stay was higher amongst CIH (26 days,
range 1-64) compared to non-CIH group (5 days, range 1-28), [p=0.0001].
Both univariate and multivariate analyses were carried out to ascertain the risk factors for CAD and CIH as detailed in Table 2.
CAD tended to be higher in those with acute leukaemia (27/102 vs 20/123
without acute leukaemia, HR 1.85 95%CI 1.0-3.5, p=0.05) Duration of
hospitalisation was a risk factor for CIH (HR 4.3 (95%CI 2.5-8.9). A
diagnosis of AML was the strongest risk factor for overall CRE
colonisation (37/58 vs 57/157 without AML, HR-2.5, 95%CI 1.1-5.6,
p=0.03).
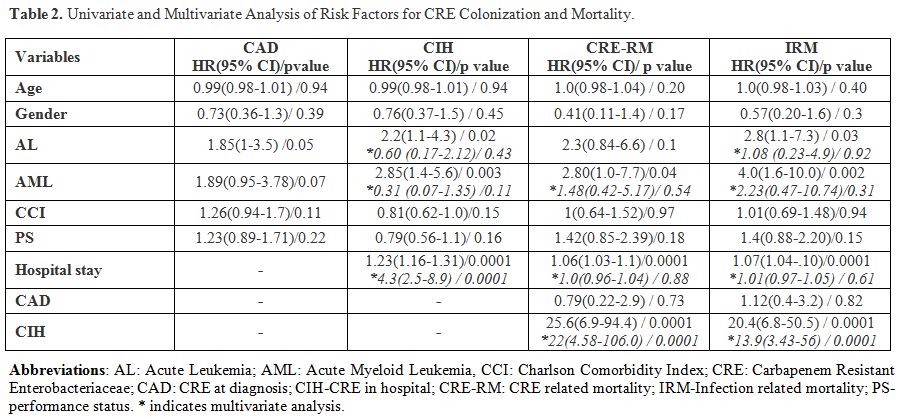 |
Table 2. Univariate and Multivariate Analysis of Risk Factors for CRE Colonization and Mortality. |
Microbiology of CRE colonisation.
Klebsiella pneumoniae (KP) was the predominant microorganism isolated
from the rectal swab sample of the patients as CRE pathogen amongst
both CAD (53%) and CIH (83%) groups. Escherichia Coli was the other
isolated organism accounting for the rest. Both pathogens were detected
in 6% and 8% in the CAD and CIH groups respectively. Thus, Klebsiella
species accounted for significantly higher colonisers amongst those
with CIH (p=0.02). All isolates were positive by susceptibility testing
as well as MHT.
All CRE isolates were resistant to all the
carbapenems tested. Twelve out of 17 patients who died of CRE had
Klebsiella species isolated from their blood culture (Table 3).
Although all the species isolated were sensitive to colistin, seven
were sensitive to tigecycline, and only one isolate was sensitive to
aminoglycosides. Among five patients who were infected with E.Coli,
four were resistant to aminoglycosides, and one was resistant to
Tigecycline. Amongst those with CIH, 12 had documented CRE bacteremia,
ten were Klebsiella species, and two were E.coli. Six of the isolates
were sensitive to colistin alone. All but one patient had received
meropenem or Imipenem in combination with aminoglycosides, tigecycline
and colistin for over 48 hours before they succumbed to the CRE
infection.
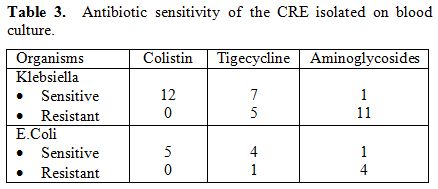 |
Table 3. Antibiotic sensitivity of the CRE isolated on blood culture. |
Infection-Related Mortality (IRM) And CRE-Related Mortality (CRE-RM).
The overall IRM over a period of 26 months was 9.5% (22 patients).
CRE-RM accounted for 17 of the 22 deaths. The other five patients
succumbed to gram-negative sepsis (n=4, Pseudomonas aureginosa-2,
Enterobacter-1, Acinetobacter Baumanii-1) and sudden cardiac death
(n=1) while on treatment for CRE. No IRM or CRE-RM was noted in
patients who were CRE negative throughout the study period. Thus, all
IRM occurred exclusively in patients colonised with CRE. IRM and CRE-RM
in CRE positive group were 24.7% (n=22/94, 95% CI 20-29.4) and 18.8%
(17/94, 95% CI 14.7-22.9) respectively.
Those with acute leukaemia had a higher IRM
(15/102, [14%; 95%CI 10.5-17.5] compared to 7/123, [5.8 %; 95%CI
3.7-7.9] in those without AL, log rank p=0.01). On subgroup analysis,
12 out of 58 with AML had IRM (22.9% 95%CI 16.8-29.0) compared to 10
out of 167 of those without AML (10/167, 5.5; 95%CI 3.7-7.3) (log rank
p=0.0001).
On further analysis, IRM was significantly
higher in the CIH group compared to CAD group (17/46, [39.6%] vs 5/48
[10.5%], p=0.0001, Figure 1).
CRE-RM was also significantly higher in the CIH group (14/46, [31.4%
(95%CI 24.4- 38.4%)] vs 3/48, [6.6% (95%CI 2.9- 10.3%)] p=0.0001)
compared to CAD group. This trend for mortality in patients with CIH
was similar in patients with and without AL. However, the incidence of
IRM was highest in those with AML and CIH (10/20) [54.9%; 95%CI
32.4-67.4%] compared to 2/17 in those with AML and CAD (11.8; 95%CI
4-18.6; p=0.0001).
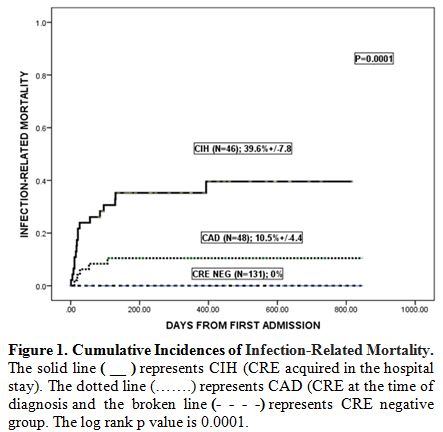 |
Figure 1. Cumulative Incidences of Infection-Related Mortality. |
CRE bacteremia
occurred exclusively in those colonised with CRE. Thus, CRE
colonisation was the most significant risk factor for CRE bacteremia
(p=0.0001). No patient with CRE infection in the above cohort survived.
Therefore, the incidence of CRE bacteremia in CRE positive patients was
18% (17/94), and mortality in those with CRE bacteremia was 100%. All
the patients were neutropenic at the time of CRE bacteremia. The median
time to the detection of bacteremia from diagnosis of CRE colonisation
was 19 days (0-41). The median time to death from the onset of the
febrile neutropenic episode was six days (1-14) and from the beginning
of severe neutropenia was four days (1-8). On multivariate analysis,
CIH was the single most important risk factor for both CRE-RM and IRM
in patients with haematological malignancies (Table 2).
Discussion
CRE, particularly the NDM-1 strain was reported to be highly prevalent in various parts of India in 2011.[4] This was confirmed by another study from South India, highlighting the prevalence of NDM as well as OXA-48 like strains.[15] However, few studies have emerged from the subcontinent highlighting the burden and the impact of CRE.[5,16,17]
Despite recognition of CRE as a global public health threat, the study
on the acquisition and the natural history of colonisation with CRE in
patients with various HM remain sparse. A review in 2014 by Satlin et
al. identified six studies reporting 35 patients of HM and HSCT in
total, with a mortality rate of 50-100%.[2] While a
few studies since then have studied the incidence CRE bacteremia and
its risk factors in both adults and children, any longitudinal research
on the incidence of colonisation and its long-term impact is lacking.[8,18-24]
We
studied a cohort of 225 patients over a 28 months period with a minimum
follow-up of 6 months. 21% of the patients were colonised with CRE at
their first visit. Due to the use of non-selective media and in the
absence of molecular typing, it is possible that we might have
under-reported the incidence of colonisation. It is not possible to
ascertain if such cases of CAD are genuinely community-acquired or
these were acquired during infrequent hospital visits before reaching a
tertiary care centre.[25] Patients with acute
leukaemia are more prone to colonisation as is evident from our data.
This could be due to multiple visits to health care set-ups before
arrival at the tertiary care centre. This is augmented by the
disease-induced neutropenia for prolonged periods in such patients.
What
was even more striking was that another 26% of patients were colonised
during the hospital stay, despite extremely stringent measures for
barrier nursing in place. Such high rates of CIH highlight the
perennial and obtrusive problem of nosocomial transmission of such
microbes. CRE have a high propensity for horizontal transmission, and
this has been highlighted in the past.[3] Colonisation
in the hospital is not a mere physical event but is contributed by
prolonged antibiotic usage, chemotherapy-induced breach of the mucosal
barrier of the gut and most importantly both disease and
therapy-induced severe and prolonged neutropenia.[18]
These factors and their combinations are unique to the patients with HM
and not generally witnessed in non-HM patients in intensive care or
solid organ transplants. The combination of these factors is probably
responsible for the high fatality rate of CRE infections in patients
with HM. This was highlighted by a multicenter study from Italy where
bloodstream infection with carbapenem-resistant KP was on the rise and
was associated with a mortality rate of greater than 50% in patients
with HM.[7]
Colonisation with CRE has been
postulated to be a risk factor for CRE bacteremia, but the data remains
scarce due to the lack of prospective nature of these studies. In a
study from Italy, 86% of patients with CRE bacteremia were found to be
colonised.[11] However, none of the studies alludes
to a longitudinal follow-up in colonised patients. In our study, 42%
patients were colonised with CRE in the study period and 18% of those
developed CRE bacteremia during a course of therapy-induced
neutropenia. CRE bacteremia was associated with 100% mortality,
although all patients colonised with CRE were initiated on colistin and
tigecycline within 24-48 hours of the onset of febrile neutropenia
along with high doses of carbapenems. Thus, a delay in initiation of
treatment is unlikely to be responsible for such high mortality. We
noted that mortality in patients with CIH was much higher than patients
with CAD. Majority of these patients succumbed within a week of the
febrile episode and onset of neutropenia. It is possible that the
nosocomial strains were more virulent as reflected by the pattern of
antibiotic sensitivity.[26,27] Very few isolates were
sensitive to aminoglycosides, and the majority of KP were resistant to
tigecycline as well. Fosfomycin, another antibiotic which has efficacy
against CRE was not available for clinical use during the study period.
Resistance to colistin as well as tigecycline is on the rise as
reported from both India as well as China.[27-31]
Hence, with extreme limitations regarding antibiotic sensitivity, the
outcome of such patients is likely to remain extremely poor.[32]
However, several newer beta-lactamases such as avibactam, vaborbactam
and relebactam in combination with ceftazidime and carbapenems might
provide an alternative for CRE infections in the near future.[33,34]
In addition, ceftolozane-tazobactam shows promise as a
carbapenem-sparing agent against both Pseudomonas as well as
enterobacteriaceae.[35]
Further to our study, we
have introduced prophylactic granulocyte infusions for all patients
colonised with CRE, who are febrile and likely to experience
neutropenia over seven days. Given the paucity of effective antibiotics
for CRE, it remains to be seen whether this approach benefits patients
with CRE colonisation. Our study has addressed the issue of gut
colonisation with CRE in patients with HM with reasonable diligence to
be able to propose the following. Half of the patients with HM are
likely to be colonised with CRE during the first few weeks of treatment
despite the best possible preventive measures. With such high incidence
of colonisation, resources are going to be severely challenged to
prevent the spread of this organism amongst patients with HM in a busy
tertiary care set-up. We are unlikely to save many such CRE infected
patients with prolonged neutropenia with a limited array of
antibiotics. Those with acute leukaemia, more so with AML remain at the
highest risk of early fatality from CRE. Gut sterilisation has stayed
unproven in such situations.[36] Rampant and random use of carbapenems is clearly responsible for the current state.[37]
Unless a concerted effort at antibiotic stewardship and regulated use
of these antibiotics are introduced with all intent and purpose in the
healthcare sectors across the globe, the problem of CRE will assume
epidemic proportions beyond geographical borders in the near future.
References
- Davies J, Davies D: Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010, 74(3):417-433. https://doi.org/10.1128/MMBR.00016-10 PMid:20805405 PMCid:PMC2937522

- Satlin
MJ, Jenkins SG, Walsh TJ: The global challenge of carbapenem-resistant
Enterobacteriaceae in transplant recipients and patients with
hematologic malignancies. Clin Infect Dis 2014, 58(9):1274-1283. https://doi.org/10.1093/cid/ciu052 PMid:24463280 PMCid:PMC4038783

- Gupta
N, Limbago BM, Patel JB, Kallen AJ: Carbapenem-resistant
Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011,
53(1):60-67. https://doi.org/10.1093/cid/cir202 PMid:21653305

- Kumarasamy
KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary
U, Doumith M, Giske CG, Irfan S et al.: Emergence of a new antibiotic
resistance mechanism in India, Pakistan, and the UK: a molecular,
biological, and epidemiological study. Lancet Infect Dis 2010,
10(9):597-602. https://doi.org/10.1016/S1473-3099(10)70143-2

- Saseedharan
S, Sahu M, Pathrose EJ, Shivdas S: Act Fast as Time Is Less: High
Faecal Carriage of Carbapenem-Resistant Enterobacteriaceae in Critical
Care Patients. J Clin Diagn Res 2016, 10(9):DC01-DC05. https://doi.org/10.7860/JCDR/2016/17638.8400

- Falagas
ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS: Antibiotic
treatment of infections due to carbapenem-resistant Enterobacteriaceae:
systematic evaluation of the available evidence. Antimicrob Agents
Chemother 2014, 58(2):654-663. https://doi.org/10.1128/AAC.01222-13 PMid:24080646 PMCid:PMC3910850

- Trecarichi
EM, Pagano L, Martino B, Candoni A, Di Blasi R, Nadali G, Fianchi L,
Delia M, Sica S, Perriello V et al: Bloodstream infections caused by
Klebsiella pneumoniae in onco-hematological patients: clinical impact
of carbapenem resistance in a multicentre prospective survey. Am J
Hematol 2016, 91(11):1076-1081. https://doi.org/10.1002/ajh.24489 PMid:27428072

- Montagnani
C, Prato M, Scolfaro C, Colombo S, Esposito S, Tagliabue C, Lo Vecchio
A, Bruzzese E, Loy A, Cursi L et al: Carbapenem-resistant
Enterobacteriaceae Infections in Children: An Italian Retrospective
Multicenter Study. Pediatr Infect Dis J 2016, 35(8):862-868. https://doi.org/10.1097/INF.0000000000001188 PMid:27100130

- Zhang
R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S:
Nationwide Surveillance of Clinical Carbapenem-resistant
Enterobacteriaceae (CRE) Strains in China. EBioMedicine 2017,
19:98-106. https://doi.org/10.1016/j.ebiom.2017.04.032 PMid:28479289 PMCid:PMC5440625

- Caselli
D, Cesaro S, Fagioli F, Carraro F, Ziino O, Zanazzo G, Meazza C,
Colombini A, Castagnola E, Infectious Diseases Study Group of the
Italian Association of Pediatric H et al: Incidence of colonization and
bloodstream infection with carbapenem-resistant Enterobacteriaceae in
children receiving antineoplastic chemotherapy in Italy. Infect Dis
(Lond) 2016, 48(2):152-155. https://doi.org/10.3109/23744235.2015.1087647 PMid:26393496

- Micozzi
A, Gentile G, Minotti C, Cartoni C, Capria S, Ballaro D, Santilli S,
Pacetti E, Grammatico S, Bucaneve G et al: Carbapenem-resistant
Klebsiella pneumoniae in high-risk haematological patients: factors
favouring spread, risk factors and outcome of carbapenem-resistant
Klebsiella pneumoniae bacteremias. BMC Infect Dis 2017, 17(1):203. https://doi.org/10.1186/s12879-017-2297-9 PMid:28283020 PMCid:PMC5345173

- CLSI:
Performance Standards for Antimicrobial Susceptibility Testing. In:
Enterobacteriaceae. Clinical and laboratory Standard Institute; 2016:
52-59.
- Cohen Stuart J, Leverstein-Van
Hall MA, Dutch Working Party on the Detection of Highly Resistant M:
Guideline for phenotypic screening and confirmation of carbapenemases
in Enterobacteriaceae. Int J Antimicrob Agents 2010, 36(3):205-210. https://doi.org/10.1016/j.ijantimicag.2010.05.014 PMid:20598859

- Girlich
D, Poirel L, Nordmann P: Value of the modified Hodge test for detection
of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol
2012, 50(2):477-479. https://doi.org/10.1128/JCM.05247-11 PMid:22116154 PMCid:PMC3264163

- Bakthavatchalam
YD, Anandan S, Veeraraghavan B: Laboratory Detection and Clinical
Implication of Oxacillinase-48 like Carbapenemase: The Hidden Threat. J
Glob Infect Dis 2016, 8(1):41-50. https://doi.org/10.4103/0974-777X.176149 PMid:27013843 PMCid:PMC4785756

- Datta
P, Gupta V, Singla N, Chander J: Asymptomatic colonization with
carbapenem resistant enterobacteriaceae (CRE) in ICU patients and its
associated risk factors: Study from North India. Indian J Med Microbiol
2015, 33(4):612-613. https://doi.org/10.4103/0255-0857.167316 PMid:26470985

- Rai
S, Das D, Niranjan DK, Singh NP, Kaur IR: Carriage prevalence of
carbapenem-resistant Enterobacteriaceae in stool samples: A
surveillance study. Australas Med J 2014, 7(2):64-67. https://doi.org/10.4066/AMJ.2014.1926 PMid:24611074 PMCid:PMC3941578

- Pouch SM, Satlin MJ: Carbapenem-resistant Enterobacteriaceae
in special populations: Solid organ transplant recipients, stem cell
transplant recipients, and patients with hematologic malignancies.
Virulence 2017, 8(4):391-402. https://doi.org/10.1080/21505594.2016.1213472 PMid:27470662 PMCid:PMC5477691

- Rodrigues
Perez LR: Carbapenem-Resistant Enterobacteriaceae: A Major Prevalence
Difference due to the High Performance of Carbapenemase Producers when
compared to the Nonproducers. Infect Control Hosp Epidemiol 2015,
36(12):1480-1482. https://doi.org/10.1017/ice.2015.227 PMid:26424090

- Satlin
MJ, Cohen N, Ma KC, Gedrimaite Z, Soave R, Askin G, Chen L, Kreiswirth
BN, Walsh TJ, Seo SK: Bacteremia due to carbapenem-resistant
Enterobacteriaceae in neutropenic patients with hematologic
malignancies. J Infect 2016, 73(4):336-345. https://doi.org/10.1016/j.jinf.2016.07.002 PMid:27404978 PMCid:PMC5026910

- Schwartz-Neiderman
A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V: Risk Factors
for Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae
(CP-CRE) Acquisition Among Contacts of Newly Diagnosed CP-CRE Patients.
Infect Control Hosp Epidemiol 2016, 37(10):1219-1225. https://doi.org/10.1017/ice.2016.153 PMid:27452597

- Swaminathan
M, Sharma S, Poliansky Blash S, Patel G, Banach DB, Phillips M,
LaBombardi V, Anderson KF, Kitchel B, Srinivasan A et al: Prevalence
and risk factors for acquisition of carbapenem-resistant
Enterobacteriaceae in the setting of endemicity. Infect Control Hosp
Epidemiol 2013, 34(8):809-817. https://doi.org/10.1086/671270 PMid:23838221

- van
Loon K, Voor In 't Holt AF, Vos MC: Clinical epidemiology of
carbapenem-resistant Enterobacteriaceae: a systematic review and
meta-analyses. Antimicrob Agents Chemother 2017. https://doi.org/10.1128/AAC.01730-17 PMid:29038269

- Weber
DJ, Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE:
Carbapenem-resistant Enterobacteriaceae: frequency of hospital room
contamination and survival on various inoculated surfaces. Infect
Control Hosp Epidemiol 2015, 36(5):590-593. https://doi.org/10.1017/ice.2015.17 PMid:25661968

- Bar-Yoseph
H, Hussein K, Braun E, Paul M: Natural history and decolonization
strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage:
systematic review and meta-analysis. J Antimicrob Chemother 2016,
71(10):2729-2739. https://doi.org/10.1093/jac/dkw221 PMid:27317444

- Manoharan
A, Barla GS, Peter R, Sugumar M, Mathai D: Multidrug resistance
mediated by co-carriage of extended-spectrum beta-lactamases, AmpC and
New Delhi metallo-beta-lactamase-1 genes among carbapenem-resistant
Enterobacteriaceae at five Indian medical centres. Indian J Med
Microbiol 2016, 34(3):359-361. https://doi.org/10.4103/0255-0857.188350 PMid:27514962

- Chen L, Kreiswirth BN: Convergence of carbapenem-resistance and hypervirulence in Klebsiella pneumoniae. Lancet Infect Dis 2017.

- Khare
V, Gupta P, Haider F, Begum R: Study on MICs of Tigecycline in Clinical
Isolates of Carbapenem Resistant Enterobacteriaceae (CRE) at a Tertiary
Care Centre in North India. J Clin Diagn Res 2017, 11(3):DC18-DC21. https://doi.org/10.7860/JCDR/2017/24594.9629

- Kumar
M: Colistin and Tigecycline Resistance in Carbapenem-Resistant
Enterobacteriaceae: Checkmate to Our Last Line Of Defense. Infect
Control Hosp Epidemiol 2016, 37(5):624-625. https://doi.org/10.1017/ice.2016.31 PMid:27077365

- Pogue
JM, Marchaim D, Abreu-Lanfranco O, Sunkara B, Mynatt RP, Zhao JJ,
Bheemreddy S, Hayakawa K, Martin ET, Dhar S et al: Fosfomycin activity
versus carbapenem-resistant Enterobacteriaceae and vancomycin-resistant
Enterococcus, Detroit, 2008-10. J Antibiot (Tokyo) 2013,
66(10):625-627. https://doi.org/10.1038/ja.2013.56 PMid:23715037

- Poirel
L, Kieffer N, Liassine N, Thanh D, Nordmann P: Plasmid-mediated
carbapenem and colistin resistance in a clinical isolate of Escherichia
coli. Lancet Infect Dis 2016, 16(3):281. https://doi.org/10.1016/S1473-3099(16)00006-2

- Livermore
DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N: What remains
against carbapenem-resistant Enterobacteriaceae? Evaluation of
chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline,
nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents
2011, 37(5):415-419. https://doi.org/10.1016/j.ijantimicag.2011.01.012 PMid:21429716

- Zhanel
GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M,
Lagace-Wiens PRS, Walkty A, Denisuik A, Golden A et al:
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel
Carbapenem-beta-Lactamase Inhibitor Combinations. Drugs 2018,
78(1):65-98. https://doi.org/10.1007/s40265-017-0851-9 PMid:29230684

- van
Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA,
Kalayjian RC, Watkins RR, Doi Y et al: Colistin Versus
Ceftazidime-Avibactam in the Treatment of Infections Due to
Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis 2018,
66(2):163-171. https://doi.org/10.1093/cid/cix783 PMid:29020404

- Giacobbe
DR, Bassetti M, De Rosa FG, Del Bono V, Grossi PA, Menichetti F, Pea F,
Rossolini GM, Tumbarello M, Viale P et al: Ceftolozane/tazobactam:
place in therapy. Expert Rev Anti Infect Ther 2018:1-14. https://doi.org/10.1080/14787210.2018.1447381 PMid:29493397

- Rieg
S, Kupper MF, de With K, Serr A, Bohnert JA, Kern WV: Intestinal
decolonization of Enterobacteriaceae producing extended-spectrum
beta-lactamases (ESBL): a retrospective observational study in patients
at risk for infection and a brief review of the literature. BMC Infect
Dis 2015, 15:475. https://doi.org/10.1186/s12879-015-1225-0 PMid:26511929 PMCid:PMC4624661

- Van
Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA,
Laxminarayan R: Global antibiotic consumption 2000 to 2010: an analysis
of national pharmaceutical sales data. Lancet Infect Dis 2014,
14(8):742-750. https://doi.org/10.1016/S1473-3099(14)70780-7

[TOP]









































