Davide Facchinelli1, Gessica Marchesini1, Gianpaolo Nadali1 and Livio Pagano2.
1 Hematology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
2 Institute of Haematology, Fondazione Policlinico A. Gemelli- IRCCS- Università Cattolica S. Cuore, Rome, Italy.
Correspondence to: Livio Pagano. Institute of Haematology, Fondazione
Policlinico A. Gemelli- IRCCS- Università Cattolica S. Cuore, Rome,
Italy. E-mail:
livio.pagano@unicatt.it
Published: November 1, 2018
Received: September 9, 2018
Accepted: September 15, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018063 DOI
10.4084/MJHID.2018.063
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
This
review summarizes the more recent evidence about epidemiology and risk
factors for invasive fungal infections (IFI) in patients affected by
Chronic Lymphocytic Leukemia (CLL), indolent Non Hodgkin Lymphoma
(iNHL) and Multiple Myeloma (MM).
Despite advances in the
prognosis and treatment of hematological malignancies in recent years,
susceptibility to infection remains a significant challenge to patient
care. A large amount of data regarding patients with acute leukemia has
been published while little information is available on the incidence
of IFI in chronic lymphoproliferative disorders (CLD).
New drugs
are now available for treatment of lymphoproliferative disorders which
may cause suppression of humoral immunity, cellular immunity, and
deficiency of white blood cells, increasing the risk for infections
which remain the leading cause of mortality in these patients.
|
Introduction
Infections
are the leading cause of death in patients with CLL and MM, and
effective strategies for managing infections remain a crucial aspect of
disease management.
Secondary immunodeficiency develops in
patients with hematological malignancies as a result of disruption to
the immune system from an early stage and even patients who do not
require treatment for their malignancy have been shown to be at greater
risk of serious infections than the general population.[1,2]
Data published about mycoses in CLD are scanty. Fungal infections in CLL patients range between 0.5 to 18%[3-14]
according to treatment received and to selected populations. The
majority of studies are retrospective, inclusion criteria heterogeneous
and they often included also possible IFI.[9,15] Noteworthy, the incidence of IFI in CLL appears to increase over the years.
Prolonged
neutropenia, age, prior IFI, lymphocytopenia or lymphocyte dysfunction,
the stage and state of the underlying malignancy (relapsed or
progressive disease), corticosteroid use and presence of
Graft-Versus-Host Disease (as expected in transplanted patients), were
factors more often associated to a higher risk of IFI in these patients.
Interestingly,
CLLs with an unfavorable prognostic profile were more often affected by
IFI. In particular CD38 expression, genetic analysis (p53, ATM or 12+)
and IgvH mutation status represented biological risk factors for IFI.[4,14]
Visentin et al. in 2017 demonstrated that at time of IFI, patients had
lower levels of IG as compared with those subjects who experienced
bacterial infections or did not have any infections, even if the number
of patients analyzed is small.
In the last years, new drugs for
the treating CLL have been introduced in clinical practice (e.g.,
ibrutinib, idelalisib, venetoclax). Recent studies, all retrospective,
suggest that patients with lymphoid malignancies receiving ibrutinib
are at risk for a variety of serious infections, including IFI, even if
they are often pretreated patients.[11,13,16-17]
These data are not yet sufficient to give strong recommendations for
prophylaxis, but as the indications for ibrutinib use continue to
expand, this highlights the need for further studies to define those
most likely to benefit from close clinical monitoring for infectious
complications and from targeted prophylaxis strategies.
Pathogenesis of Opportunistic Infections in CLL
Patients
with CLL are at increased risk for infections because of their
compromised immune function. The pathogenesis of infections in CLL is
multifactorial, and the major risk factors in these patients are immune
defects related to the primary disease and the consequences of therapy.[1]
Disease-related
defects include hypogammaglobulinemia, which occurs in virtually all
patients with CLL and correlates with disease duration and stage.[18,19] Even with therapeutic response, there is little improvement in the underlying defect.
Another
disease related defect is connected to the innate immunity.
Quantitative and qualitative neutrophil and monocyte defects are found
in CLL patients. Although the absolute number of neutrophils is normal
or slightly decreased in untreated patients, defects in phagocytic and
bactericidal activity, have been demonstrated.[18]
Decreased
levels of components of complement (e.g., properdin) are documented in
patients with CLL. Defects in complement activation and binding and
reduced expression of complement receptors on CLL B cells have been
also reported.[18]
Major infections are reported to occur at least once in >50% of CLL patients contributing to 30% - 50% of deaths.[20-22]
Most
data about infections in CLL patients are reported from clinical trials
or retrospective analyses at referral centers, not necessarily
representative of the overall CLL population.[21]
There
are limited data on infections in treatment-naive CLL patients who are
at increased risk mainly for bacterial infections caused by common
pathogens such as Staphylococcus aureus, Streptococcus pneumoniae,
Haemophilus influenzae, Escherichia coli, Klebsiella pneumoniae, and
Pseudomonas aeruginosa.
Epidemiology of Fungal Infections in CLL
Limited information is available on the epidemiology of IFI in CLL.[3,5,9,10,12]
The
overall incidence of IFI is reported from 1,3% to 7,8%. Nevertheless,
the estimated mortality is extremely high (up to 80%).[5]
Invasive
aspergillosis is the most frequent IFI observed. Risk factors are
mainly related to disease status (highest risk in relapsed/refractory
disease), a number of previous chemotherapy regimens and Ig levels.[12]
Whereas bacterial infections predominate during neutropenia, invasive
fungal infections start to develop as neutropenia persists.
Invasive
mold infections, due primarily to aspergillus species, are the most
frequent cause of serious, often life-threatening infections in
patients with neutropenia that persists for more than two weeks.[23]
Other risk factors include impaired cellular immunity, prolonged
corticosteroid administration, allogeneic stem cell transplantation and
advanced age.[6]
Treatment Related Infections
Therapy
related immunosuppression has a further impact on immune function in
CLL patients, and the infectious complications have evolved in relation
to the specific agents.
Alkylators.
Chlorambucil has been used for many years as standard therapy for CLL
patients. As for treatment-naive patients, the majority of infections
are bacterial of mucosal origin and when they occur this is related to
neutropenia. Fungal and viral infections are infrequent.
Cyclophosphamide is rarely used as a single agent and is commonly part of combination therapy (see below).
Bendamustine.
It is an alkylating agent able to induce a high number of remissions in
CLL and more effective than chlorambucil when compared in clinical
trials.[24]
Infections during bendamustine
treatment may be related to neutropenia and are prevalently bacterial.
In untreated patients, when bendamustine was combined with rituximab,
infections were the leading cause of non-hematological toxicity (12
grade 3; three grade 4, mainly febrile neutropenias and pneumonia not
otherwise specified) with a total incidence of 7.7%.[25] In the setting of refractory/relapsed patients, no opportunistic infections were reported.[26]
Fludarabine.
Purine analogs determine quantitative and qualitative T cell
abnormalities giving rise to a wider spectrum of infections compared to
reported with alkylating agents.
Fungal infections as Nocardia, Candida, Aspergillus, Cryptococcus, Pneumocystis have been reported.[21,27]
The addition of glucocoticoids increases the risk of the opportunistic infections and should be avoided.
Pneumocystis
prophylaxis in patients receiving single fludarabine agent is suggested
by some experts and guidelines, usually until CD4 count is >200
cells/microL (NCCN Guidelines Insights: Non-Hodgkin's Lymphomas,
Version 3.2016.)
Other authors have suggested Pneumocystis
Jiroveci Pneumonia (PJP) prophylaxis only when fludarabine is used in
combination with other alkylating agents as cyclophosphamide or
bendamustine.[21]
Also for patients treated with
combinations of fludarabine and rituximab (FR), the PJP prophylaxis is
recommended alongside with antivirals.
The
fludarabine-cyclophosphamide-rituximab (FCR) combination has been used
as salvage therapy for relapsed or refractory CLL patients with
antiviral and anti PJP prophylaxis. In this setting major infections
occurred in 16% of cases[28] while in naive treatment patients where 3%.[29] A small number of opportunistic infections occurred (PJP, Aspergillus, Candida glabrata).
Anti-CD20 monoclonal antibodies.
Rituximab has been used as a single agent in CLL but is more commonly
used as part of combination therapy (FR). With this combination, the
rate of severe infections is reported up to 20%, mainly of viral
origin.[30]
Rituximab has been associated with hepatitis B reactivation and multifocal leukoencephalopathy (PML).
Ofatumumab and Obinutuzumab have an infection profile similar to Rituximab.[28,1]
Most
of the infections occurring with obinutuzumab in combination with
chlorambucil were of bacterial origin, and opportunistic infections
were uncommon.[31]
Alemtuzumab.
Although nowadays seldom used for CLL treatment, this anti CD-52
antibody is associated with profound defects in cell-mediated immunity.
Reductions in B, T and NK cells occur early in treatment and persist
for up to 9 months after discontinuation of therapy.
Alemtuzumab
has been associated with a wide range of infections, including
bacterial, viral, fungal and protozoal, although CMV
reactivation/disease is the most significant infectious complication
particularly in previously treated patients.[32]
When used in combination regimens, anti PJP and anti-viral prophylaxis is suggested by experts[33]
and guidelines (Prevention and Treatment of Cancer-Related Infections,
Version 2.2016, NCCN Clinical Practice Guidelines in Oncology).
Idelalisib.
Idelalisib is a reversible inhibitor of phosphatidylinositol 3-kinase
(PI3K) which is a cytoplasmic tyrosine kinase involved in various
signaling pathways, most importantly activating the AKT/mTOR pathway.
The delta isoform is ubiquitously expressed in leukocytes.
Inhibition of PI3Kdelta induces disruption of interactions between malignant B cells and the microenvironment.
In
a trial of idelalisib in combination with rituximab for relapsed CLL,
pneumonia occurred in 6% of patients and PJP in 3% (grade 1 or 2
toxicity).[34]
In 2016, an increase in serious
adverse events and fatalities was reported from different clinical
trials of idelalisib used in combination with other agents. In
particular, an increase in cases of PJP and CMV infections was
observed.
The overall incidence of PJP was 2.5% in patients on
idelalisib ± co-therapy vs 0.2% in patients receiving only anti-CD20
antibody alone or bendamustine and rituximab (relative risk = 12.5). A
correlation between CD4 count (<200 cells/mcL) and the increased
risk of PJP was not observed.[35]
The manufacturer of idelalisib suggested that PJP prophylaxis should be considered for patients receiving Idelalisib.
Currently,
patients treated with idelalisib (with or without rituximab) are
considered at high risk of PJP and prophylaxis is recommended at least
through active treatment.[36] TMP/SMX is the drug of choice because of its activity against other pathogens like nocardia, toxoplasma, and listeria.
Ibrutinib. PJP and other fungal infections have been reported in small case series and case reports on ibrutinib treated patients.[37,38] In particular, invasive aspergillosis (IA) and cryptococcosis have been described.[39,40]
Recent combination study in patients with primary central nervous system lymphoma reported IA in 39% (7/18) of all patients.[41] Earlier single agent study reported aspergillosis in 5-11% of patients[42,43] suggesting that the combination therapy may account for the observed differences.
In
a recently published retrospective survey, an alarming 40% of cerebral
localizations of IA has been reported in patients, mainly with
CLL, treated with ibrutinib.[17]
How ibrutinib
may decrease antifungal immunity remains to be clarified. The molecular
target of the drug, the Bruton tyrosine kinase (BTK), is present in
normal lymphocytes and can be involved in the exertion of deleterious
off-target effects also on the T cell-macrophage axis.
The rate of
fungal infections raises concern over the role of BTK and
Interleukin-2-inducible kinase (ITK) inhibition and their clinical
relevance in antifungal immunity.
BTK is an indispensable
component of the B-cell receptor signaling pathway through which it
mediates B-cell growth, adhesion and survival.[44]
BTK is also found in neutrophils, monocytes and macrophages where it
mediates pathways involving innate and adaptive immunity.[44]
Interleukin-2-inducible
kinase (ITK), found in T cells, has significant homology with BTK and
is irreversibly inhibited by ibrutinib.[45] ITK
functions downstream of the T-cell receptor playing a central role in
inflammatory responses and T-cell maturation. In the absence of ITK,
CD4+ T cells fail to differentiate into Th2 effector cells effectively
and are unable to mount a protective response to pathogens.[45]
ITK
inhibition by ibrutinib may contribute to the opportunistic infections
seen with the use of this drug: a definitive answer to this question
would require a carefully designed trial comparing ibrutinib to more
specific inhibitors of BTK such as acalabrutinib.
Another relevant
issue is related to the important pharmacokinetic interactions between
ibrutinib and other drugs metabolized by CYP3A4 (e.g., 2nd
generation triazoles like voriconazole and posaconazole). The ibrutinib
dose should be reduced to 140 mg (a quarter of maximal prescribed dose)
when coadministered with moderate CYP3A4 inhibitors so that exposures
remain within observed ranges at therapeutic doses.[46]
New triazoles (e.g., isavuconazole, ravuconazole) will be probably
increasingly used in this setting for their more favourable toxicity
profile and pharmacokinetic characteristics.
However, ibrutinib
also allows for partial reconstitution of humoral immunity (in
particular serum IgA levels), and the infection rate in patients with
CLL is reported to decrease with time.[8]
Lenalidomide.
Lenalidomide is an immunomodulatory agent used in monotherapy or in
combination with anti CD20 monoclonals or steroids. There is no clear
evidence of specific immune dysfunction capable of increasing the risk
of opportunistic infections in patients treated with lenalidomide.
Anti
PJP and antiviral prophylaxis is suggested only for patients receiving
lenalidomide in combination with fludarabine and rituximab.
Venetoclax.
Venetoclax is a B cell leukemia/lymphoma-2 (BCL-2) inhibitor used as a
single agent or in combination with anti CD20 monoclonals for
pretreated patients with CLL and unfavourable citogenetics (17p
deletion). Most trials were conducted in patients with relapsed and
refractory disease. In a recently published phase II trial, Grade 3 or
4 adverse events (AEs) were primarily hematologic. The infection rate
was 81% for AEs of any grade. Forty patients (25%) had grade ≥ 3
infection (four cases were fatal: RSV, Klebsiella
sepsis, septic shock, and pneumonia). Seven patients had opportunistic
infections, with three grade 3 or 4 events (2 PJP and 1 herpes zoster).[47]
Antiviral, antifungal and anti PJP prophylaxis is therefore suggested
on a case-by-case basis in settings of prior opportunistic infections
or immune defects related to previous treatment.
Epidemiology of Fungal Infections in Indolent NHL (iNHL)
All together 17% of IFI in haematological diseases occur in patients affected by NHL.[23]In iNHL the incidence of IFI is reported from 0.5% to 4%.[3,5,7-11,23,48-53] Most
of the reports concern the epidemiology of IFI in all subtypes of
lymphomas, and few data are available about the incidence of IFI in
patients with indolent lymphomas that mainly include Follicular
lymphomas (FL), mantle cell lymphomas (MCL) and Waldenstrom
macroglobulinemia (WM). Moreover, most of them are retrospective and
monocentric regarding a limited number of patients. In Table 1 incidence of mold infection in NHL and iNHL.
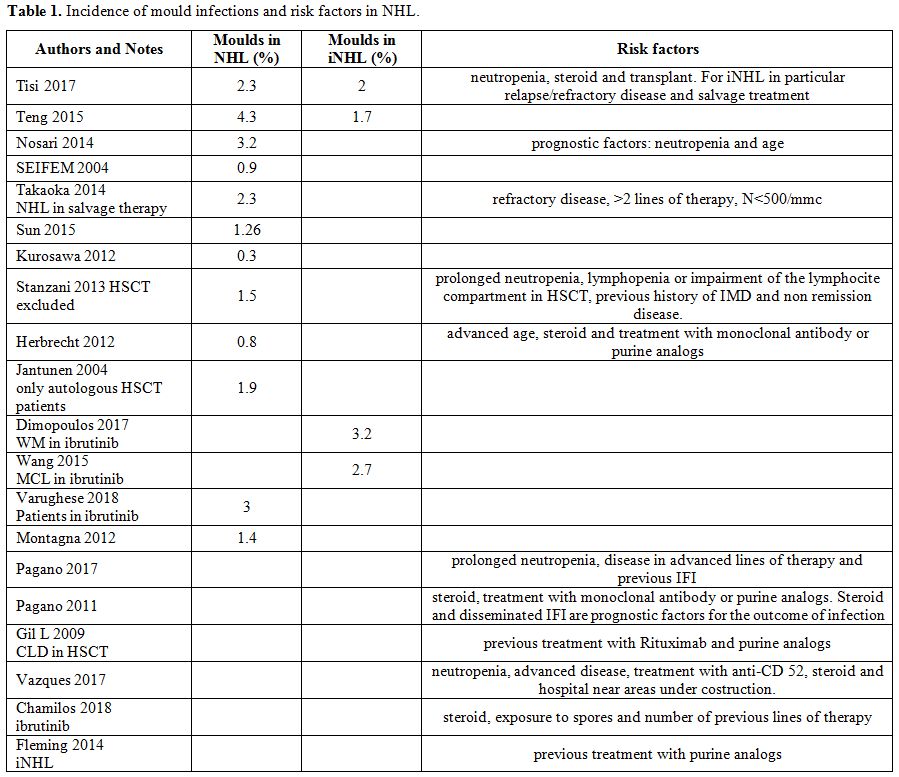 |
Table
1. Incidence of mould infections and risk factors in NHL. |
The rate of IFI in aggressive lymphomas (aNHL) is higher (2.3-4.3%) compared to the incidence in iNHL (1.7-2%).[9,10] This is likely related to the more aggressive treatment delivered to these patients.[9,10]According to recent recommendations,[4]
lymphomas are allocated in the low risk category, whereas
relapsed/refractory Diffuse Large B Cell Lymphomas (DLBCL) and patients
undergoing autologous/allogeneic stem cell transplantation (HSCT)
belong to the intermediate risk category. Although
transplanted patients are considered at increased risk of IFI, two
different studies have shown that the incidence of IFI is comparable in
NHL patients undergoing autologous transplantation (1.9%) or not
(1.7%).[7,50] The
lung was most frequently involved (88.5%) while other sites of
infection were paranasal sinuses, the central nervous system (CNS) and
abdomen.[54]Invasive aspergillosis is the most frequent IFI observed (90%), followed by Mucor and Fusarium infection.[5,51]
The attributable mortality rate for mold infections seems to be lower
in chronic lymphoproliferative disorders (CLD) (16%), compared to what
reported in acute myeloid leukemia (AML) (27%).[3,55] Compared
to AML patients, who recover their immunologic competence as soon as
disease remission is achieved, the immune system of patients with CLD
remains blunted for a long period, probably due to treatments with
purine analogs or monoclonal antibodies.[3,56]The
incidence of IFI in NHL is also related to age. An Italian study showed
that in pediatric patients with NHL no one had developed mold
infections.[52]There is a rising trend of IFIs in patients with CLD: from the reported 1.6% in 2004[5] to the 4.3% in 2014.[3]
This could be related to the introduction of new drugs potentially
leading to a greater immune deficiency causing a modification on
infectious epidemiology.[4]
Risk Factors for IFI in iNHL
Several
studies highlighted the presence of risk factors for IFIs in patients
with NHL. The most important are related to disease status (higher risk
in relapse/refractory and advance stage disease) and type of treatment
(higher risk for steroid administration, intensive chemotherapy with
prolonged neutropenia, monoclonal antibody and purine analogs).[10,57-59]These data were recently confirmed by a Delphi-like analysis in haematological patients published by Vazquez et al.[60] which showed that most experts agree that these are the most relevant risk factors.Stanzani
M et al. developed a score for IFI in patients with hematological
malignancies and identified four main risk factors: prolonged
neutropenia, lymphopenia or impairment of the lymphocyte compartment in
allogenic HSCT patient, previous history of IFI and non remission
disease. The score can discriminate patients who have a low or high
probability of developing IFI within 90 days of hospitalization. A
limitation of this study is related to the presence of some confounding
factors such as the different use of antifungal prophylaxis and the
underestimation of factors such as GVHD and steroid administration for
which it would be necessary to make a special study on patients
undergoing allogeneic transplantation.[7]A
similar score was proposed by Takaoka et al. for patients with
lymphomas in rescue therapy and identified as main risk factors the
refractory disease, more than two lines of therapy and neutropenia
inducing treatments (Table 2). On this basis, high risk patients have an IFI incidence of 9% and low risk patients an incidence of 0.19%.[48]
In Table 1, the main risk factors for mold infection in NHL patients.
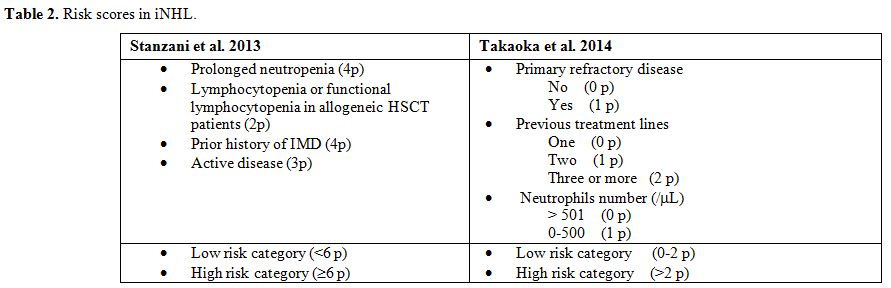 |
Table 2. Risk scores in iNHL. |
IFI Prevention in iNHL
Patients
with iNHL are considered to be at low risk for IFI, and therefore there
is no consensus for biomarkers monitoring (GM or BDG assays) or need
for prophylaxis.4 Nevertheless, their use at presentation is variable
and depends largely on treating physician’s opinion.[3]These
patients can move into the intermediate risk category for the following
factors: relapse-refractory disease, use of high dose chemotherapy,
steroid and therapy with T cell damaging agents, stem cell
transplantation.[61]Therefore prophylaxis is not indicated for iNHL who receive standard chemotherapy[57]
and there is no indication also for patients undergoing autologous
transplantation with the exception of conditioning regimens that can
cause mucositis. If needed, the recommended drug is fluconazole.[36]
New Treatments
Recently,
new drugs have been introduced for treatment of iNHL. They act blocking
the B cell receptor signal transduction system (BCR) at different
levels. So far, there have been several reports of IFI in patients
treated with these molecules so that the Infectious Diseases Working
Party of the German Society for Haematology and Medical Oncology
pointed out that BTK, bcl2 and PI3K inhibitors result in an increased
incidence of severe mold infection but it is still unclear if
prophylaxis is indicated or not.[62] This new scenario requires revision of the epidemiology and specific risk factors for IFIs also among patients with CLD.The
European Society of Clinical Microbiology and Infectious Diseases
(ESCMID) Study Group for Infections in Compromised Hosts (ESGICH)
investigated the potential infectious risks related to the new
molecules highlighting that the drugs related to the increased IFI
incidence are BTK inhibitors, followed by PI3K inhibitors while, at the
moment, the bcl2 inhibitors do not seem to be related to this type of
infections. Anyway, antifungal prophylaxis is not indicated in any
group.[63] Some
groups have attempted to identify risk factors for IFIs in this new
subset of patients. For ibrutinib, simultaneous treatment with
steroids, exposure to spores and previous lines of therapy have been
identified as increasing the risk for IFI.[38] A
recent report has shown how IA during ibrutinib treatment
occurred with a median of three months after starting the drug and
moreover, unlike what seen with standard treatments, about 40% of
infections are localized to the CNS.[17]The
most common risk factors for IFIs in NHL (neutropenia, lymphopenia, and
treatment with purine analogs) are no more valid for patients treated
with ibrutinib, and the incidence of IFI is similar in first or
advanced line of therapy (4%), showing that the drug itself can
increase the risk of IFI regardless of previous treatments.[11]
An increased rate of IFI is also reported in patients treated with
ibrutinib for other diseases (e.g., 2.7% in MCL, 3.2% in WM).[53,64]
Epidemiology of Fungal Infections in MM
The overall incidence of IFI in MM ranges from 0.5% to 12.3% and the incidence of mold infections from 0,3% to 3,3% (Table 3).
In the Italian multicentric retrospective study SEIFEM-2004, the
overall incidence of IFI in MM was 0.5% (7 cases in 1616 patients), and
molds accounted for more than a half of the infections (4 of the 7
cases). The causative agent was aspergillus in all cases.[5]
IFI occurred mostly during disease progression or following the second
or third autograft, underlining the relevance of the defining a high
risk category that could benefit from active mold prophylaxis.[65,66]
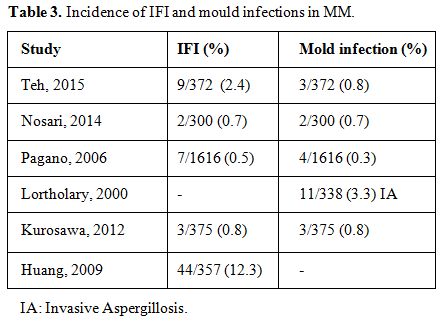 |
Table 3. Incidence of IFI and mould infections in MM. |
Two French studies[67,68]
indicate chronic lymphoproliferative disorders as a new high risk group
for invasive aspergillosis (IA) following acute leukemia and allogeneic
stem cell transplant (HSCT). Liu et al. showed that the incidence of
IFI was 3.8% per chemotherapy course. Most of the infections were
possible IFI according to EORTC criteria.[69] Sun et
al. in 2015 in a multicentric study, reported three cases of IFI in 395
patients with MM enrolled. The incidence of proven/probable cases was
0.68%.[15]
Risk Factors in MM
In
the majority of studies, MM is considered a low risk disease although
only a few of them have evaluated the impact of newly available drugs
as well as the extensive use of autologous stem cell transplant (ASCT).
Immunomodulatory drugs and proteasome inhibitors are less
myelosuppressive than the conventional chemotherapy which now tends to
be used only in refractory or progressive disease. This could explain
why most of the IFI occurred during disease progression or treatment of
refractory disease.Neutropenia is the risk factor reported in the majority of the studies and seems to be related to its duration and severity.[49]
Teh et al. reported, as main risk factors, neutropenia less than 0.5 x
10^9/L for ten days or more, corticosteroid therapy (>= 0.5
mg/kg/day of prednisolone equivalent over four weeks) and T cell
suppressive chemotherapy before the diagnosis of IFI.[65] In
multivariate analysis, only the number (3 or more) of lines of therapy
was independently related with an increased risk of developing IFI,
whereas the use of bortezomib retained significance only in univariate
analysis.[65] Together
with a high dosage of glucocorticoids and intensive chemotherapy, other
risk factors such as the use of bortezomib, thalidomide, lenalidomide
or transplant procedures (HSCT) were reported as significant risk
factors.[70]The
use of broad- spectrum antibiotics, diabetes, dialysis and the use of
fludarabine are also reported to increase the risk of IFI
significantly.[66] The
presence of central vein catheterization, the use of broad spectrum
antibiotics for > 7 days, hepatic dysfunction, decreased serum
albumin and antifungal prophylaxis did not emerge as significant risk
factors. Only a prior history of IFI was confirmed to predict the onset
of a new IFI significantly.[69]
In Table 4 main risk factors for IFI in MM patients.
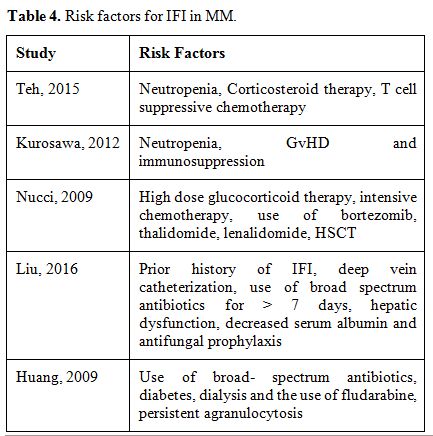 |
Table 4. Risk factors for IFI in MM. |
IFI Prevention in MM
Patients
with MM are generally considered at low risk for IFI, and so far there
is no consensus for antifungal prophylaxis. Nevertheless, several
studies tried to identify subgroups that are likely to benefit from it.
The most common prophylactic agents are triazoles in many centers.The
Hema e-chart study underlined that the type of antifungal prophylaxis
was correlated with the number of IFI diagnosed because the
galactomannan test (GM) was positive in a significantly lower
proportion of proven/probable mould infections when active mould
prophylaxis, like itraconazole or posaconazole, was used. This could be
due to the reduced sensitivity of the GM test during prophylaxis, that
could lead to underscoring IA as possible infections with subsequent
insufficient antifungal treatment.[56]In
the study by Liu et al. the incidence of IFI was higher in
patients who received antifungal prophylaxis probably because these
patients had more risk factors at baseline and were considered as
having a much higher risk to develop IFI. The mortality rate for
patients with probable or possible IFI was 11,7%.[69] In Table 5, data about the mortality rate in different studies are reported.
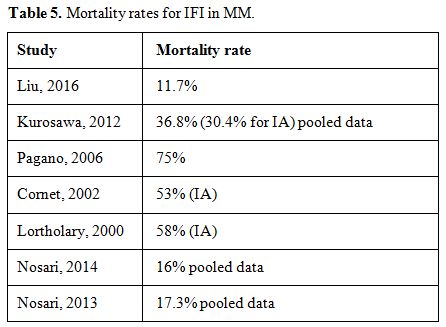 |
Table 5. Mortality rates for IFI in MM. |
The
parameters associated with an increased risk of death were older age, a
diagnosis based on positive culture together with two positive GM
detections in serum samples, the presence of pleural effusion or CNS
involvement, while an initial antifungal treatment including
voriconazole was associated with a decreased risk of death.[68]
Conclusions
CLDs
are rather common haematological malignancies, nevertheless, the real
incidence of fungal infections in these patients is largely unknown.
The majority of published data are case reports or monocentric studies,
and therefore the landscape is quite heterogeneous. In CLL the incidence of IFI is reported up to 7,8%.[5]
Risk factors are mainly related to disease status, with the highest
risk in relapsed/refractory disease, some previous chemotherapy
regimens and immunoglobulin levels.[12] Depending on
treatment administered, the risk is different: IFIs are mainly
associated with the use of monoclonal anti-CD20 antibodies, purine
analogs and BTK inhibitors.[30,38] In
NHL the overall incidence of IFI is reported up to 4% although in
aggressive lymphomas the rate is higher (2.3-4.3%) compared to what
observed in iNHL (1.7-2%).[10] Risk factors are
related either to disease status (higher risk in relapse/refractory and
advance stage disease) or to the modality of treatment (steroid use,
intensive chemotherapy with prolonged neutropenia, monoclonal antibody
and purine analogs).[58]Patients
with iNHL are considered to be at low risk for IFI, and therefore there
is no consensus for biomarkers monitoring (GM or BDG assays) or need
for prophylaxis.[4] An increased rate of IFI is also reported in patients treated with ibrutinib (2.7% in MCL, 3.2% in WM).[53,64] In MM the incidence of IFI is reported from 0.5% to 12.3% with a mortality rate for probable or possible IFI of 11,7%.[69]
IFI occurred mostly during disease progression or following the second
or third autograft, underlining the relevance of the definition of a
high risk category that could benefit from prophylaxis.[65,66]
The main risk factors are prolonged neutropenia, steroid therapy, and T
cell suppressive chemotherapy before the diagnosis of IFI.[65]
Immunomodulatory drugs and proteasome inhibitors are less
myelosuppressive compared to conventional chemotherapy which now is
mainly used in refractory or progressive disease, and this could
explain why most of the IFI occurred during treatment of advanced or
refractory cases. The
epidemiology of fungal infections in CLD is changing over time, and
this mutation is apparently related to the new treatments recently
introduced into clinical practice. The new “target drugs” not only act
on neoplastic cells but also on the healthy counterpart, interacting
with the normal functioning of the immune system. This could lead to an
increase in the risk of infections including IFI. Longer follow-up and
larger studies are warranted to define better the risk in CLD patients
treated with the new molecules, to identify those who could benefit
from adequate prophylaxis. References
- Forconi F. and P. Moss, Perturbation of the normal immune system in patients with CLL. Blood, 2015. 126(5): p. 573-81. https://doi.org/10.1182/blood-2015-03-567388 PMid:26084672
- Compagno, N. et al., Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol, 2014. 5: p. 626. https://doi.org/10.3389/fimmu.2014.00626 PMid:25538710 PMCid:PMC4259107
- Nosari
A.M. et al., Invasive fungal infections in lymphoproliferative
disorders: a monocentric retrospective experience. Leuk Lymphoma, 2014.
55(8): p. 1844-8. https://doi.org/10.3109/10428194.2013.853299 PMid:24138328
- Pagano
L. et al., Risk stratification for invasive fungal infections in
patients with hematological malignancies: SEIFEM recommendations. Blood
Rev, 2017. 31(2): p. 17-29. https://doi.org/10.1016/j.blre.2016.09.002 PMid:27682882
- Pagano
L. et al., The epidemiology of fungal infections in patients with
hematologic malignancies: the SEIFEM-2004 study. Haematologica, 2006.
91(8): p. 1068-75. PMid:16885047
- Safdar
A. and D. Armstrong, Infections in patients with hematologic neoplasms
and hematopoietic stem cell transplantation: neutropenia, humoral, and
splenic defects. Clin Infect Dis, 2011. 53(8): p. 798-806. https://doi.org/10.1093/cid/cir492 PMid:21890754
- Stanzani
M. et al., A risk prediction score for invasive mold disease in
patients with hematological malignancies. PLoS One, 2013. 8(9): p.
e75531. https://doi.org/10.1371/journal.pone.0075531 PMid:24086555 PMCid:PMC3784450
- Sun
C. et al., Partial reconstitution of humoral immunity and fewer
infections in patients with chronic lymphocytic leukemia treated with
ibrutinib. Blood, 2015. 126(19): p. 2213-9. https://doi.org/10.1182/blood-2015-04-639203 PMid:26337493 PMCid:PMC4635117
- Teng
J.C., et al., Epidemiology of invasive fungal disease in
lymphoproliferative disorders. Haematologica, 2015. 100(11): p. e462-6.
https://doi.org/10.3324/haematol.2015.126698 PMid:26206797 PMCid:PMC4825301
- Tisi
M.C., et al., Invasive fungal infections in chronic lymphoproliferative
disorders: a monocentric retrospective study. Haematologica, 2017.
102(3): p. e108-e111. https://doi.org/10.3324/haematol.2016.151837 PMid:27856512 PMCid:PMC5394967
- Varughese
T. et al., Serious Infections in Patients Receiving Ibrutinib for
Treatment of Lymphoid Malignancies. Clin Infect Dis, 2018. https://doi.org/10.1093/cid/ciy175 PMid:29509845
- Visentin
A. et al., Epidemiology and risk factors of invasive fungal infections
in a large cohort of patients with chronic lymphocytic leukemia.
Hematol Oncol, 2017. 35(4): p. 925-928. https://doi.org/10.1002/hon.2343 PMid:27641225
- Williams
A.M. et al., Analysis of the risk of infection in patients with chronic
lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma,
2018. 59(3): p. 625-632. https://doi.org/10.1080/10428194.2017.1347931 PMid:28696801
- Francis,
S., et al., The effect of immunoglobulin VH gene mutation status and
other prognostic factors on the incidence of major infections in
patients with chronic lymphocytic leukemia. Cancer, 2006. 107(5): p.
1023-33. https://doi.org/10.1002/cncr.22094 PMid:16862572
- Sun
Y. et al., Invasive fungal infection in patients receiving chemotherapy
for hematological malignancy: a multicenter, prospective, observational
study in China. Tumour Biol, 2015. 36(2): p. 757-67. https://doi.org/10.1007/s13277-014-2649-7 PMid:25293517
- Ruchlemer
R., R. Ben Ami and T. Lachish, Ibrutinib for Chronic Lymphocytic
Leukemia. N Engl J Med, 2016. 374(16): p. 1593-4. PMid:27096597
- Ghez
D. et al., Early-onset invasive aspergillosis and other fungal
infections in patients treated with ibrutinib. Blood, 2018. 131(17): p.
1955-1959. https://doi.org/10.1182/blood-2017-11-818286 PMid:29437588
- Wadhwa P.D. and V.A. Morrison, Infectious complications of chronic lymphocytic leukemia. Semin Oncol, 2006. 33(2): p. 240-9. https://doi.org/10.1053/j.seminoncol.2005.12.013 PMid:16616071
- Ravandi
F. and S. O'Brien, Immune defects in patients with chronic lymphocytic
leukemia. Cancer Immunol Immunother, 2006. 55(2): p. 197-209. https://doi.org/10.1007/s00262-005-0015-8 PMid:16025268
- Moreira
J. et al., Infectious complications among individuals with clinical
monoclonal B-cell lymphocytosis (MBL): a cohort study of newly
diagnosed cases compared to controls. Leukemia, 2013. 27(1): p. 136-41.
https://doi.org/10.1038/leu.2012.187 PMid:22781591
- Morrison
V.A. et al., Impact of therapy With chlorambucil, fludarabine, or
fludarabine plus chlorambucil on infections in patients with chronic
lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B
9011. J Clin Oncol, 2001. 19(16): p. 3611-21. https://doi.org/10.1200/JCO.2001.19.16.3611 PMid:11504743
- Hensel
M. et al., Disease activity and pretreatment, rather than
hypogammaglobulinaemia, are major risk factors for infectious
complications in patients with chronic lymphocytic leukaemia. Br J
Haematol, 2003. 122(4): p. 600-6. https://doi.org/10.1046/j.1365-2141.2003.04497.x PMid:12899715
- Steinbach
W.J. et al., Clinical epidemiology of 960 patients with invasive
aspergillosis from the PATH Alliance registry. J Infect, 2012. 65(5):
p. 453-64. https://doi.org/10.1016/j.jinf.2012.08.003 PMid:22898389
- Knauf
W.U. et al., Phase III randomized study of bendamustine compared with
chlorambucil in previously untreated patients with chronic lymphocytic
leukemia. J Clin Oncol, 2009. 27(26): p. 4378-84. https://doi.org/10.1200/JCO.2008.20.8389 PMid:19652068
- Fischer
K. et al., Bendamustine in combination with rituximab for previously
untreated patients with chronic lymphocytic leukemia: a multicenter
phase II trial of the German Chronic Lymphocytic Leukemia Study Group.
J Clin Oncol, 2012. 30(26): p. 3209-16. https://doi.org/10.1200/JCO.2011.39.2688 PMid:22869884
- Fischer
K. et al., Bendamustine combined with rituximab in patients with
relapsed and/or refractory chronic lymphocytic leukemia: a multicenter
phase II trial of the German Chronic Lymphocytic Leukemia Study Group.
J Clin Oncol, 2011. 29(26): p. 3559-66. https://doi.org/10.1200/JCO.2010.33.8061 PMid:21844497
- Anaissie
E.J. et al., Infections in patients with chronic lymphocytic leukemia
treated with fludarabine. Ann Intern Med, 1998. 129(7): p. 559-66. https://doi.org/10.7326/0003-4819-129-7-199810010-00010 PMid:9758577
- Wierda
W. et al., Chemoimmunotherapy with fludarabine, cyclophosphamide, and
rituximab for relapsed and refractory chronic lymphocytic leukemia. J
Clin Oncol, 2005. 23(18): p. 4070-8. https://doi.org/10.1200/JCO.2005.12.516 PMid:15767647
- Keating
M.J. et al., Early results of a chemoimmunotherapy regimen of
fludarabine, cyclophosphamide, and rituximab as initial therapy for
chronic lymphocytic leukemia. J Clin Oncol, 2005. 23(18): p. 4079-88. https://doi.org/10.1200/JCO.2005.12.051 PMid:15767648
- Byrd
J.C. et al., Randomized phase 2 study of fludarabine with concurrent
versus sequential treatment with rituximab in symptomatic, untreated
patients with B-cell chronic lymphocytic leukemia: results from Cancer
and Leukemia Group B 9712 (CALGB 9712). Blood, 2003. 101(1): p. 6-14. https://doi.org/10.1182/blood-2002-04-1258 PMid:12393429
- Goede
V. et al., Obinutuzumab plus chlorambucil in patients with CLL and
coexisting conditions. N Engl J Med, 2014. 370(12): p. 1101-10. https://doi.org/10.1056/NEJMoa1313984 PMid:24401022
- Martin
S.I. et al., Infectious complications associated with alemtuzumab use
for lymphoproliferative disorders. Clin Infect Dis, 2006. 43(1): p.
16-24. https://doi.org/10.1086/504811 PMid:16758413
- Elter
T. et al., Management of infections in patients with chronic
lymphocytic leukemia treated with alemtuzumab. Ann Hematol, 2009.
88(2): p. 121-32. https://doi.org/10.1007/s00277-008-0566-9 PMid:18682948
- Furman
R.R. et al., Idelalisib and rituximab in relapsed chronic lymphocytic
leukemia. N Engl J Med, 2014. 370(11): p. 997-1007. https://doi.org/10.1056/NEJMoa1315226 PMid:24450857 PMCid:PMC4161365
- Sehn L.H., Introduction to a review series: the paradox of indolent B-cell lymphoma. Blood, 2016. 127(17): p. 2045-6. https://doi.org/10.1182/blood-2016-03-692442 PMid:26989203
- Baden
L.R. et al., Prevention and Treatment of Cancer-Related Infections,
Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl
Compr Canc Netw, 2016. 14(7): p. 882-913. https://doi.org/10.6004/jnccn.2016.0093 PMid:27407129
- Ahn,
I.E. et al., Atypical Pneumocystis jirovecii pneumonia in previously
untreated patients with CLL on single-agent ibrutinib. Blood, 2016.
128(15): p. 1940-1943. https://doi.org/10.1182/blood-2016-06-722991 PMid:27503501 PMCid:PMC5064717
- Chamilos
G., M.S. Lionakis, and D.P. Kontoyiannis, Call for Action: Invasive
Fungal Infections Associated With Ibrutinib and Other Small Molecule
Kinase Inhibitors Targeting Immune Signaling Pathways. Clin Infect Dis,
2018. 66(1): p. 140-148. https://doi.org/10.1093/cid/cix687 PMid:29029010
- Arthurs
B. et al., Invasive aspergillosis related to ibrutinib therapy for
chronic lymphocytic leukemia. Respir Med Case Rep, 2017. 21: p. 27-29.
PMid:28377877 PMCid:PMC5369366
- Baron
M. et al., Fungal infections in patients treated with ibrutinib: two
unusual cases of invasive aspergillosis and cryptococcal
meningoencephalitis. Leuk Lymphoma, 2017. 58(12): p. 2981-2982. https://doi.org/10.1080/10428194.2017.1320710 PMid:28554246
- Lionakis
M.S. et al., Inhibition of B Cell Receptor Signaling by Ibrutinib in
Primary CNS Lymphoma. Cancer Cell, 2017. 31(6): p. 833-843 e5.
- Choquet
S. et al., Efficacy and safety of rituximab in B-cell
post-transplantation lymphoproliferative disorders: results of a
prospective multicenter phase 2 study. Blood, 2006. 107(8): p. 3053-7. https://doi.org/10.1182/blood-2005-01-0377 PMid:16254143
- Grommes C. and L.M. DeAngelis, Primary CNS Lymphoma. J Clin Oncol, 2017. 35(21): p. 2410-2418. https://doi.org/10.1200/JCO.2017.72.7602 PMid:28640701 PMCid:PMC5516483
- Tillman
B.F. et al., Systematic review of infectious events with the Bruton
tyrosine kinase inhibitor ibrutinib in the treatment of hematologic
malignancies. Eur J Haematol, 2018. 100(4): p. 325-334. https://doi.org/10.1111/ejh.13020 PMid:29285806
- Dubovsky
J.A. et al., Ibrutinib is an irreversible molecular inhibitor of ITK
driving a Th1-selective pressure in T lymphocytes. Blood, 2013.
122(15): p. 2539-49. https://doi.org/10.1182/blood-2013-06-507947 PMid:23886836 PMCid:PMC3795457
- de
Zwart L. et al., Ibrutinib Dosing Strategies Based on Interaction
Potential of CYP3A4 Perpetrators Using Physiologically Based
Pharmacokinetic Modeling. Clin Pharmacol Ther, 2016. 100(5): p.
548-557. https://doi.org/10.1002/cpt.419 PMid:27367453
- Stilgenbauer
S. et al., Venetoclax for Patients With Chronic Lymphocytic Leukemia
With 17p Deletion: Results From the Full Population of a Phase II
Pivotal Trial. J Clin Oncol, 2018. 36(19): p. 1973-1980. https://doi.org/10.1200/JCO.2017.76.6840 PMid:29715056
- Takaoka
K. et al., A novel scoring system to predict the incidence of invasive
fungal disease in salvage chemotherapies for malignant lymphoma. Ann
Hematol, 2014. 93(10): p. 1637-44. https://doi.org/10.1007/s00277-014-2093-1 PMid:24908330
- Kurosawa
M. et al., Epidemiology and treatment outcome of invasive fungal
infections in patients with hematological malignancies. Int J Hematol,
2012. 96(6): p. 748-57. https://doi.org/10.1007/s12185-012-1210-y PMid:23111539
- Jantunen
E. et al., Invasive fungal infections in autologous stem cell
transplant recipients: a nation-wide study of 1188 transplanted
patients. Eur J Haematol, 2004. 73(3): p. 174-8. https://doi.org/10.1111/j.1600-0609.2004.00273.x PMid:15287914
- Herbrecht
R. et al., Risk stratification for invasive aspergillosis in
immunocompromised patients. Ann N Y Acad Sci, 2012. 1272: p. 23-30. https://doi.org/10.1111/j.1749-6632.2012.06829.x PMid:23231711
- Montagna,
M.T., et al., Invasive fungal infections in patients with hematologic
malignancies (aurora project): lights and shadows during 18-months
surveillance. Int J Mol Sci, 2012. 13(1): p. 774-87. https://doi.org/10.3390/ijms13010774 PMid:22312285 PMCid:PMC3269719
- Dimopoulos
M.A. et al., Ibrutinib for patients with rituximab-refractory
Waldenstrom's macroglobulinaemia (iNNOVATE): an open-label substudy of
an international, multicentre, phase 3 trial. Lancet Oncol, 2017.
18(2): p. 241-250. https://doi.org/10.1016/S1470-2045(16)30632-5
- Klingspor
L. et al., Epidemiology and outcomes of patients with invasive mould
infections: a retrospective observational study from a single centre
(2005-2009). Mycoses, 2015. 58(8): p. 470-7. https://doi.org/10.1111/myc.12344 PMid:26152371
- Pagano
L. et al., Invasive aspergillosis in patients with acute myeloid
leukemia: a SEIFEM-2008 registry study. Haematologica, 2010. 95(4): p.
644-50. https://doi.org/10.3324/haematol.2009.012054 PMid:19850903 PMCid:PMC2857195
- Nosari
A.M. et al., Hema e-Chart registry of invasive fungal infections in
haematological patients: improved outcome in recent years in mould
infections. Clin Microbiol Infect, 2013. 19(8): p. 757-62. https://doi.org/10.1111/1469-0691.12014 PMid:23279327
- Fleming
S. et al., Consensus guidelines for antifungal prophylaxis in
haematological malignancy and haemopoietic stem cell transplantation,
2014. Intern Med J, 2014. 44(12b): p. 1283-97. https://doi.org/10.1111/imj.12595 PMid:25482741
- Pagano
L. et al., Risk assessment and prognostic factors for mould-related
diseases in immunocompromised patients. J Antimicrob Chemother, 2011.
66 Suppl 1: p. i5-14. https://doi.org/10.1093/jac/dkq437 PMid:21177404
- Gil
L. et al., Increased risk for invasive aspergillosis in patients with
lymphoproliferative diseases after autologous hematopoietic SCT. Bone
Marrow Transplant, 2009. 43(2): p. 121-6. https://doi.org/10.1038/bmt.2008.303 PMid:18794866
- Vazquez
L. et al., Delphi-based study and analysis of key risk factors for
invasive fungal infection in haematological patients. Rev Esp
Quimioter, 2017. 30(2): p. 103-117. PMid:28198173
- van
Hal S.J. et al., Survey of antifungal prophylaxis and fungal diagnostic
tests employed in malignant haematology and haemopoietic stem cell
transplantation (HSCT) in Australia and New Zealand. Intern Med J,
2014. 44(12b): p. 1277-82. https://doi.org/10.1111/imj.12594 PMid:25482740
- Mellinghoff
S.C. et al., Primary prophylaxis of invasive fungal infections in
patients with haematological malignancies: 2017 update of the
recommendations of the Infectious Diseases Working Party (AGIHO) of the
German Society for Haematology and Medical Oncology (DGHO). Ann
Hematol, 2018. 97(2): p. 197-207. https://doi.org/10.1007/s00277-017-3196-2 PMid:29218389 PMCid:PMC5754425
- Reinwald
M. et al., ESCMID Study Group for Infections in Compromised Hosts
(ESGICH) Consensus Document on the safety of targeted and biological
therapies: an infectious diseases perspective (Intracellular signaling
pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect,
2018. 24 Suppl 2: p. S53-S70. https://doi.org/10.1016/j.cmi.2018.02.009 PMid:29454849
- Wang
M.L. et al., Long-term follow-up of MCL patients treated with
single-agent ibrutinib: updated safety and efficacy results. Blood,
2015. 126(6): p. 739-45. https://doi.org/10.1182/blood-2015-03-635326 PMid:26059948 PMCid:PMC4528064
- Teh
B.W. et al., Invasive fungal infections in patients with multiple
myeloma: a multi-center study in the era of novel myeloma therapies.
Haematologica, 2015. 100(1): p. e28-31. https://doi.org/10.3324/haematol.2014.114025 PMid:25304609 PMCid:PMC4281332
- Huang
B.H. et al., [The clinical features and risk factors for invasive
fungal infection in multiple myeloma.]. Zhonghua Nei Ke Za Zhi, 2009.
48(12): p. 1026-30. PMid:20193522
- Cornet
M. et al., Epidemiology of invasive aspergillosis in France: a six-year
multicentric survey in the Greater Paris area. J Hosp Infect, 2002.
51(4): p. 288-96. https://doi.org/10.1053/jhin.2002.1258 PMid:12183144
- Lortholary
O. et al., Epidemiological trends in invasive aspergillosis in France:
the SAIF network (2005-2007). Clin Microbiol Infect, 2011. 17(12): p.
1882-9. https://doi.org/10.1111/j.1469-0691.2011.03548.x PMid:21668573
- Liu
J. et al., Epidemiology and treatment of invasive fungal diseases in
patients with multiple myeloma: findings from a multicenter prospective
study from China. Tumour Biol, 2016. 37(6): p. 7893-900. https://doi.org/10.1007/s13277-015-4441-8 PMid:26700667
- Nucci
M. and E. Anaissie, Infections in patients with multiple myeloma in the
era of high-dose therapy and novel agents. Clin Infect Dis, 2009.
49(8): p. 1211-25. https://doi.org/10.1086/605664 PMid:19769539
[TOP]




