Giuseppe Leone and Livio Pagano
Istituto di Ematologia, Università Cattolica del Sacro Cuore, Roma, Italy.
Corresponding
author: Giuseppe Leone. Istituto di Ematologia, Università Cattolica del Sacro Cuor e, Roma. Italy. E-mail:
giuseppe.leone@unicatt.it
Published: July 1, 2018
Received: June 11, 2018
Accepted: June 12, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018039 DOI
10.4084/MJHID.2018.039
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Infections
remain a significant problem in myelodysplastic syndromes (MDS) in
treated as well in non-treated patients and assume a particular
complexity. The susceptibility to infections is due, in the absence of
intensive chemotherapies, mainly to functional defects in the myeloid
lineage with or without neutropenia. Furthermore, MDS includes a
heterogeneous group of patients with very different prognosis, therapy
and risk factors regarding survival and infections. You should
distinguish risk factors related to the disease, like as neutrophils
function impairment, neutropenia, unfavorable cytogenetics and bone
marrow insufficiency; factors related to the patient, like as age and
comorbidities, and factors related to the therapy. When the patients
with MDS are submitted to intensive chemotherapy with and without
hematopoietic stem cell transplantation (HSCT), they have a risk factor
for infection very similar to that of patients with acute myeloid
leukemia (AML), and mostly related to neutropenia. Patients with MDS
treated with supportive therapy only or with demethylating agent or
lenalidomide or immunosuppressive drugs should have a tailored
approach. Most of the infections in MDS originate from bacteria, and
the main risk factors are represented by neutropenia, thrombocytopenia,
and unfavorable cytogenetics. Thus, it is reasonable to give
antibacterial prophylaxis to patients who start the therapy with
demethylating agents with a number of neutrophils <500 x 109/L
, or with thrombocytopenia and unfavorable cytogenetics. The antifungal
prophylaxis is not considered cost/benefit adequate and should be taken
into consideration only when there is an antecedent fungal infection or
presence of filamentous fungi in the surveillance cultures. Subjects
submitted to immunosuppression with ATG+CSA have a high rate of
infections, and when severely neutropenic should ideally be nursed in
isolation, should be given prophylactic antibiotics and antifungals,
regular mouth care including an antiseptic mouthwash.
|
Introduction
Infections
remain a significant problem in myelodysplastic syndromes (MDS)
patients treated as well non-treated, even if in reduction as a cause
of death in the high-risk group.[1,2,3] At variance with acute myeloid
leukemia (AML) the susceptibility to infections is due, in the
absence of intensive chemotherapies, mainly to functional defects in
the myeloid lineage[4-8] with or without neutropenia, which become
essential risk factor when worsened by the treatments.[9]
In the
study of Fianchi et al.,[6] the in vitro bactericidal and fungicidal
activities of neutrophils isolated from 16 MDS patients showed a
significantly reduced killing activity against Escherichia coli, against Lactococcus lactis, and more against Candida albicans
in comparison to those from healthy individuals. The same patients were
observed at a median time of 11 months (range 0–54) from the initial
diagnosis; during this period, recurrent infectious episodes were
recorded in 6 of them. No significant correlations were observed
between the number and severity of infectious incidents and neutrophil
counts. Interestingly, some functional defects could be
reversed, a Maitake mushroom extract, administered to 21 patients
with MDS, was able to enhance in vitro neutrophil and monocyte function
in 18 of them.[7]
Accordingly, Merkel et al[8] in patients treated
with azacytidine and decitabine, at the dose employed in MDS, found
that platelet (PLT) count lower than 20 x 109/L,
Hb level lower than 10 g/dL, and poor cytogenetics were the only
statistically significant risk factors for infection. A low PLT count
appeared to be the most significant risk factor, resulting in a
2.26-fold increase in infection risk, while poor cytogenetics and low
Hb were associated with a 1.77- and 1.75-fold rise in infection rate,
respectively. Surprisingly, low neutrophil count did not come up as one
of the significant factors, at least in multivariate analysis.
In
the past, most of the patients with MDS were treated with supportive
therapies only. However the infections, bacterial, fungal and viral
were frequently present, also independently from
neutropenia.[1,2,6,8,10] The risk is significant in both high and
low/intermediate risk MDSs.[1,2,10-14] In the series of M. D. Anderson
Cancer Center[11] from 1980 to 2004, including 903 patients with
low/intermediate MDS (median age at presentation of 66 years) in
supportive care only, the causes of death (CODs) MDS-related was
defined as infection, bleeding, transformation to AML, or disease
progression. Remaining CODs were classified as non–MDS-related. The COD
was identified as MDS-related in 230 of 273 (84%) patients. The most
common disease-related CODs were infections (38%), transformation to
AML (15%), and hemorrhage (13%). The most frequent non–disease-related
COD was cardiovascular events (19 of 43 patients). Thus, the majority
of patients with low- or intermediate-1 risk MDS will die because of
causes related to their underlying disease.
In the Dusseldorf
registry,[2,3] including low/intermediate and high-risk patients, of
1665 patients with a clearly documented cause of death, 1388 patients
(83.4%) succumbed directly disease-related: AML (46.6%), infection
(27.0%), bleeding (9.8%). Whereas, 277 patients (16.6%) died for
reasons not directly related with MDS, including 132 patients with
cardiac failure, 77 non-disease-related reasons, 23 patients with solid
tumors, and 45 patients with possibly disease-related causes like
hemochromatosis. By dividing the patients according to the WHO
classification, infections were the cause of death in about the 30% of
patients with very low, low and intermediate risk and about 15% with
high and very high risk.[3] It is noteworthy that, in this same
registry,[2] analyzing the survival and rate of leukemic progression of
4147 patients diagnosed during the last 30 years, an improvement of
survival was found in those patients diagnosed after 2002 (30 vs. 23
months, p<0.0001). In detail, the improvement of the prognosis was
restricted to high-risk MDS patients diagnosed between 2002 and 2014 in
comparison to the patient group diagnosed between 1982 and 2001 (19 vs.
13 months, p<0.001), whereas the prognosis of low-risk MDS patients
did not change significantly. This improvement was attributed primarily
to a reduction of the death from infections.[2,3] Infections are
bacterial mostly, but fungal infections are not rare, and the organs
more frequently interested are the lungs, the skin and the gut (Table 1). Sepsis and bacteremia are also frequent.[1]
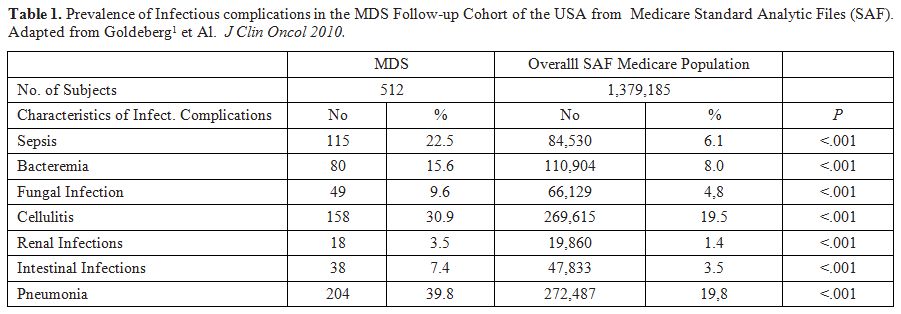 |
Table 1. Prevalence of
Infectious complications in the MDS Follow-up Cohort of the USA
from Medicare Standard Analytic Files (SAF). Adapted from
Goldeberg et Al.[1] J Clin Oncol 2010. |
Recently
appeared three exhaustive reviews on infections in MDS, they represent
an important contribution to understanding this pathology.[12-14]
However, they were not focused on the different therapies and stages of
the disease. In this current review, considering the heterogeneity of
this nosographic group, we have tried to relate on the risk of
infections to the stages of the disease as evaluated by the
International Prognostic Scoring System (IPSS) and the effect of the
different therapies administered. So, we report the incidence of
infections in the myelodysplastic syndromes classified according to the
IPSS and its variations,[14-15] and the subsequent therapies by
consulting the current English literature present in PUBMED, SCOPUS,
and WEB of Science.
Low and Intermediate Risk Myelodysplastic Syndrome
Patients
with low and intermediate risk MDS have been treated in the past only
with supportive therapy or by adding the Erythropoiesis-Stimulating
Agents (ESAs), with or without granulocytic/granulocytic-monocytic
growth factors, G-CSF or GM-CSF.[17,18] Also at present, this
approach is considered the standard therapy excluding the 5q-
syndrome,[19] which is generally treated with lenolidomide[19-22] and
the hypoplastic forms, which can respond to immunosuppression with
anti-thymocyte globulin, cyclosporine, and alemtuzumab.[23-29] After
the failure of ESAs, other therapies can be instituted based on
lenalidomide,[30] demethylating agents[9,31-33] and others not yet
approved drugs. There is no clear guidance regarding the choice of
lenalidomide or an HMA as initial disease-modifying therapy for
patients with non-del(5q) LR-MDS, who mainly require treatment to
reduce anemia and the need for transfusions.
The addition of G-CSF
and Gm-CSF, even if is a common practice in myelodysplastic
patients[9,17,18] with marked neutropenia has not a proved efficacy in
preventing infections, and other drugs reducing granulocytes
malfunctions should be tried.[34]
Recently, to avoid iron
overload and organ damage has been proposed the addiction of chelation,
particularly to low or intermediate 1 risk MDS patients.[35,36]
Patients with iron overload disorders are known to be susceptible to
lethal infections with bacteria that are considered only moderately
pathogenic in other settings.[37] Two species of “siderophilic”
bacteria are characteristic of such infections Vibrio vulnificus and Yersinia enterocolitica.
These infections have been described in thalassemia or hemochromatosis
patients with tremendous iron overload treated with deferoxamine but
not in MDS.[37-39] It is possible that the infections from
“siderophilic” bacteria in thalassemia and hemochromatosis can be in
part attributed to the use of deferoxamine which, at variance with
deferasirox and deferiprone enhances the growth of Yersinia
in vitro or in vivo.[37]. On the contrary, the use of iron-chelators
could provide a complementary approach to overcome drug resistance in
pathogenic bacteria by reducing the iron available by siderophilic
bacteria.[39] However, the addition of iron-chelators seems to improve
overall survival without reducing the deaths from
infections,[35,36,40,41] even if a recent paper suggests that the time
to its first manifestation was significantly longer in chelated
patients.[37,42] Also, the hepcidin could play a role reducing
infection by lowering the iron-free plasma level.[43]
Low grade/intermediate risk MDS treated with Immunosuppression.
Immunosuppressive treatment may be a therapeutic option for selected
patients with myelodysplastic syndrome characterized by hypoplastic
bone marrow. Following the immunosuppressive therapy, authors reported
a response between 30 to 60%.[23-29]
The most significant
factors favoring the response to treatment are younger age, hypoplastic
bone marrow, HLA-DR15 positivity and combination anti-thymocyte
globulin (ATG) plus cyclosporine A (CsA) treatment.[23,24] The
infections represented the primary cause of death, particularly in
nonresponsive patients. Sloan et al[23] reported response in 30% of 129
patients treated; 59 patients died, whom 33% died from leukemia and 61%
from bleeding/and or infection consecutive to marrow failure. In the
study of 27 patients reported by Komrokji et al.[29] three died, of
whom one of a preexisting line infection and one of pulmonary
aspergillosis. The survey of Passweg et al.[24] is the only reporting a
control group, treated with the only supportive therapy. This trial
included mostly patients with low or intermediate[1] risk group (80%),
the response was about 30% versus 4% of the control. The incidence of
neutropenia was the same in the two groups. In addition to the 40
deaths, 20 serious adverse events (SAEs) were reported (16 in the
ATG+CSA arm and four in the Best Supportive Care (BSC) arm; P=.005).
The deaths from infections were four in ATG+CSA arm and 2 in BSC arm.
Low grade/intermediate risk MDS treated with Lenolamide.
Lenalidomide is considered the drug of choice in MDS patients with 5q
deletion.[19-21]. Neutropenia and thrombocytopenia are the most
treatment-associated adverse events and in the pivotal trial of List et
al.[20] are reported respectively in 54.7% and 43.9% of subjects
treated. Grade 4 (<500 x 109/L)
was more common among patients receiving continuous daily dosing than
among those receiving 21-day dosing (44.1% vs.17.4 %, P<0.001).
However, neutropenia was accompanied by fever in only 4.1% of patients.
During this trial, 11 patients died while receiving treatment or within
30 days after the last dose of lenalidomide; 3 deaths, attributed to
neutropenic infection, were judged to be possibly treatment related by
the treating physician. All other deaths were considered unrelated to
the treatment. There were three deaths from congestive heart failure,
one death from ischemic colitis, one death from AML, one death from
procedure-associated intestinal perforation, one death from
subarachnoid hemorrhage, and one sudden death. In the study of Fenaux
et al.[21] grade 3 or 4 neutropenia and thrombocytopenia generally
occurred within the first two cycles and subsequently decreased and
were also the most common reasons for lenalidomide dose reductions.
Furthermore, infection and febrile neutropenia were significant grade 3
or 4 adverse events. Also, in the evaluation of the same study made by
Giagounidis et al.,[21] the most common grade 3–4 adverse event in
patients treated with lenalidomide was myelosuppression. Grade 3–4
neutropenia was reported more frequently (75%) in patients treated with
lenalidomide than in placebo group. So, infection (any grade) was
reported in about 60% in lenolamide groups and about 30% of patients
in placebo groups. In this study, there were no treatment-related
deaths because of neutropenic infection at variance with the study of
List et al.[20] This difference was attributed to improved monitoring
of neutropenia and management of febrile neutropenia, the dose
reduction rules implemented, and possibly the use of G-CSF or GM-CSF.
In the recent experience reported by Fenaux et al[44] comparing the
behavior of patients at different ages, the adverse events (AEs) in the
≥75 years group were compared with the <65 years group. The most
common grade 3–4 AEs were neutropenia and thrombocytopenia. The
incidence of grade 3–4 thrombocytopenia was significantly lower in
patients aged <65 years than in patients aged ≥65 to <75 years.
However, the incidence of grade 3–4 neutropenia was significantly lower
in patients aged ≥75 years than in patients aged ≥65 to <75 years (p
= 0.041). Dose reductions due to thrombocytopenia were more common in
the ≥75 years group compared with that <65 years. G-CSF prophylaxis
for neutropenia did not differ significantly across the age groups. The
lower rates of neutropenia in the ≥75 years group may reflect the
reduced total dose of lenalidomide in this age group rather than
variations in G-CSF use. Although grade 3–4 neutropenia occurred less
frequently in patients aged ≥75 years, infectious episodes were more
common, a disparity possibly related to the known natural deterioration
of the immune response in older individuals.[44]
Lenalidomide has
also been utilized in patients with low-intermediate risk MDS without
5q deletion previously treated or not treated with ESA, with and
without ESA.[29,45-47] The results are better if the patients were not
previously treated or resistant to ESA.[29,36] Furthermore, the
addition of ESA seems to improve the erythropoietic response.[45,46]
Moreover in patients with non-del(5q) lower-risk MDS previously treated
with ESAs, none of the most commonly used second-line treatments
(demethylating agents and lenalidomide) improved OS.[47]
Also in
these patients, lenalidomide, compared with placebo, was associated
with a higher incidence of grade 3-4 treatment-emergent adverse events
(TEAEs; 86% vs. 44%, and among them neutropenia was prevalent, 30%),
but with not a major risk of infection (p = .817). Only the frequency
of pneumonitis could be major in patients treated with lenalidomide
(5.6% vs. 2.5%).[48]
Low grade/intermediate risk MDS treated with Demethylating Agents.
Azacytidine (AZA) and decitabine (DAC) are approved in the USA both for
low Intermediate-1 as well intermediate-high risk MDS in Europe only
for Intermediate-2 and high-risk MDS. In patients with low/intermediate
risk, they have been utilized mostly as second-line therapy in patients
primarily or secondarily resistant to ESA, and transfusion dependent
(TD).[9,31,32,47,49,50] There are some difficulties in understanding
the role of the demethylating agents in infections in this setting of
patients. In fact, in the American literature, most trials reporting
AZA or DAC experience include low-risk and high-risk patients without
distinguishing the response and the side effects of the therapy in the
two settings. Furthermore, in the abundant word literature is rare to
find demethylating trials in which there is a control group treated
with the supportive therapy only. So, even if there is concordance in
finding that neutropenia is the most important hematological toxicity,
hitting about 35% of all patients treated and that the infection is the
main cause of death, it is difficult to understand how many patients
acquired an infection because of the therapy, being evident that there
is not, in MDS, a strict correlation between neutropenia and
infections. The prospective phase II study of Tubiasson et al.[49]
evaluated the efficacy of AZA in 30 patients with MDS low/intermediate
risk, refractory to full-dose Epo+/-granulocyte colony stimulation
factors for 48 weeks a and with a transfusion need of >4 units over
eight weeks. AZA 75 mg/sqm days for 5 days each 28-day cycle, was given
for six cycles; non-responding patients received another three cycles
combined with Epo 60.000 units per week. The most important
hematological toxicity was neutropenia. Nineteen patients suffered from
severe neutropenia (ANC<0.5x109/L)
at any time point during treatment, four of which were severely
neutropenic before the treatment was started. The most commonly
reported non-hematological adverse events were infections (n=30) and
the related adverse events were neutropenic fever (n=12) and fever
(n=6). Thirty-eight serious adverse events were reported in 18 patients
during the study period. The main serious adverse event criterion
(n=36) was in-patient hospitalization. The vast majority (n=28) of the
serious adverse event was related to infection with or without
neutropenia. Two patients died. Cause of death for the first patient is
unknown; he suffered a sudden death after two cycles of AZA and had at
the onset of the disease a moderate cytopenia aggravated during
treatment. Cause of death for the second patient was septicemia with
Escherichia coli during AZA-associated severe neutropenia. Authors
conclude that AZA can induce transfusion independence (TI) in severely
anemic MDS patients, but efficacy is limited, toxicity substantial and
most responses of short duration. Thus, this treatment cannot generally
be recommended in lower-risk MDS. The study of Fili et al[30]
prospectively evaluated the efficacy and safety of AZA, administered at
a lower cumulative monthly dose [5-days AZA (5d-AZA); 75 mg/sqm days
for 5 days each 28-day cycle,], in 32 patients with IPSS low- or
Int-1–risk MDS who were symptomatic and/or unresponsive to previous
treatments. The overall response rate was 47% (15 of 32) on
intention-to-treat and 58% (15 of 26) for patients completing the
treatment program. In this latter group, 5 (19%) achieved complete
remission (CR), and 10 (38%) had hematologic improvement, according to
the International Working Group (IWG) criteria. Neutropenia, observed
in 15 of 32 patients (47%), was the most common hematologic toxicity,
and four patients died for infections and/or bleeding. In the
experience of Sanchez-Garcia et al.,[50] 40 patients with MDS (IPSS score
low or Int-1), with the absence of del5q, transfusion dependent (TD)
anemia, and unresponsive to ESAs were assigned randomly to supportive
therapy or to AZA 75mg/sqm, subcutaneously for 5 days of each
28-day cycle for nine cycles. Though the erythroid hematological
improvement (HI-E) was confirmed in 44.4% of randomized to AZA and in
5.5% of patients receiving best supportive cure (p< .01), the
event-free survival was not different between the two groups.
Manageable hematological toxicity was seen in 52.2% of patients in AZA
arm with seven patients experiencing severe AEs. In the BSC arm, eight
patients also developed AEs related to the natural course of MDS. In
particular, febrile neutropenia and/or pneumonia were reported in 22%
of patients treated with AZA and in 11% of those treated with
supportive treatment only.
The patients with low-risk MDS can
also respond to a low dose of demethylating agents. Jabour et al[51]
compared the safety and efficacy of low-dose DAC vs. low-dose AZA in
this group of patients. Adults with low- or intermediate-1 risk MDS or
MDS/myeloproliferative neoplasm (MPN), including chronic myelomonocytic
leukemia, were randomly assigned using a Bayesian adaptive design to
receive either AZA 75 mg/sqm intravenously/subcutaneously daily or DAC
20 mg/m2 intravenously daily for
three consecutive days on a 28-day cycle. More myelosuppression was
encountered in patients treated with DAC, resulting in cycle delays and
dose reductions, however, the ORR was better in them, being 70% respect
49% in patients treated with AZA (P = .03). Cycle delays and dose
reduction were required in 38% and 12% of patients treated with DAC and
20% and 5% of patients treated with AZA. The number of infections was
not so different in the two groups. Infection or neutropenic fever
occurred in 7% and 5% of patients treated with DAC and AZA,
respectively.
A large cooperative study evaluated the
outcome of low-risk MDS patients 5q-negative after the failure of
ESA.[52] Out of 653 subjects failing or relapsing after ESA, 450 were
treated with second-line therapy. Of them, 194 received hypomethylating
agents (HMA), 148 lenalidomide and 108 another treatment. None of these
treatments improved the overall survival significantly. In all three
groups, the infections were the predominant cause of death, 26% in
patients treated with HMA, 23% in those treated with lenalidomide and
22% in the third group. In conclusion at variance with
high/intermediate-2 MDS patients with low-risk MDS, resistant to ESAs,
do not have any advantage from demethylating agents, and probably the
advantage in remission is counterbalanced by an increased rate of
infections. The reduction of infections as a cause of deaths could be a
way to improve the prognosis.
Intermediate 2 and High Risk MDS
The
standard treatment for Intermediate 2 and high risk MDS is represented
by the AZA and DAC.[53-56] The approval by the US Food and Drug
Administration (FDA) of the hypomethylating agents (HMAs) AZA and DAC
was made in 2004 and 2006, and by the European Medicines Agency (EMA)
in 2009 and 2012 respectively. However, patients without comorbidities,
particularly if young, can also be treated with intensive therapies,
mainly to obtain a remission before being submitted to hematopoietic
stem cell transplantation.[55] However, recently, even AZA has also
been utilized pre-transplant in order to achieve a remission or a
hematologic improvement.[55,57] Only recently have been published
some studies dedicated explicitly to infections in patients treated
with AZA[58-65] or DAC.[67]
Patients treated with AZA.
In the first trials demonstrating the superiority of the AZA in high
risk MDS versus supportive therapy[53] or the best current
therapy[54,55] it was shown a reduced or comparable rate of infections
in the patients treated with AZA. In the Silverman et al[53]
experience, the rate of infection per patient-year was 0.64 in the AZA
group and 0.95 in the observation group. Clinically significant
infections were similar to the most common sites of infection (lung,
urinary tract, and the bloodstream, skin) typically observed in
patients with MDS, with no apparent increase in the AZA group. In the
observation group, infection with pneumonia/sepsis was the cause of
death in month 3 of one (2%) of the 41 observation patients who did not
cross over during the study. Among 150 AZA-treated patients, infections
were the cause of death in three patients (2%). In the trial of Fenaux
et al.[54], the most common grade 3–4 events were peripheral blood
cytopenias for all treatments. The rate of infections treated with
intravenous antimicrobials per patient-year in the AZA group was 0·60
(95% CI 0·49–0·73) compared with 0·92 (0·74–1·13) in the conventional
care group (relative risk 0·66, 95% CI 0·49–0·87; p=0·0032). The
advantage of in term of infection of AZA respect to any other therapy
was particularly evident in high risk MDS having a percentage of blasts
between 20 and 30% in the bone marrow, and so classified at present as
AML according to WHO.[66]
A few studies have been dedicated
specifically to the incidence and risk factors of infections in
patients treated with AZA.[8,58-66]
In the retrospective study of
Merkel et al.[8] aimed to evaluate the incidence and predisposing risk
factors for infections in AZA-treated, were included 184 patients [157
high-risk MDS and 27 AML, with a median age of 71.6], treated with AZA
in 18 Israeli medical institutions between 2008 and 2011. Overall, 153
infectious events were reported during 928 treatment cycles
administered to 100 patients. One hundred fourteen, (75%) events
required hospitalization and 30 (19.6%) were fatal. In a univariate
analysis, unfavorable cytogenetics, low neutrophil, hemoglobin and
platelet counts were found to be associated with infections in
multivariate analysis, only low Hb level, low PLT count, and
unfavorable cytogenetics remained significant. Before therapy, poor
cytogenetics, PLT count below 20 x 109/L and a neutrophil count below 0.5 x 109/L were predictive of the risk of infection during the first two cycles of therapy (Table 2).
Infectious events were more frequent after doses of 75 mg/sqm for seven
days than 75 mg/sqm for five days, regardless of the patient’s age.[58]
In this study, the causative pathogen was identified as bacterial in 25
(54.3%) and as viral or fungal in 2 (4.3%) and 2 (4.3%) cases,
respectively.
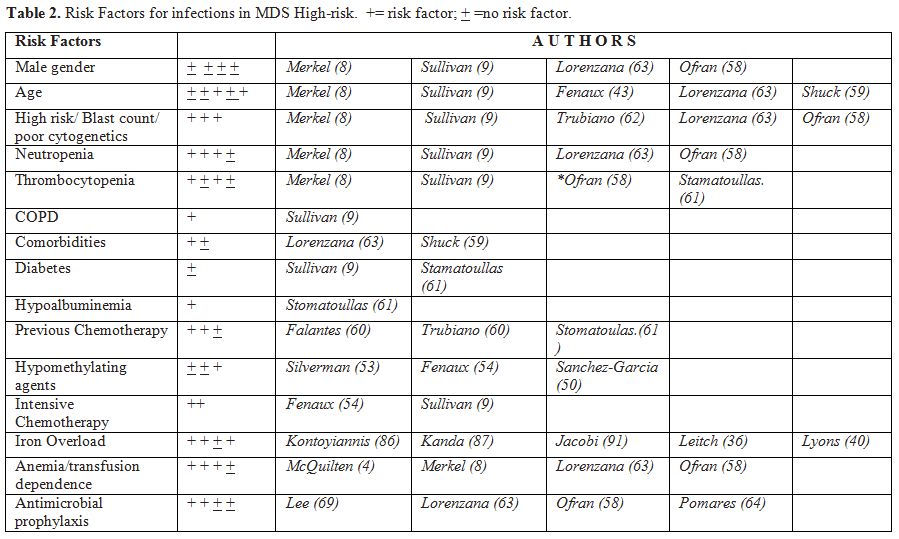 |
Table 2. Risk Factors for infections in MDS High-risk. += risk factor; + =no risk factor. |
No
pathogen was identified in 17 (37%) cases. Infections were
significantly more prevalent among patients who presented with platelet
counts < 20,000 (43.6% vs. 23.6%; P < .012) and poor risk
cytogenetics (40.7% vs. 19.8%; P < .008). Patients treated with AZA
who previously received intensive chemotherapy seem to be at the
highest risk for fungal infection (invasive aspergillosis; (p .015), so
primary antifungal prophylaxis might be recommended in this group of
patients.[60]
The importance of a previous therapy was not
confirmed in a multivariate analysis by Stamatoullas et al.,[62] who
found a major risk of infection in subjects with hypoalbuminemia and
hypergammaglobulinemia. However, Trubiano et Al., in a paper of 2017,
report a retrospective review of patients receiving ≥1 cycle of AZA for
MDS (49), or AML (19). Sixty-eight patients received 884 AZA cycles.
Bacterial infections occurred in 25% of cycle-1 and 27% of cycle-2 AZA
therapy. Febrile neutropenia complicated 5.3% of AZA cycles, bacteremia
2%, and invasive Aspergillosis 0.3%. Using Poisson modeling, a very
high IPSS-R (RR 10.26, 95% CI 1.20, 87.41, p= .033) was identified as
an independent risk factor for infection. Infection-related
attributable mortality was 23%. In this series the burden of infection
is high in AZA-treated patients and is associated with high
attributable mortality. Over 25% of AZA cycles 1 and 2 were complicated
by infection, predominantly bacterial, rates dropping to <10% after
cycle-5 (Table 3, Figure 1). Among the microbiologically-confirmed infections were prevalent the bacteria (49) in this order E. coli, Coagulase-negative Staphylococcus, Enterococcus spp., Staphylococcus aureus, Pseudomonas spp., Clostridium difficile, Stenotrophomonas maltophilia; and among the fungal infections Aspergillus spp was the most common. (Table 4)
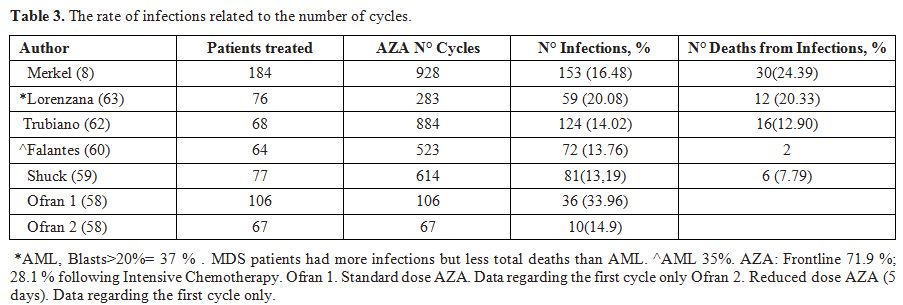 |
Table 3.
The rate of infections related to the number of cycles. |
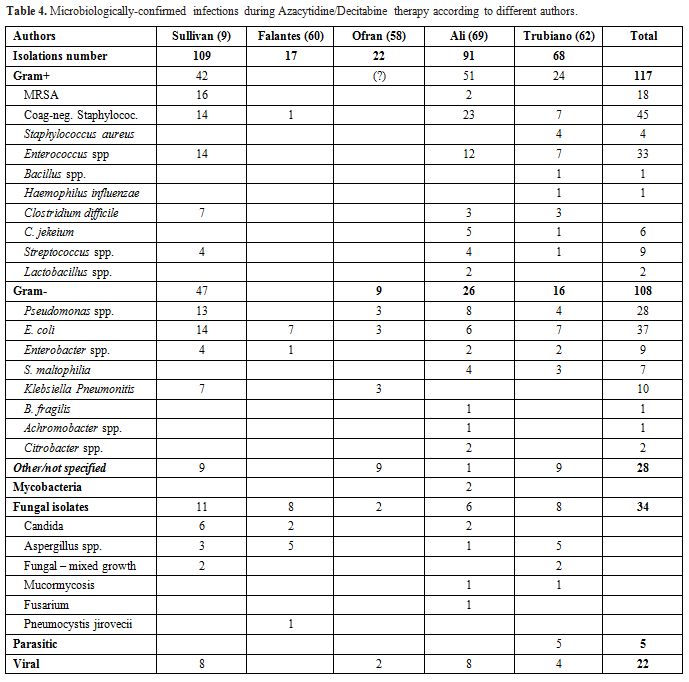 |
Table 4. Microbiologically-confirmed infections during Azacytidine/Decitabine therapy according to different authors. |
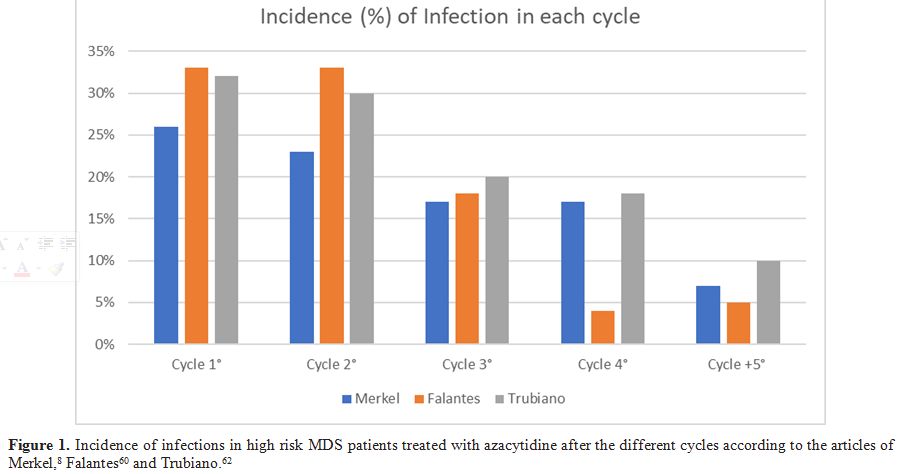 |
Figure 1. Incidence of infections in high
risk MDS patients treated with azacytidine after the different cycles
according to the articles of Merkel,[8] Falantes[60] and Trubiano.[62] |
Shuck
et al.[59] of Dusseldorf group retrospectively evaluated the clinical
course of 77 patients with MDS treated with AZA between 2004 and 2015
(median age 69 years). In total, 614 AZA cycles were administered, and
81 cycles with at least one infection complication (IC) were
individuated. The median number of cycles was 6 (range 1–43). Median OS
after the start of AZA was 17 months (range 1–103). Infection rates
were higher in the first 3 cycles with bacterial infections leading (Table 3), (Figure 1).
The better patients' hematological response to AZA with less IC
occurred, and fewer days with antimicrobial treatment were needed.
Compared to progressive disease, the stable disease made no significant
improvement in the occurrence of IC and days in the hospital. Older age
was associated with more IC and longer time in the hospital.
Comorbidities or IPSS-R did not influence IC. The incidence of IC
correlated with hematological response and age. The stable disease led
to longer OS, but the incidence of IC was comparable to progressive
disease and survival seemed to be bought by a considerable number of
IC. IC rates were highest in the first 3 cycles (Figure 1 and Table 3).
Taking
into account the high risk of infection bacterial and antifungal
prophylaxis has been suggested in different protocols, but there is not
a randomized trial demonstrating the utility of antibacterial and
antifungal prophylaxis during AZA treatment. Lorenzana et Al. compared
in a retrospective, single-center study, the impact of prophylaxis on
the incidence of infection and morbidity in all consecutive higher-risk
MDS and AML patients, during the first 4 AZA cycles. Seventy-six
patients, corresponding to 283 AZA cycles, were studied. Antimicrobial
prophylaxis was administered in 117 cycles (41%). There were
significant differences between the cycles with and without
prophylaxis. Cycles with prophylaxis showed lower neutrophil counts and
more severe disease characteristics. The majority of patients (75%)
received combination therapy with quinolones and antifungals. There
were infectious events in 43% of the patients. Globally, prophylaxis
did not decrease the incidence of infection (17 vs 24%, p = 0.22).
However, when only cycles starting with a neutrophil count below 0.5 ×
109/L were analyzed, the incidence of
infection was significantly lower (16 vs. 51%, p < 0.001). Risk
factors for infection were neutropenia (OR 9.6 [2.63–34.7], p <
0.001) and comorbidity index (OR 1.62 [1.02–2.56], p = 0.003).
Prophylaxis decreased the risk of infection (OR 0.13 [0.03–0.56], p =
0.006), with a significant interaction with neutropenia (OR 16.7
[2.5–109.8], p = 0.003). Median overall survival was comparable between
patients with or without infections. However, the development of
infections led to more hospital admissions, increased red blood cells
and platelet requirements, and a delay in subsequent cycles. In the
multivariate analysis, a neutrophil count below 0.5 × 109/L
(OR 12.5 [2.6-50]) and antimicrobial prophylaxis (OR 0.1 [0.02-04])
were independent factors for the development of infection. Authors
conclude that infectious events have a significant impact on the early
clinical course of AZA-treated patients by increasing hospital
admissions and transfusion requirements. Antimicrobial prophylaxis may
prevent infections, leading to a decreased need for supportive care in
these patients with poor outcome. On the contrary, Pomares et al.[64]
found a very low risk of fungal infection in patients with high-risk
MDS and AML treated with AZA, since the incidence rate of
proven/probable invasive fungal infection (IFI) was 0.21% per treatment
cycle and 1.6% per patient treated for the whole series, and 0.73% per
treatment cycle and 4.1% per patient treated in those with severe
neutropenia MDS. Therefore, they think that this very low risk of IFI
does not justify the use of antifungal prophylaxis.
Patients treated with Decitabine (DAC).
In the randomized trial[67] comparing low-dose DAC versus best
supportive care in elderly patients with intermediate- or high-risk MDS
ineligible for intensive chemotherapy grade 3 to 4 febrile neutropenia
occurred in 25% of patients on DAC versus 7% of patients on BSC. Grade
3 to 4 infections occurred in 57% and 52% of patients on DAC and BSC,
respectively. This trial did not demonstrate the superiority of DAC in
overall survival; however, this treatment was associated with
improvements in patient-reported quality-of-life (QOL) parameters. The
type of infection found in 27 patients with MDS and 58 with AML (older
or unfit) treated with DAC low dose ten days was investigated in a
prospective clinical study of Washington University School of
Medicine.[68] Prophylactic antimicrobial therapy was recommended, but
not stipulated as part of the study. Recommended prophylaxis consisted
of acyclovir, ciprofloxacin, and fluconazole Culture results were
available for 163 infection-related complications that occurred in 70
patients. Ninety (55.2%) events were culture-negative, 32 (19.6%) were
gram-positive bacteria, 20 (12.3%) were gram-negative bacteria, 12
(7.4%) were mixed, 6 (3.7%) were viral, 2 (1.2%) were fungal, and 1
(0.6%) was mycobacterial. Infection-related mortality occurred in 3/24
(13%) of gram-negative events, and 0/51 gram-positive events. (Table 3)
On average, nearly one-third of patients experienced an
infection-related complication with each cycle, and the incidence did
not decrease during later cycles. In summary, in patients receiving
10-day DAC, infectious complications are common and may occur during
any cycle of therapy. Although febrile events are commonly
culture-negative, gram+ infections are the most frequent source of
culture-positive infections, but gram-negative infections represent a
significant risk of mortality in AML and MDS patients treated with DAC.
Comparing the infections incidences, a higher incidence of
infections was noted in MDS patients (96.3%) respect to AML patients
(77.5%, P = 0.032). However, AML patients also had shorter
survival compared with MDS patients.
The role of antibiotic
prophylaxis during DAC treatment for MDS was studied in a group of 28
MDS patients treated with DAC in a University Hospital of Seoul
(Korea).[69] The primary endpoint was the incidence of febrile
episodes. The total number of DAC cycles given to 28 patients was 131,
and febrile episodes occurred in 15 cycles (11.5%). Antibiotic
prophylaxis was given orally in 95 cycles (72.5%). Febrile episodes
were significantly less frequent among patients who received antibiotic
prophylaxis (7.4%) than in those without prophylaxis (22.2%) (P =
0.017). Causative microbial agents were isolated in 6 cycles:
methicillin-sensitive Staphylococcus aureus in 2 (blood in 1 and central venous catheter (CVC) in 1) and each one of Staphylococcus epidermidis (blood), Klebsiella peumoniae (urine), Enterococcus faecalis (urine), and Stenotrophomonas maltophilia (sputum) (Table 4).
According to this report, antibiotic prophylaxis reduces the incidence
of febrile episodes in patients who received DAC treatment for MDS,
especially at earlier cycles and in the presence of severe
cytopenia.[69]
Intensive Treatment.
Like AML, high-risk MDS commonly require intensive chemotherapy to
achieve disease complete remission. In the past high dose chemotherapy
was the standard therapy for fit patients, with age <60.[70] The
main cause of infections after intensive chemotherapy in both MDS
and AML is the neutropenia, so in this circumstance, there is no a
difference in prevention and treatment of infections between patients
affected by MDS and AML.[70-74] It is noteworthy that most of the
fungal infections are reported in MDS patients with high blasts
infiltration treated with intensive chemotherapy.[75] So the
experiences with intensive therapy of patients with MDS or AML are
frequently reported together, furthermore, before the WHO
classification of 2008, the subjects with blast infiltration between 20
and 30% were classified in the MDSs.[76] However, the rate of the
relapse of patients with MDS was very high, so, similarly
to leukemia, trials were made utilizing intensive chemotherapy as a
pre-transplant procedure followed by an allogeneic hematopoietic stem
cell transplant (HSCT)[70,77] However, the pretreatment with high dose
chemotherapy of patients with MDS entails a series of side effects,
among them, infections are prevalent, which reduce the number of
patients susceptible to stem cell transplantations.[77] Furthermore,
the patients pretreated with chemotherapy have an overall survival
similar to those transplanted upfront.[78,79] Therefore, even if
the subjects in remission have a better prognosis, today the upfront
transplant is preferred and the pretreatment with chemotherapy is
suggested only in presence of a percentage of bone marrow blasts
>10, when the reduced dose conditioning regimen is
chosen.[80] To decrease the toxicity a reduced intensity
conditioning (RIC) regimen is becoming more and more frequent,[80-82]
even if a recent study of Seattle group[81] suggests that the
eradicating regimen should be considered the standard. RIC has a
higher relapse rate than standard conditioning but a lower the toxicity
and non-relapse mortality.[80,81] Bacterial complications are more
frequently observed in eradicating regimens, whereas no differences
have been reported in CMV reactivation, EBV reactivation, or other
viral o fungal infections.[81] According to some investigators the
antifungal prophylaxis with fluconazole could be not necessary,[83] in
patients treated with RIC but in general it is applied[82] and
antifungal prophylaxis should be performed with posaconazole
delayed-release tablets during remission induction chemotherapy.[84]
Relapse rate is generally is higher in RIC regimens. Some particular
risk factors for infection have been reported in the patients
transplanted because of MDS. The Seattle group reported increased
infection-related mortality in patients with MDSs with neutropenia <
1.5 x 109/L at baseline.[85] All
patients included in this analysis received a myeloablative
conditioning regimen. All patients were monitored for the onset of
infections during the first 100 days after HSCT. Monitoring included
bacterial and fungal blood cultures and chest radiographs when patients
developed a fever (38.3°C, orally). Additionally, all patients
receiving >0.5 mg/kg of corticosteroid therapy were monitored with
weekly bacterial and fungal blood cultures and chest radiographs. For Pneumocystis jiroveci
prophylaxis all patients received trimethoprim/sulfamethoxazole as
first-line therapy, dapsone as second-line therapy, from the time of
engraftment until six months after HSCT or until six weeks after all
immunosuppressive medications had been discontinued. All patients
received fluconazole or itraconazole for prevention of candidiasis from
the time of conditioning until day 75 after HSCT. Infections were
considered causes of death when they occurred in the absence of GVHD,
relapse, graft failure, and graft rejection. Overall, the neutropenic
cohort had significantly increased rates of bacterial and fungal
infections in comparison to non-neutropenic patients within the first
100 days after HSCT (rate ratio [RR] 1.59, P = .001 and RR =
2.89, P = .01, respectively). Most fungal infections were caused by the
Aspergillus species (27 of 32), and the remaining fungal infections were because of Candida glabrata (2 of 32) and Mucorales
spp. (3 of 32). The propensity for neutropenic patients to develop
bacterial infections varied by type of organism. There was an increase
in the rate of infections with gram-positive organisms but not with
gram-negative rods. The increased rate of fungal and gram-positive
bacterial infections among the neutropenic patients was most prominent
more than 60 days after HSCT. The rate ratio for fungal infections
remained unchanged after adjustment for aGVHD grades II-IV (RR 5 2.76,
95% confidence interval [CI] 1.1-6.7, P 5 .01), indicating there was no
evidence of confounding by aGVHD. Another important risk factor
advocated for an increased peritransplant mortality, and in particular
due to the infections is the iron overload.[86-91] The ferritinemia
(SF) is considered the standard method for measuring iron overload.
However, the optimal parameters and time points for the measurement of
iron overload (IO) in allogeneic stem cell transplantation (ASCT)
patients are still under discussion. Non-transferrin-bound iron (NBTI)
could be a better marker to predict the effect for a higher risk of
bloodstream infections than SF, as well the superconducting quantum
interference device (SQUID) biomagnetic liver susceptometry correlates
with ferritinemia and a significant association between SQUID,
measured before HSCT and fungal infection was also found.[90,91]
Another
possibility to improve the response to HSCT, reducing the toxicity and
the infections associated with high dose chemotherapy, is the
pretreatment of high risk MDs patients with demethylating agent.[92,93]
At variance with intensive chemotherapy[77] pretreatment with
demethylating agents does not reduce the number of patients susceptible
to HSCT significantly.[92,93] In a recent trial Voso et al. for
the Italian group GITMO demonstrate that HSCT is feasible after AZA in
the majority of patients with HR-MDS/AML/CMML-2 (74 % of subjects
with donor enrolled in the trial). Causes of death in the non-HSCT
group were disease progression or relapse (16 of 26 patients, 61.5%),
followed by infectious (7 patients), and hemorrhagic complications (3
patients). Serious adverse events impeded HSCT in three patients and
consisted of infection in two cases and an intra-abdominal hemorrhage
in one patient. Mortality was transplant-related in 16 patients (30%,
GVHD: 4 patients, infectious complication: 6 patients, multi-organ
failure: 4 patients, other causes: 2 patients), disease relapse in 9
patients (17%), and second malignant disease in 1 patient. So, in this
experience, the infections were the causes of deaths in 15 patients out
of 97 patients enrolled. Similarly, in the more restricted pilot study
of Tampa group (25 patients whom 21 transplanted), toxicities of 5-AZA
treatment were low and included febrile neutropenia (5%), Clostridium difficile
colitis (5%), nodular pneumonia (presumed fungal, 5%), perirectal
abscess (5%), deep venous thrombosis (5%), and cerebrovascular accident
(5%), without mortality. Causes of death of transplanted patients
included four disease relapses, three infectious complications, and
three with GVHD and infections. Central line-associated bloodstream
infections commonly complicate the care of patients with AML and MDS
after HSCT. However, you should distinguish between pathogens usually
acquired following high dose chemotherapy because of disruption of
mucosal barriers during the vulnerable neutropenic period, such as
enteric gram-negative bacilli and Streptococcus viridans,
that afterward localize in a central line, and pathogens which
localized directly in the central line.[95] Although both types of
central venous catheter (CVC) infection are characterized by a high
rate of mortality (>70) the time of insurgency, the species of
bacteria and fungus are different, and so should be the modality of
prevention.[95]
Conclusions
Infections
remain a major problem in MDSs and assume a particular complexity. In
fact, MDS include a heterogeneous group of patients with very different
prognosis, different therapy and different risk factors regarding
survival and infections. About this last point, we should distinguish
risk factors related to the disease, like as neutrophils function
impairment, neutropenia, unfavorable cytogenetics and bone marrow
insufficiency; factors related to the patient, like as age and
comorbidities, factors related to the therapy. When the patients with
MDS are submitted to intensive chemotherapy with and without HSCT, they
have a risk factor for infection very similar to that of patients with
AML. The age and comorbidities should be considered the most
important risk factor, and you should follow the same guideline for the
acute myeloid leukemia patients. Patients with MDS treated with
supportive therapy only or with demethylating agent or lenalidomide or
immunosuppressive drugs should have a tailored approach. Considering
that most (about 80%) of the infections in MDS originate from bacteria,
and the major risk factors are represented by neutropenia,
thrombocytopenia and unfavorable cytogenetics, it is reasonable to give
an antibacterial prophylaxis in patients who start the therapy with
demethylating agents with a number of neutrophils <500, or with
thrombocytopenia and unfavorable cytogenetics. This recommendation is
imperative in the first cycles of therapy during which the infections
are more frequent. The antifungal prophylaxis is not considered
cost/benefit adequate and should be taken into consideration only when
there is an antecedent fungal infection or presence of filamentous
fungi in the surveillance cultures. Subjects submitted to
immunosuppression with ATG+CSA have a high number of infections,
although there are no guidelines we think that they should be treated
like with aplastic anemia. Therefore, patients who are severely
neutropenic should ideally be nursed in isolation when in hospital, and
likely, should be given prophylactic antibiotics and antifungals,
regular mouth care including an antiseptic mouthwash (such as
chlorhexidine or saline). Prophylactic antibiotics, either two
non-absorbable (e.g., colistin and neomycin) or quinolones (e.g.,
ciprofloxacin), may be initiated but the preference should be according
to local policy. A mold-active azole, preferably itraconazole or
posaconazole, should be used as prophylaxis, in the presence of
positive surveillance cultures. The use of lenalidomide, although can
give neutropenia, which in general is not durable, does not increase
the infection rate. An unresolved problem is how to prevent infections
in low-risk MDS on no therapy or supportive therapy. Patients
with MDS low-risk transfusion dependent frequently have iron overload
and are more at risk of infection. Chelating agents can reduce iron
overload and so probably increase the overall survival. However, no
convincing data are demonstrating a decrease of infections after
chelation therapy also in the presence of a decrement of ferritin
level. Pharmacological enhancement of some neutrophil functions is
possible and could be a new tool to reduce infections.
References
- Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N,
Pecora AL, Laouri M. Incidence and clinical complications of
myelodysplastic syndromes among United States Medicare beneficiaries. J
Clin Oncol. 2010; 28(17):2847-52.. https://doi.org/10.1200/JCO.2009.25.2395

- Neukirchen
J, Nachtkamp K, Schemenau J, Aul C, Giagounidis A, Strupp C, Kuendgen
A, Kobbe G, Haas R, Germing U. Change of prognosis of patients with
myelodysplastic syndromes during the last 30 years. Leuk Res. 2015
Jul;39(7):679-83. https://doi.org/10.1016/j.leukres.2015.04.001

- Nachtkamp
K, Stark R, Strupp C, Kündgen A, Giagounidis A, Aul C, Hildebrandt B,
Haas R, Gattermann N, Germing U. Causes of death in 2877 patients with
myelodysplastic syndromes. Ann Hematol. 2016 May;95(6):937-44. https://doi.org/10.1007/s00277-016-2649-3

- Prodan
M, Tulissi P, Perticarari S, Presani G, Franzin F, Pussini E, Pozzato G
(1995) Flow cytometric assay for the evaluation of phagocytosis and
oxidative burst of polymorphonuclear leukocytes and monocytes in
myelodysplastic disorders. Haematologica 80:212–218. PMid:7672714

- Fuhler
GM, Drayer AL, Olthof SG, Schuringa JJ, Coffer PJ, Vel- lenga E (2008)
Reduced activation of protein kinase B, Rac, and F-actin polymerization
contributes to an impairment of stromal cell derived factor-1 induced
migration of CD34+ cells from patients with myelodysplasia. Blood
111:359–368. https://doi.org/10.1182/blood-2006-11-060632

- Fianchi
L, Leone G, Posteraro B, Sanguinetti M, Guidi F, Valentini CG, Voso MT,
Pagano L (2012) Impaired bactericidal and fungicidal activities of
neutrophils in patients with mye-lodysplastic syndrome. Leuk Res
36:331–333. https://10.1016/j.leukres.2011.11.012

- Wesa
KM, Cunningham-Rundles S, Klimek VM, Vertosick E, Coleton MI, Yeung KS,
Lin H, Nimer S, Cassileth BR. Maitake mushroom extract in
myelodysplastic syndromes (MDS): a phase II study. Cancer Immunol
Immunother. 2015;64(2):237-47. Epub 2014 Oct 29. https://doi.org/10.1007/s00262-014-1628-6

- Merkel
D, Filanovsky K, Gafter-Gvili A, Vidal L, Aviv A, Gatt ME, Silbershatz
I, Herishanu Y, Arad A, Tadmor T, Dally N, Nemets A, Rouvio O, Ronson
A, Herzog-Tzarfati K, Akria L, Braester A, Hellmann I, Yeganeh S,
Nagler A, Leiba R, Mittelman M, Ofran Y. Predicting infections in
high-risk patients with myelodysplastic syndrome/acute myeloid leukemia
treated with azacitidine: a retrospective multicenter study. Am J
Hematol. 2013 Feb;88(2):130-4. PubMed PMID: 23345248. https://doi.org/10.1002/ajh.23368

- Sullivan
LR, Sekeres MA, Shrestha NK, Maciejewski JP, Tiu RV, Butler R, Mossad
SB. Epidemiology and risk factors for infections in myelodysplastic
syndromes. Transpl Infect Dis. 2013 Dec;15(6):652-7. https://doi.org/10.1111/tid.12130

- Santini
V. Treatment of low-risk myelodysplastic syndromes. Hematology Am Soc
Hematol Educ Program. 2016 Dec 2;2016(1):462-469. Review https://doi.org/10.1182/asheducation-2016.1.462 PMid:27913517

- Dayyani
F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, Faderl
S,O'Brien S, Pierce S, Kantarjian H, Garcia-Manero G. Cause of death in
patients with lower-risk myelodysplastic syndrome. Cancer. 2010 May
1;116(9):2174-9. https://doi.org/10.1002/cncr.24984

- Toma A, Fenaux P, Dreyfus F, Cordonnier C (2012) Infections in myelodysplastic syndromes. Haematologica 97:1459–1470. https://doi.org/10.3324/haematol.2012.063420

- Pagano
L, Caira M. Risks for infection in patients with myelodysplasia and
acute leukemia. Curr Opin Infect Dis. 2012 Dec;25(6):612-8. https://doi.org/10.1097/QCO.0b013e328358b000

- Caira
M, Latagliata R, Girmenia C. The risk of infections in patients with
myelodysplastic syndromes in 2016. Expert Rev Hematol. 2016
Jun;9(6):607-14. https://doi.org/10.1080/17474086.2016.1181540

- Greenberg
P, Cox C, LeBeau MM, et al: International scoring system for evaluating
prognosis in myelodysplastic syndromes. Blood 89:2079-2088, 1997
PMid:9058730

- Greenberg
PL, Tuechler H, Schanz J, et al: Revised international prognostic
scoring system for myelodysplastic syndromes. Blood 2012;
120:2454-2465, https://doi.org/10.1182/blood-2012-03-420489 PMid:22740453 PMCid:PMC4425443

- Hellstrom-Lindberg
E, Ahlgren T, Beguin Y, et al. Treatment of anemia in myelodysplastic
syndromes with granulocyte colony-stimulating factor plus
erythropoietin: results from a randomized phase II study and long-term
follow-up of 71 patients. Blood. 1998;92(1):68-75 PMid:9639501

- Park
S, Grabar S, Kelaidi C, et al; GFM group (Groupe Francophone des
Myelodysplasies). Predictive factors of response and survival in
myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM
experience. Blood. 2008;111(2):574-582 https://doi.org/10.1182/blood-2007-06-096370 PMid:17940203

- Pellagatti A., Boultwood J. Recent Advances in the 5q- Syndrome. Mediterr J Hematol Infect Dis 2015, 7(1): e2015037, https://doi.org/10.4084/mjhid.2015.037

- List
A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell
B,Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, Schmidt
M, Zeldis J, Knight R; Myelodysplastic Syndrome-003 Study
Investigators. Lenalidomide in the myelodysplastic syndrome with
chromosome 5q deletion. N Engl J Med. 2006 Oct 5;355(14):1456-65 https://doi.org/10.1056/NEJMoa061292 PMid:17021321

- Fenaux
P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M,
Muus P, Te Boekhorst P, Sanz G, Del Ca-izo C, Guerci-Bresler A, Nilsson
L,Platzbecker U, Lübbert M, Quesnel B, Cazzola M, Ganser A, Bowen D,
Schlegelberger B, Aul C, Knight R, Francis J, Fu T, Hellström-Lindberg
E; MDS-004 Lenalidomide del 5q Study Group. A randomized phase 3 study
of lenalidomide versus placebo in RBC transfusion-dependent patients
with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q.
Blood. 2011 Oct 6;118(14):3765-76. https://doi.org/10.1182/blood-2011-01-330126

- Giagounidis
A, Mufti GJ, Mittelman M, Sanz G, Platzbecker U, Muus P, SelleslagD,
Beyne-Rauzy O, te Boekhorst P, del Ca-izo C, Guerci-Bresler A, Nilsson
L, Lübbert M, Quesnel B, Ganser A, Bowen D, Schlegelberger B, Göhring
G, Fu T Benettaib B, Hellström-Lindberg E, Fenaux P. Outcomes in RBC
transfusion-dependent patients with Low-/Intermediate-1-risk
myelodysplastic syndromes with isolated deletion 5q treated with
lenalidomide: a subset analysis from the MDS-004 study. Eur J Haematol.
2014 Nov;93(5):429-38. https://doi.org/10.1111/ejh.12380

- Sloand
EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response
and survival in patients with myelodysplasia treated with
immunosuppressive therapy. J Clin Oncol. 2008 May 20;26(15):2505-11. https://doi.org/10.1200/JCO.2007.11.9214

- Passweg
JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M,
Ossenkoppele G, Hofmann WK, Schilling K, Tichelli A, Ganser A.
Immunosuppressive therapy for patients with myelodysplastic syndrome: a
prospective randomized multicenter phase III trial comparing
antithymocyte globulin plus cyclosporine with best supportive
care--SAKK 33/99. J Clin Oncol. 2011 Jan 20;29(3):303-9. https://doi.org/10.1200/JCO.2010.31.2686

- Hata
T, Tsushima H, Baba M, Imaizumi Y, Taguchi J, Imanishi D, Nagai
K,Tomonaga M, Miyazaki Y. Long-term outcome of immunosuppressive
therapy for Japanese patients with lower-risk myelodysplastic
syndromes. Int J Hematol. 2013 Dec;98(6):687-93. https://doi.org/10.1007/s12185-013-1468-8

- Neukirchen
J, Platzbecker U, Sockel K, Tsamaloukas A, Haas R, Germing U. Real life
experience with alemtuzumab treatment of patients with lower-risk MDS
and a hypocellular bone marrow. Ann Hematol. 2014 Jan;93(1):65-9. https://doi.org/10.1007/s00277-013-1859-1

- Parikh
AR, Olnes MJ, Barrett AJ. Immunomodulatory treatment of myelodysplastic
syndromes: antithymocyte globulin, cyclosporine, and alemtuzumab. Semin
Hematol. 2012;49(4):304-11. doi: 10.1053/j.seminhematol.2012.07.004.
Review. https://doi.org/10.1053/j.seminhematol.2012.07.004

- Haider
M, Al Ali N, Padron E, Epling-Burnette P, Lancet J, List A, Komrokji R.
Immunosuppressive Therapy: Exploring an Underutilized Treatment Option
for Myelodysplastic Syndrome. Clin Lymphoma Myeloma Leuk. 2016 Aug;16
Suppl:S44-8. https://doi.org/10.1016/j.clml.2016.02.017

- Komrokji
RS, Mailloux AW, Chen DT, Sekeres MA, Paquette R, Fulp WJ, Sugimori C,
Paleveda-Pena J, Maciejewski JP, List AF, Epling-Burnette PK. A phase
II multicenter rabbit anti-thymocyte globulin trial in patients with
myelodysplastic syndromes identifying a novel model for response
prediction. Haematologica. 2014;99:1176-83. https://doi.org/10.3324/haematol.2012.083345

- Raza
A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, Dreisbach
L,Schiffer CA, Stone RM, Greenberg PL, Curtin PT, Klimek VM, Shammo JM,
Thomas D, Knight RD, Schmidt M, Wride K, Zeldis JB, List AF. Phase 2
study of lenalidomide in transfusion-dependent, low-risk, and
intermediate-1 risk myelodysplastic syndromes with karyotypes other
than deletion 5q. Blood. 2008 Jan 1;111(1):86-93. https://doi.org/10.1182/blood-2007-01-068833 PMid:17893227

- Filì
C, Malagola M, Follo MY, Finelli C, Iacobucci I, Martinelli G, Cattina
F, Clissa C, Candoni A, Fanin R, Gobbi M, Bocchia M, Defina M, Spedini
P, Skert C, Manzoli L, Cocco L, Russo D. Prospective phase II Study on
5-days azacitidine for treatment of symptomatic and/or erythropoietin
unresponsive patients with low/INT-1-risk myelodysplastic syndromes.
Clin Cancer Res. 2013 Jun15;19(12):3297-308. doi: https://doi.org/10.1158/1078-0432

- Komrokji
R, Swern AS, Grinblatt D, Lyons RM, Tobiasson M, Silverman LR, Sayar H,
Vij R, Fliss A, Tu N, Sugrue MM. Azacitidine in Lower-Risk
Myelodysplastic Syndromes: A Meta-Analysis of Data from Prospective
Studies. Oncologist. 2017 Nov8. pii: theoncologist.2017-0215. https://doi.org/10.1634/theoncologist.2017-0215

- Sibon
D, Cannas G, Baracco F, Prebet T, Vey N, Banos A, Besson C, Corm S
Blanc M, Slama B, Perrier H, Fenaux P, Wattel E; Groupe Francophone des
Myélodysplasies. Lenalidomide in lower-risk myelodysplastic syndromes
with karyotypes other than deletion 5q and refractory to
erythropoiesis-stimulating agents. Br J Haematol. 2012
Mar;156(5):619-25. https://doi.org/10.1111/j.1365-2141.2011.08979.x

- Hutzschenreuter
F, Monsef I, Kreuzer KA, Engert A, Skoetz N. Granulocyte and
granulocyte macrophage colony stimulating factors for newly diagnosed
patients with myelodysplastic syndromes. Cochrane Database Syst Rev.
2016 Feb 16;2:CD009310. https://doi.org/10.1002/14651858.CD009310.pub2

- Neukirchen
J, Fox F, Kündgen A, Nachtkamp K, Strupp C, Haas R, Germing U,
Gattermann N. Improved survival in MDS patients receiving iron
chelation therapy - a matched pair analysis of 188 patients from the
Düsseldorf MDS registry. Leuk Res. 2012 ;36(8):1067-70. https://doi.org/10.1016/j.leukres.2012.04.006

- Angelucci
E, Urru SA, Pilo F, Piperno A. Myelodysplastic Syndromes and Iron
Chelation Therapy. Mediterr J Hematol Infect Dis. 2017 Mar 1;9(1):
e2017021. https://doi.org/10.4084/mjhid.2017.021

- Ganz
T. Iron and infection. Int J Hematol. 2018 ;107(1):7-15.
doi:10.1007/s12185-017-2366-2. Epub 2017 Nov 16. Review. Erratum in:
Int J Hematol.2017 Dec 2;:. PubMed PMID: 29147843. https://doi.org/10.1007/s12185-017-2366-2

- Chan
GC, Chan S, Ho PL, Ha SY. Effects of chelators (deferoxamine,
deferiprone and deferasirox) on the growth of Klebsiella pneumoniae and
Aeromonas hydrophila isolated from transfusion-dependent thalassemia
patients. Hemoglobin. 2009;33(5):352-60. https://doi.org/10.3109/03630260903211888

- Gokarn
K, Pal RB. Activity of siderophores against drug-resistant
Gram-positive and Gram-negative bacteria. Infect Drug Resist. 2018 Jan
9;11:61-75. https://doi.org/10.2147/IDR.S148602

- Leitch
HA, Parmar A, Wells RA, Chodirker L, Zhu N, Nevill TJ, Yee KWL, Leber
B, Keating MM, Sabloff M, St Hilaire E, Kumar R, Delage R, Geddes M,
Storring JM, Kew A, Shamy A, Elemary M, Lenis M, Mamedov A, Ivo J,
Francis J, Zhang L, Buckstein R. Overall survival in lower IPSS risk
MDS by receipt of iron chelation therapy, adjusting for patient-related
factors and measuring from time of first red blood cell transfusion
dependence: an MDS-CAN analysis. Br J Haematol.
2017;179(1):83-97. https://doi.org/10.1111/bjh.14825

- Lyons
RM, Marek BJ, Paley C, Esposito J, McNamara K, Richards PD, DiBella N,
Garcia-Manero G. Relation between chelation and clinical outcomes in
lower-risk patients with myelodysplastic syndromes: Registry analysis
at 5 years. Leuk Res. 2017 May; 56:88-95. https://doi.org/10.1016/j.leukres.2017.01.033

- Wong
CAC, Wong SAY, Leitch HA. Iron overload in lower international
prognostic scoring system risk patients with myelodysplastic syndrome
receiving red blood cell transfusions: Relation to infections and
possible benefit of iron chelation therapy. Leuk Res. 2018 Feb 10; 67:
75-81. https://doi.org/10.1016/j.leukres.2018.02.005

- Stefanova
D, Raychev A, Arezes J, Ruchala P, Gabayan V, Skurnik M, Dillon BJ,
Horwitz MA, Ganz T, Bulut Y, Nemeth E. Endogenous hepcidin and its
agonist mediate resistance to selected infections by clearing
non-transferrin-bound iron. Blood. 2017 Jul 20;130(3):245-257. https://doi.org/10.1182/blood-2017-03-772715

- Fenaux
P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mittelman M, Muus P,Nimer
SD, Hellström-Lindberg E, Powell BL, Guerci-Bresler A, Sekeres MA, Deeg
HJ,Del Ca-izo C, Greenberg PL, Shammo JM, Skikne B, Yu X, List AF.
Clinical characteristics and outcomes according to age in
lenalidomide-treated patients with RBC transfusion-dependent lower-risk
MDS and del(5q). J Hematol Oncol. 2017 Jun 26;10(1):131. https://doi.org/10.1186/s13045-017-0491-2

- Toma
A, Kosmider O, Chevret S, Delaunay J, Stamatoullas A, Rose C,
Beyne-RauzyO, Banos A, Guerci-Bresler A, Wickenhauser S, Caillot D,
Laribi K, De Renzis B,Bordessoule D, Gardin C, Slama B, Sanhes L,
Gruson B, Cony-Makhoul P, Chouffi B, Salanoubat C, Benramdane R, Legros
L, Wattel E, Tertian G, Bouabdallah K, Guilhot F, Taksin AL, Cheze S,
Maloum K, Nimuboma S, Soussain C, Isnard F, Gyan E, Petit R, Lejeune J,
Sardnal V, Renneville A, Preudhomme C, Fontenay M, Fenaux P,Dreyfus F.
Lenalidomide with or without erythropoietin in
transfusion-dependenterythropoiesis-stimulating agent-refractory
lower-risk MDS without 5q deletion. Leukemia. 2016 Apr;30(4):897-905. https://doi.org/10.1038/leu.2015.296

- Santini
V, Almeida A, Giagounidis A, Gröpper S, Jonasova A, Vey N, Mufti
GJ,Buckstein R, Mittelman M, Platzbecker U, Shpilberg O, Ram R, Del
Ca-izo C,Gattermann N, Ozawa K, Risue-o A, MacBeth KJ, Zhong J, Séguy
F, Hoenekopp A, Beach CL, Fenaux P. Randomized Phase III Study of
Lenalidomide Versus Placebo in RBC Transfusion-Dependent Patients With
Lower-Risk Non-del(5q) Myelodysplastic Syndromes and Ineligible for or
Refractory to Erythropoiesis-Stimulating Agents. J Clin Oncol. 2016 Sep
1;34(25):2988-96. https://doi.org/10.1200/JCO.2015.66.0118

- Park
S, Hamel JF, Toma A, Kelaidi C, Thépot S, Campelo MD, Santini V,
Sekeres MA, Balleari E, Kaivers J, Sapena R, Götze K, Müller-Thomas C,
Beyne-Rauzy O,Stamatoullas A, Kotsianidis I, Komrokji R, Steensma DP,
Fensterl J, Roboz GJ,Bernal T, Ramos F, Calabuig M, Guerci-Bresler A,
Bordessoule D, Cony-Makhoul P,Cheze S, Wattel E, Rose C, Vey N, Gioia
D, Ferrero D, Gaidano G, Cametti G, Pane F, Sanna A, Germing U, Sanz
GF, Dreyfus F, Fenaux P. Outcome of Lower-RiskPatients With
Myelodysplastic Syndromes Without 5q Deletion After Failure of
Erythropoiesis-Stimulating Agents. J Clin Oncol. 2017 May
10;35(14):1591-1597. https://doi.org/10.1200/JCO.2016.71.3271

- Almeida
A, Fenaux P, Garcia-Manero G, Goldberg SL, Gröpper S, Jonasova A, Vey
N, Castaneda C, Zhong J, Beach CL, Santini V. Safety profile of
lenalidomide in patients with lower-risk myelodysplastic syndromes
without del(5q): results of a phase 3 trial. Leuk Lymphoma. 2018 Jan
11:1-9. https://doi.org/10.1080/10428194.2017.1421758

- Tobiasson
M, Dybedahl I, Holm MS, Karimi M, Brandefors L, Garelius H, Grövdal M,
Högh-Dufva I, Grønbæk K, Jansson M, Marcher C, Nilsson L, Kittang AO,
Porwit A, Saft L, Möllgård L, Hellström-Lindberg E. Limited clinical
efficacy of azacitidine in transfusion-dependent, growth
factor-resistant, low- and Int-1-risk MDS: Results from the nordic
NMDSG08A phase II trial. Blood Cancer J. 2014 Mar 7;4:e189. https://doi.org/10.1038/bcj.2014.8

- Sanchez-Garcia
J, Falantes J, Medina Perez A, Hernandez-Mohedo F, Hermosin L, Torres
Sabariego A, Bailen A, Hernandez-Sanchez JM, Solé Rodriguez M, Casa-o
FJ, Calderon C, Labrador M, Vahí M, Serrano J, Lumbreras E,
Hernández-Rivas JM; Grupo Andaluz SMD. Prospective randomized trial of
5 days azacitidine versus supportive care in patients with lower-risk
myelodysplastic syndromes without 5q deletion and transfusion-dependent
anemia. Leuk Lymphoma. 2017 Aug 24:1-10. https://doi.org/10.1080/10428194.2017.1366998

- Jabbour
E, Short NJ, Montalban-Bravo G, Huang X, Bueso-Ramos C, Qiao W, Yang H,
Zhao C, Kadia T, Borthakur G, Pemmaraju N, Sasaki K, Estrov Z, Cortes
J, Ravandi F, Alvarado Y, Komrokji R, Sekeres MA, Steensma DP, DeZern
A, Roboz G, Kantarjian H, Garcia-Manero G. Randomized phase 2 study of
low-dose decitabine vs low-dose azacitidine in lower-risk MDS and
MDS/MPN. Blood. 2017 Sep 28;130(13):1514-1522. https://doi.org/10.1182/blood-2017-06-788497

- Park
S, Hamel JF, Toma A, Kelaidi C, Thépot S, Campelo MD, Santini V,
Sekeres MA, Balleari E, Kaivers J, Sapena R, Götze K, Müller-Thomas C,
Beyne-Rauzy O, Stamatoullas A, Kotsianidis I, Komrokji R, Steensma DP,
Fensterl J, Roboz GJ, Bernal T, Ramos F, Calabuig M, Guerci-Bresler A,
Bordessoule D, Cony-Makhoul P, Cheze S, Wattel E, Rose C, Vey N, Gioia
D, Ferrero D, Gaidano G, Cametti G, Pane F, Sanna A, Germing U, Sanz
GF, Dreyfus F, Fenaux P. Outcome of Lower-Risk Patients With
Myelodysplastic Syndromes Without 5q Deletion After Failure of
Erythropoiesis-Stimulating Agents. J Clin Oncol. 2017 May
10;35(14):1591-1597 https://doi.org/10.1200/JCO.2016.71.3271

- Silverman
LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL,
Larson RA; Cancer and Leukemia Group B. Further analysis of trials with
azacitidine in patients with myelodysplastic syndrome: studies 8421,
8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006
Aug 20;24(24):3895-903. https://doi.org/10.1200/JCO.2005.05.4346 PMid:16921040

- Fenaux
P, Mufti GJ, Hellstrom-Lindberg E, et al: Efficacy of azacitidine
compared with that of conventional care regimens in the treatment of
higher-risk myelodysplastic syndromes: A randomised, open-label, phase
III study. Lancet Oncol 10: 223-232, 2009 https://doi.org/10.1016/S1470-2045(09)70003-8

- Garcia-Manero
G, Fenaux P. Hypomethylating agents and other novel strategies in
myelodysplastic syndromes. J Clin Oncol. 2011 Feb 10;29(5):516-23.
https://doi.org/10.1200/JCO.2010.31.0854

- Odenike
O. Incorporating novel approaches in the management of MDS beyond
conventional hypomethylating agents. Hematology Am Soc Hematol Educ
Program. 2017 Dec 8;2017(1):460-469. doi:
10.1182/asheducation-2017.1.460. Review.

- Voso
MT, Leone G, Piciocchi A, Fianchi L, Santarone S, Candoni A, Criscuolo
M,Masciulli A, Cerqui E, Molteni A, Finelli C, Parma M, Poloni A,
Carella AM, SpinaF, Cortelezzi A, Salvi F, Alessandrino EP, Rambaldi A,
Sica S. Feasibility of allogeneic stem-cell transplantation after
azacitidine bridge in higher-riskmyelodysplastic syndromes and low
blast count acute myeloid leukemia: results of the BMT-AZA prospective
study. Ann Oncol. 2017 Jul 1;28(7):1547-1553. https://doi.org/10.1093/annonc/mdx154

- Ofran
Y, Filanovsky K, Gafter-Gvili A, Vidal L, Aviv A, Gatt ME, Silbershatz
I, Herishanu Y, Arad A, Tadmor T, Dally N, Nemets A, Rouvio O, Ronson
A, Herzog-Tzarfati K, Akria L, Braester A, Hellmann I, Yeganeh S,
Nagler A, Leiba R, Mittelman M, Merkel D. Higher infection rate after
7- compared with 5-day cycle of azacitidine in patients with
higher-risk myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2015
Jun;15(6): e95-9. https://doi.org/10.1016/j.clml.2015.02.030

- Schuck
A, Goette MC, Neukirchen J, Kuendgen A, Gattermann N, Schroeder T,
Kobbe G, Germing U, Haas R. A retrospective study evaluating the impact
of infectious complications during azacitidine treatment. Ann Hematol.
2017Jul;96(7):1097-1104. https://doi.org/10.1007/s00277-017-3001-2

- Falantes
JF, Calderón C, Márquez-Malaver FJ, Aguilar-Guisado M, Martín-Pe-a A,
Martino ML, Montero I, González J, Parody R, Pérez-Simón JA, Espigado
I. Patterns of infection in patients with myelodysplastic syndromes and
acute myeloid leukemia receiving azacitidine as salvage therapy.
Implications for primary antifungal prophylaxis. Clin Lymphoma Myeloma
Leuk. 2014 Feb;14(1):80-6. https://doi.org/10.1016/j.clml.2013.09.014

- Stamatoullas
A, Rezine I, Mareschal S, Ménard AL, Lanic H, David M, Daliphard S,
Penther D, Lemasle E, Cassuto O, Lenain P, Contentin N, Lepretre S,
Jardin F, Bastard C, Tilly H. Hypoalbuminemia and
hypergammaglobulinemia are associated with an increased infection risk
in patients with myeloid malignancies treated with azacitidine. A
3-year monocentric retrospective study. Leuk Lymphoma.
2016;57(6):1491-3. https://doi.org/10.3109/10428194.2015.1101096

- Trubiano
JA, Dickinson M, Thursky KA, Spelman T, Seymour JF, Slavin MA, Worth
LJ. Incidence, etiology and timing of infections following azacitidine
therapy for myelodysplastic syndromes. Leuk Lymphoma. 2017
Oct;58(10):2379-2386. https://doi.org/10.1080/10428194.2017.1295141

- Lorenzana
N, Avila LF, Alonso S, Colado E, Bernal T. The impact of antimicrobial
prophylaxis in morbidity and infections during azacitidine treatment.
Ann Hematol. 2017 Nov;96(11):1833-1840. https://doi.org/10.1007/s00277-017-3091-x

- Pomares
H, Arnan M, Sánchez-Ortega I, Sureda A, Duarte RF. Invasive fungal
infections in AML/MDS patients treated with azacitidine: a risk worth
considering antifungal prophylaxis? Mycoses. 2016 Aug;59(8):516-9. https://doi.org/10.1111/myc.12500

- Radsak
M, Platzbecker U, Schmidt CS, Hofmann WK, Nolte F.
Infectiouscomplications in patients with myelodysplastic syndromes: A
review of the literature with emphasis on patients treated with
5-azacitidine. Eur J Haematol. 2017;99(2):112-118. https://doi.org/10.1111/ejh.12883

- Fenaux
P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Sanz G,
List AF, Gore SD, Seymour JF, Backstrom J, Zimmerman L, McKenzie D,
Beach CL, Silverman LB. Azacitidine prolongs overall survival and
reduces infections and hospitalizations in patients with WHO-defined
acute myeloid leukaemia compared with conventional care regimens: an
update. Ecancermedicalscience. 2008; 2:121. https://doi.org/10.3332/ecancer.2008.121

- Lübbert
M, Suciu S, Baila L, Rüter BH, Platzbecker U, Giagounidis A, Selleslag
D, Labar B, Germing U, Salih HR, Beeldens F, Muus P, Pflüger KH, Coens
C, Hagemeijer A, Eckart Schaefer H, Ganser A, Aul C, de Witte T,
Wijermans PW. Low-dose decitabine versus best supportive care in
elderly patients with intermediate- or high-risk myelodysplastic
syndrome (MDS) ineligible for intensive chemotherapy: final results of
the randomized phase III study of the European Organisation for
Research and Treatment of Cancer Leukemia Group and the German MDS
Study Group. J Clin Oncol. 2011 May 20;29(15):1987-96. https://doi.org/10.1200/JCO.2010.30.9245

- Ali
AM, Weisel D, Gao F, Uy GL, Cashen AF, Jacoby MA, Wartman LD, Ghobadi
A, Pusic I, Romee R, Fehniger TA, Stockerl-Goldstein KE, Vij R, Oh ST,
Abboud CN,Schroeder MA, Westervelt P, DiPersio JF, Welch JS. Patterns
of infectious complications in acute myeloid leukemia and
myelodysplastic syndromes patients treated with 10-day decitabine
regimen. Cancer Med. 2017 Dec;6(12):2814-2821. https://doi.org/10.1002/cam4.1231

- Lee
JH, Lee KH, Lee JH, Kim DY, Kim SH, Lim SN, Kim SD, Choi Y, Lee SM,
LeeWS, Choi MY, Joo YD. Decreased incidence of febrile episodes with
antibiotic prophylaxis in the treatment of decitabine for
myelodysplastic syndrome. Leuk Res. 2011 Apr;35(4):499-503. https://doi.org/10.1016/j.leukres.2010.07.006

- Oosterveld
M, Muus P, Suciu S, Koller C, Verhoef G, Labar B, Wijermans P, Aul C,
Fière D, Selleslag D, Willemze R, Gratwohl A, Ferrant A, Mandelli F,
Cortes J, de Witte T, Estey E; EORTC, EBMT, SAKK, GIMEMA Leukemia
Groups and the MD Anderson Cancer Center. Chemotherapy only compared to
chemotherapy followed by transplantation in high risk myelodysplastic
syndrome and secondary acute myeloid leukemia; two parallel studies
adjusted for various prognostic factors. Leukemia. 2002
Sep;16(9):1615-21. https://doi.org/10.1038/sj.leu.2402591 PMid:12200672

- Freifeld
AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the
use of antimicrobial agents in neutropenic patients with cancer: 2010
update by the infectious diseases society of America. Clin Infect Dis
2011;52:e56–93. https://doi.org/10.1093/cid/cir073 PMid:21258094

- Barreto
JN, Beach CL, Wolf RC, Merten JA, Tosh PK, Wilson JW, Hogan WJ, Litzow
MR. The incidence of invasive fungal infections in neutropenic patients
with acute leukemia and myelodysplastic syndromes receiving primary
antifungal prophylaxis with voriconazole. Am J Hematol. 2013
Apr;88(4):283-8. https://doi.org/10.1002/ajh.23388

- Walter
RB, Lee SJ, Gardner KM, Chai X, Shannon-Dorcy K, Appelbaum FR, EsteyEH.
Outpatient management following intensive induction chemotherapy for
myelodysplastic syndromes and acute myeloid leukemia: a pilot study.
Haematologica. 2011 Jun;96(6):914-7. https://doi.org/10.3324/haematol.2011.040220

- Vaughn
JE, Othus M, Powell MA, Gardner KM, Rizzuto DL, Hendrie PC, Becker PS,
Pottinger PS, Estey EH, Walter RB. Resource Utilization and Safety of
Outpatient Management Following Intensive Induction or Salvage
Chemotherapy for Acute Myeloid Leukemia or Myelodysplastic Syndrome: A
Nonrandomized Clinical Comparative Analysis. JAMA Oncol. 2015
Nov;1(8):1120-7. https://doi.org/10.1001/jamaoncol.2015.2969

- Mele
L, Ricci P, Nosari A, Tonso A, Fianchi L, Cudillo L, Pagano
L.Filamentous fungi infection in patients with myelodysplastic
syndrome. A report of twelve cases. Leuk Lymphoma. 2002
Jul;43(7):1421-5. https://doi.org/10.1080/1042819022386743 PMid:12389623

- Vardiman J. The classification of MDS: from FAB to WHO and beyond. Leuk Res. 2012 Dec;36(12):1453-8. https://doi.org/10.1016/j.leukres.2012.08.008

- de
Witte T, Suciu S, Verhoef G, Labar B, Archimbaud E, Aul C, Selleslag
D,Ferrant A, Wijermans P, Mandelli F, Amadori S, Jehn U, Muus P,
Boogaerts M, Zittoun R, Gratwohl A, Zwierzina H, Hagemeijer A, Willemze
R. Intensive chemotherapy followed by allogeneic or autologous stem
cell transplantation for patients with myelodysplastic syndromes (MDSs)
and acute myeloid leukemia following MDS. Blood. 2001 Oct
15;98(8):2326-31. https://doi.org/10.1182/blood.V98.8.2326 PMid:11588026

- Nakai
K, Kanda Y, Fukuhara S, Sakamaki H, Okamoto S, Kodera Y, Tanosaki R,
Takahashi S, Matsushima T, Atsuta Y, Hamajima N, Kasai M, Kato S. Value
of chemotherapy before allogeneic hematopoietic stem cell
transplantation from an HLA-identical sibling donor for myelodysplastic
syndrome. Leukemia. 2005 Mar;19(3):396-401. https://doi.org/10.1038/sj.leu.2403640 PMid:15674354

- Alessandrino
EP, Della Porta MG, Pascutto C, Bacigalupo A, Rambaldi A. Should
cytoreductive treatment be performed before transplantation in patients
withhigh-risk myelodysplastic syndrome? J Clin Oncol. 2013 Jul
20;31(21):2761-2. https://doi.org/10.1200/JCO.2012.48.0525

- de
Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I,
Mufti GJ, Fenaux P, Sanz G, Martino R, Alessandrino EP, Onida F,
Symeonidis A, Passweg J, Kobbe G, Ganser A, Platzbecker U, Finke J, van
Gelder M, van de Loosdrecht AA, Ljungman P, Stauder R, Volin L, Deeg
HJ, Cutler C, Saber W, Champlin R, Giralt S, Anasetti C, Kröger N.
Allogeneic hematopoietic stem cell transplantation for MDS and CMML:
recommendations from an international expert panel. Blood. 2017 Mar
30;129(13):1753-1762. https://doi.org/10.1182/blood-2016-06-724500

- Scott
BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, Maziarz
RT,Warlick ED, Fernandez HF, Alyea EP, Hamadani M, Bashey A, Giralt S,
Geller NL,Leifer E, Le-Rademacher J, Mendizabal AM, Horowitz MM, Deeg
HJ, Horwitz ME. Myeloablative Versus Reduced-Intensity Hematopoietic
Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic
Syndromes. J Clin Oncol. 2017;35(11):1154-1161. https://doi.org/10.1200/JCO.2016.70.7091

- Kröger
N, Iacobelli S, Franke GN, Platzbecker U, Uddin R, Hübel K, Scheid C,
Weber T, Robin M, Stelljes M, Afanasyev B, Heim D, Deliliers GL, Onida
F, Dreger P, Pini M, Guidi S, Volin L, Günther A, Bethge W, Poiré X,
Kobbe G, van Os M, Brand R, de Witte T. Dose-Reduced Versus Standard
Conditioning Followed by Allogeneic Stem-Cell Transplantation for
Patients With Myelodysplastic Syndrome: A Prospective Randomized Phase
III Study of the EBMT (RICMAC Trial). J Clin Oncol. 2017 Jul
1;35(19):2157-2164. https://doi.org/10.1200/JCO.2016.70.7349

- Brissot
E, Cahu X, Guillaume T, Delaunay J, Ayari S, Peterlin P, Le Bourgeois
A, Harousseau JL, Milpied N, Bene MC, Moreau P, Mohty M, Chevallier P.
Initial fluconazole prophylaxis may not be required in adults with
acute leukemia or myelodysplastic/myeloproliferative disorders after
reduced intensity conditioning peripheral blood stem cell allogeneic
transplantation. Ann Hematol. 2015 Apr;94(4):663-9
 .
.
- Mellinghoff
SC, Panse J, Alakel N, Behre G, Buchheidt D, Christopeit M,Hasenkamp J,
Kiehl M, Koldehoff M, Krause SW, Lehners N, von Lilienfeld-Toal
M,Löhnert AY, Maschmeyer G, Teschner D, Ullmann AJ, Penack O, Ruhnke M,
Mayer K,Ostermann H, Wolf HH, Cornely OA. Primary prophylaxis of
invasive fungal infections in patients with haematological
malignancies: 2017 update of the recommendations of the Infectious
Diseases Working Party (AGIHO) of the German Society for Haematology
and Medical Oncology (DGHO). Ann Hematol. 2018; 97(2):197-207. https://doi.org/10.1007/s00277-017-3196-2

- Scott
BL, Park JY, Deeg HJ, Marr KA, Boeckh M, Chauncey TR, Appelbaum FR,
Storb R, Storer BE. Pretransplant neutropenia is associated with
poor-risk cytogenetic features and increased infection-related
mortality in patients with myelodysplastic syndromes. Biol Blood Marrow
Transplant. 2008 ;14(7):799-806. https://doi.org/10.1016/j.bbmt.2008.04.011

- Kontoyiannis
DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad II et al. Increased
bone marrow iron stores is an independent risk factor for invasive
aspergillosis in patients with high-risk hematologic malignancies and
recipients of allogeneic hematopoietic stem cell transplantation.
Cancer 2007; 110: 1303–1306. https://doi.org/10.1002/cncr.22909 PMid:17614303

- Kanda
J, Mizumoto C, Ichinohe T, Kawabata H, Saito T, Yamashita K et al.
Pretransplant serum ferritin and C-reactive protein as predictive
factors for early bacterial infection after allogeneic hematopoietic
cell transplantation. Bone Marrow Transplant 2011; 46: 208–216 https://doi.org/10.1038/bmt.2010.108 PMid:20436524

- Tachibana
T, Tanaka M, Takasaki H, Numata A, Ito S, Watanabe R et al.
Pretransplant serum ferritin is associated with bloodstream infections
within 100 days of allogeneic stem cell transplantation for myeloid
malignancies. Int J Hematol 2011; 93: 368–374. https://doi.org/10.1007/s12185-011-0784-0 PMid:21331523

- Ohmoto
A, Fuji S, Miyagi-Maeshima A, Kim SW, Tajima K, Tanaka T, Okinaka K,
Kurosawa S, Inamoto Y, Taniguchi H, Fukuda T. Association between
pretransplant iron overload determined by bone marrow pathological
analysis and bacterial infection. Bone Marrow Transplant. 2017;
52(8):1201-1203. https://doi.org/10.1038/bmt.2017.93

- Hilken
A, Langebrake C, Wolschke C, Kersten JF, Rohde H, Nielsen P, Kröger N.
Impact of non-transferrin-bound iron (NTBI) in comparison to serum
ferritin on outcome after allogeneic stem cell transplantation (ASCT).
Ann Hematol. 2017 Aug;96(8):1379-1388. https://doi.org/10.1007/s00277-017-3034-6

- Jacobi
N, Herich L. Measurement of liver iron concentration by superconducting
quantum interference device biomagnetic liver susceptometry validates
serum ferritin as prognostic parameter for allogeneic stem cell
transplantation. Eur J Haematol. 2016 Oct;97(4):336-41. https://doi.org/10.1111/ejh.12734

- Gerds
AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL.
Pretransplantation therapy with azacitidine vs induction chemotherapy
and posttransplantation outcome in patients with MDS. Biol Blood Marrow
Transplant. 2012 Aug;18(8):1211-8. https://doi.org/10.1016/j.bbmt.2012.01.009

- Voso
MT, Leone G, Piciocchi A, Fianchi L, Santarone S, Candoni A, Criscuolo
M, Masciulli A, Cerqui E, Molteni A, Finelli C, Parma M, Poloni A,
Carella AM, Spina F, Cortelezzi A, Salvi F, Alessandrino EP, Rambaldi
A, Sica S. Feasibility of allogeneic stem-cell transplantation after
azacitidine bridge in higher-risk myelodysplastic syndromes and low
blast count acute myeloid leukemia: results of the BMT-AZA prospective
study. Ann Oncol. 2017 Jul 1;28(7):1547-1553. https://doi.org/10.1093/annonc/mdx154

- Nishihori
T, Perkins J, Mishra A, Komrokji R, Kim J, Kharfan-Dabaja MA, Perez L,
Lancet J, Fernandez H, List A, Anasetti C, Field T. Pretransplantation
5-azacitidine in high-risk myelodysplastic syndrome. Biol Blood Marrow
Transplant. 2014 Jun;20(6):776-80. https://doi.org/10.1016/j.bbmt.2014.02.008

- Lukenbill
J, Rybicki L, Sekeres MA, Zaman MO, Copelan A, Haddad H, Fraser
T,DiGiorgio MJ, Hanna R, Duong H, Hill B, Kalaycio M, Sobecks R,
Bolwell B, Copelan E. Defining incidence, risk factors, and impact on
survival of central line-associated blood stream infections following
hematopoietic cell transplantation in acute myeloid leukemia and
myelodysplastic syndrome. Biol Blood Marrow Transplant. 2013
May;19(5):720-4. https://doi.org/10.1016/j.bbmt.2013.01.022

[TOP]

























































































 .
. 










