Mohamed A. Yassin1, Ashraf T. Soliman2, Vincenzo De Sanctis3, Radwa M. Hussein4, Randa Al-Okka4, Nancy Kassem4, Rula Ghasoub4, Ahmed Basha4, Abdulqadir J. Nashwan5 and Ahmad M. Adel4.
1 Hematology Section, National Center for Cancer Care and Research, Hamad Medical Corporation, (HMC), Doha, Qatar.
2 Departments of Pediatrics, University of Alexandria, Alexandria, Egypt.
3 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
4
Pharmacists, Department of Pharmacy, National Center for Cancer Care
and Research, Hamad Medical Corporation (HMC), Doha, Qatar.
5 Nurse Research Scientist, Cancer Clinical Trials Unit, National Center for Cancer Care and Research, Hamad
Medical Corporation (HMC), Doha, Qatar.
Correspondence to: Mohamed A Yassin, MD. Consultant Hematologist,
Hematology Section, National Center for Cancer Care and Research, Hamad
Medical Corporation, Doha (Qatar). E-mail:
Yassinmoha@gmail.com
Published: November 1, 2018
Received: August 8, 2018
Accepted: October 10, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018064 DOI
10.4084/MJHID.2018.064
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction:
Due to the chronic nature of chelation therapy and the adverse
consequences of iron overload, patient adherence to therapy is an
important issue. Jadenu® is a new oral formulation of deferasirox
(Exjade®) tablets for oral suspension. While Exjade® is a dispersible
tablet that must be mixed in liquid and taken on an empty stomach,
Jadenu ® can be taken in a single step, with or without a light meal,
simplifying administration for the treatment of patients with chronic
iron overload. This may significantly improve the compliance to
treatment of patients with β-thalassemia major (BMT). The aim of this
study was to evaluate the drug tolerability and the effects of
chelation therapy on serum ferritin concentration, liver iron
concentration (LIC) and biochemical profiles in patients with BMT and
iron overload.
Patients and Methods:
Twelve selected adult patients BMT (mean age: 29 years; range: 15-34
years) were enrolled in the study. All patients were on monthly regular
red cell transfusion therapy to keep their pre-transfusional hemoglobin
(Hb) level not less than 9 g/dL. They were on Exjade® therapy (30 mg/kg
per day) for two years or more before starting Jadenu® therapy (14-28
mg/kg/day). The reason for shifting from Deferasirox® to Jadenu®
therapy was lack of tolerability, as described by patients, such as
nausea, vomiting, diarrhea, stomach pain. Most of them also reported
that Deferasirox® was not palatable. Lab investigations included
monthly urine analysis and measurement of their serum concentrations of
creatinine, fasting blood glucose (FBG), serum ferritin, alkaline
phosphatase (ALP), alanine transferase (ALT), aspartate transferase
(AST) and albumin concentrations. LIC was measured using FerriScan®.
Thyroid function, vitamin D and serum parathormone, before and one year
after starting Jadenu® therapy, were also assessed.
Results:
Apart from some minor gastrointestinal complaints reported in 3 BMT
patients that did not require discontinuation of therapy, other side
effects were not registered during the treatment. Subjectively,
patients reported an improvement in the palatability of Jadenu®
compared to Exjade® therapy in 8 out of 12 BMT patients. A
non-significant decrease in LIC measured by Ferriscan® and serum
ferritin levels was observed after one year of treatment with Jadenu®.
A significant positive correlation was found between serum ferritin
level and LIC measured by the FerriScan® method. LIC and serum ferritin
level correlated significantly with ALT level (r = 0.31 and 0.45
respectively, p < 0.05). No significant correlation was detected
between LIC and other biochemical or hormonal parameters. Conclusion:
Our study shows that short-term treatment with Jadenu® is safe but is
associated with a non-significant decrease in LIC and serum ferritin
levels. Therefore, there is an urgent need for adequately-powered and
high-quality trials to assess the clinical efficacy and the long- term
outcomes of new deferasirox formulation.
|
Introduction
In
patients with β-thalassemia major (BTM), iron overload is the joint
outcome of multiple blood transfusions and an inappropriately increased
iron absorption. In BTM patients, the rate of transfusional and
gastro-intestinal (GI) tract iron accumulation is generally 0.3-0.6
mg/kg per day.[1] Increased GI tract iron absorption can result from
severe anemia and ineffective erythropoiesis (IE), which down-regulate
the synthesis of hepcidin, a protein that controls iron absorption from
the GI tract and the release of recycled iron from macrophages.[2]
Without correction, iron overload can lead to end-organ damage,
resulting in cardiac, hepatic, and endocrine dysfunction/ failure.
Iron
chelation has been proven to decrease organ dysfunction and to improve
survival in certain transfusion-dependent anemias, such as β-
thalassemia.[3] To date, there are 3 major classes of iron chelators:
hexadentate (deferoxamine [DFO], Desferal®, Novartis Pharma AG, Basel,
Switzerland), in which 1 atom of iron is bound to
1 DFO molecule; bidentate (deferiprone, [DFP] Ferriprox®, Apotex
Inc.,
Toronto, ON, Canada), in which 1 atom of iron is bound to 3 DFP
molecules; and tridentate (deferasirox [DFX], Exjade® and Jadenu®,
Novartis Pharma AG, Basel, Switzerland) , in which 1 atom of iron is
bound to 2 DFX molecules.[4] The intensive demands
and uncomfortable side effects of therapy can have a negative impact on
daily activities and well-being, which may affect adherence to
treatment.[5]
Exjade® is a once-daily, oral iron chelator that was
developed out of a need for a long-acting, conveniently-administered
chelator for patients with transfusional hemosiderosis. The approved
mode of administration requires taking Exjade® on an empty stomach with
water, apple juice or orange juice to limit variation in
bioavailability. Any residual medication has to be resuspended in a
small volume of liquid and taken. This procedure leads to a lengthy
mixing process and the theoretical risk of patients not completely
taking the intended dose. Additionally, one third of patients find
Exjade® as a tablet for oral suspension unpalatable.[6] Additionally, approximately one-quarter
of patients experience mild to moderate GI symptoms, which may pose
additional challenges, particularly in the younger and older age
ranges.[7]
The new tablet DFX formulation (Jadenu®) was developed in
an attempt to overcome these tolerability issues and is the only
once-daily oral iron chelator that can be swallowed with a light meal,
without the need to disperse into a suspension prior to consumption. It
was approved by the FDA on March 31, 2015.[8] The recommended initial
dose of Jadenu® for patients 2 years of age and older, with estimated
glomerular filtration rate (eGFR) greater than 60 mL/min/1.73 m2, is 14
mg/kg/body weight given orally, once daily, and titrated up by 3.5–7
mg/kg/day. In patients not adequately controlled with doses of 21 mg
per kg/day (e.g., serum ferritin levels persistently above 2,500 µg/L
and not showing a decreasing trend over time), doses of up to 28 mg per
kg may be considered. Doses above 28 mg per kg are not recommended.[9,10]
When
converting a patient from Exjade® to Jadenu®, the dosage should be
decreased by 30% because the new formulation is more bioavailable than
the original Exjade® formulation.[9,10]
Up to now, the new DFX
formulation has been evaluated in pharmacokinetic studies in healthy
volunteers in an open-label, phase II ECLIPSE study, over 24 weeks in
chelation-naïve or pre- treated patients (aged >10 years) with
transfusion- dependent thalassemia or myelodysplastic syndromes.[11]
Patients reported greater adherence and satisfaction, better
palatability and fewer concerns with Deferasirox® than Jadenu®.
Treatment compliance by pill count was higher with latter compound:
92.9% vs. 85.3%.[11]
Our study aimed to evaluate the patient'
satisfaction, the adverse events (AEs) and the effects of Jadenu®
treatment on serum ferritin concentration, liver iron concentrations
(LIC) measured by Ferriscan® and biochemical profiles in BTM patients
with iron overload.
Patients and Methods
A
pre-selected group of twelve adult patients with BTM that couldn't
tolerate Exjade® therapy were enrolled in our study. The reason for
shifting from Deferasirox® to Jadenu® therapy was the lack of
tolerability as described by patients, such as nausea, vomiting,
diarrhea, stomach pain. Most of them also reported that Deferasirox®
was not palatable.
The mean age of patients was 29 years
(range:15-34 years). Six patients were males, and 2 out of 12 patients
were splenectomized. All patients were on regular packed red cell
transfusion therapy in order to keep their pre- transfusional Hb level
not less than 9 g/dL. They were on Exjade® therapy (30 mg/kg per day)
for two years or more before substitution to Jadenu® therapy. All
patients started with a dose of 14 mg/kg/day then escalated to a
maximum dose of 28 mg/kg/day.
The efficacy and tolerability of
iron chelation therapy were regularly analyzed and recorded before the
blood transfusions. Doctors, taking care of BTM patients asked, during
the study, the patient's satisfaction, palatability of medicine, and
the presence of side effects. Safety was evaluated by monitoring and
assessing AEs, changes in laboratory parameters, and clinical
observations from the start of study treatment to 30 days after the
last intake of study drug.
Lab investigations included monthly
urine analysis and measurement of their serum concentrations of
creatinine, fasting blood glucose (FBG), serum ferritin, alkaline
phosphatase (ALP), alanine transferase (ALT),
aspartate transferase (AST) and albumin concentrations. LIC was
measured using FerriScan®.12 LIC values were expressed as mg/g /dry
weight and classified into: normal (LIC <3 mg/g /dry weight); mild
(LIC > 3 and < 7 mg/g /dry weight), moderate (LIC > 7 and <
14 mg/g /dry weight) and severe overload (LIC ≥ 15 mg Fe/g
dry weight).[12,13] In addition, thyroid function [free T4 (FT4),
thyrotropin (TSH)], 25 OH vitamin D and serum parathormone (PTH) levels
were measured before and one year after starting Jadenu® therapy. All
patients were on vitamin D (800 U/day) and folic acid (5 mg/day).
The
paired t-Student test was used to compare lab results before versus
after Jadenu® treatment. Linear regression was used to investigate a
possible relation between variables (LIC vs. serum ferritin level, LIC
vs. other biochemical or hormonal parameters). A p value < 0.05 was
considered as significant.
Ethical approval for the study was
obtained by the Ethical Committee of Hamad General Hospital. All
procedures were carried out with the adequate understanding and consent
of patients.
Results
Subjectively,
8 out of 12 BMT patients reported an improvement in the palatability of
Jadenu® compared to Exjade® therapy. Apart from some minor
gastrointestinal complaints, reported in 3 BMT patients, that did not
require discontinuation of therapy, other side effects were not
registered during the treatment.
A non-significant statistical
decrease in serum ferritin concentration and LIC values was
observed after one year of Jadenu® treatment. No significant variations
were observed for urine analysis, serum creatinine, albumin, ALP, ALT,
AST, FBG, thyroid function, vitamin D and PTH levels (Tables 1 and 2).
A
significant positive correlation was found between serum ferritin level
and LIC, measured by the FerriScan® method (r= 0.61, p: 0.03). LIC and
serum ferritin level were also correlated significantly to ALT level (r
= 0.31 and 0.45 respectively, p < 0.05). No significant correlation
was detected between LIC and other biochemical or hormonal parameters.
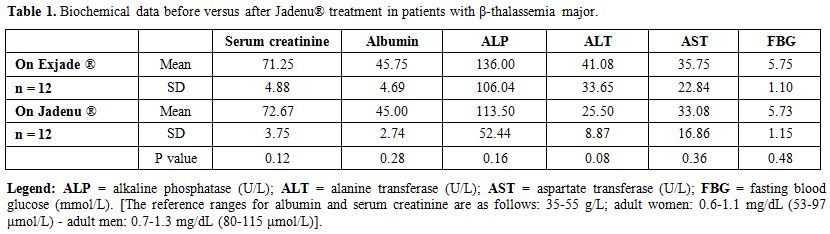 |
Table 1. Biochemical data before versus after Jadenu® treatment in patients with β-thalassemia major. |
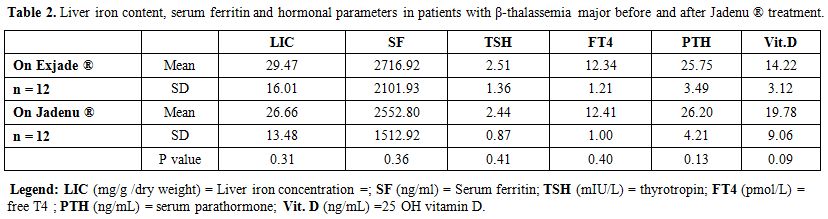 |
Table 2.
Liver iron content, serum ferritin and hormonal parameters in
patients with β-thalassemia major before and after Jadenu ® treatment. |
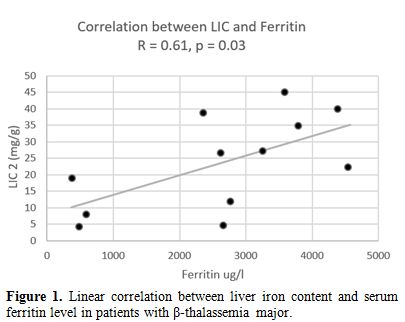 |
Figure 1. Linear correlation between liver iron content and serum ferritin level in patients with β-thalassemia major. |
Discussion
There
are currently four different types of iron chelator readily available
for patients with thalassemia: deferoxamine/DFO (branded as Desferal),
deferiprone/DFP (branded as Ferriprox®), deferasirox/DFX (branded as
Exjade® and Jadenu®). Each of the four chelators offers different
benefits and challenges to the patients.
Usage of DFP or DFX, as
oral chelator is more preferably due to its ease of use, with several
studies presenting higher compliance rate in LeoGib1707 patients with
oral chelator compared to DFO injection (sc or iv) chelator. People
treated with all chelators must be kept under close medical supervision
and treatment with DFO or DFX requires regular monitoring of neutrophil
counts or renal function, respectively.[14]
Patients taking DFX
tablets for oral suspension (Exjade®) reported superior satisfaction
scores compared to those reported by patients taking DFO, with
satisfaction rates for DFX as high as 90%.[15,16] However, palatability
studies showed that more than third of patients disliked DFX (Exjade®)
tablets for oral suspension, with self- reported adherence to the
medication varying from 67% to 85%.[17,18]
The most common side
effect with DFX tablets for oral suspension is GI discomfort, with 10%
– 33% of patients experiencing abdominal pain, diarrhea, nausea, and/or
vomiting.
However, most patients are able to tolerate these side
effects, although 7% of patients cited GI side effects as a reason for
stopping treatment.[17-19] As DFX can cause renal toxicity and
proteinuria, creatinine should be monitored twice prior to the
initiation of therapy and monthly thereafter.[9,10]
The
new DFX formulation (Jadenu®) thanks to the
simplification of its administration is hoped to improve patient
satisfaction, and thereby adherence to treatment.[8]
Subjectively,
8 out of 12 BMT patients (66.6%) enrolled in our study reported an
improvement in the palatability of Jadenu® compared to Exjade® therapy.
Three out of 12 BTM patients (25%) reported nausea and abdominal
discomfort on Jadenu® therapy, but none of these symptoms required
discontinuation of treatment. During the study period, we did not
register significant changes in serum creatinine concentrations,
albumin levels, and urine analysis, as well as for glucose levels and
thyroid function.
Liver iron overload and hepatic dysfunction are
major side effects of chronic transfusion therapy. Measurement of LIC
is the most reliable indicator of body iron load. Normal LIC values are
up to 1.8 mg/g dry weight, with levels of up to 7 mg/g dry weight seen
in some non-thalassaemic populations without apparent adverse effects.
Sustained high LIC (above 15-20 mg/g dry wt) have been linked to
worsening prognosis and liver fibrosis progression.[19,20] Adequate
control of LIC is linked to the risk of hepatic damage as well as the
risk of extrahepatic damage.
After one year of treatment with
Jadenu®, a non-significant decrease in LIC and serum ferritin levels
was observed in our patients. The term non- responder has been used to
describe individuals who fail to show a downward trend in iron balance
(changes in LIC) and extrahepatic iron distribution (myocardial T2*).
Lack of a response of an individual may result from inadequate
dosing, high transfusion requirement, poor treatment adherence, or
unfavorable pharmacology of the chelation regime.[21,22]
In our
patients, the dosage of oral iron chelation therapy was appropriate,
the blood consumption was not increased, but the compliance to
treatment was not fully evaluated, and the pharmacokinetic and
pharmacodynamic profiles of the new deferasirox formulation were not
performed. In general, the new formulation has comparable
pharmacokinetic to the dispersible tablet formulation. However, the new
formulation is more bioavailable than the original Exjade®, and the
peak serum concentrations (Cmax) is approximately 30% higher.[9,10]
A
significant correlation between LIC assessed by FerriScan® and serum
ferritin levels was observed in the current study. Although the small
numbers preclude a generalization, this is in line with the findings of
Zamani et al.[23] and Majd et al.[24] who reported that serum ferritin
is a good parameter to detect hepatic iron loading. Serum ferritin
remains an inexpensive and easily available tool for assessment of iron
overload and can be used in areas where access to liver T2 MRI
assessment is unavailable or limited. However, ferritin trends need to
be interpreted with caution before critical changes are made in the
chelation plan because trends in ferritin can be dramatically different
from changes in LIC as assessed by MRI.[25]
Conclusions
Once
the need for iron chelators is established in patients with
transfusional iron overload, the ideal agent should be determined by
the practitioner and patient. DFX (Exjade®) is a frequent choice due to
ease of once-daily oral administration, but adherence may be
hampered by palatability of the tablet for oral suspension.
Although
some limitations are present in our study: (a) it was performed only in
a single centre, and (b) the number of patients enrolled in the study
was small, our results confirm that short- term treatment with Jadenu®
is safe, has a better palatability and fewer patients' concerns versus
the original formulation. However, it was associated with a
non-significant decrease in LIC and serum ferritin levels. Therefore,
there is an urgent need for further research assessing the clinical
efficacy and the long-term outcomes of the new DFX formulation.
References
- Pippard M. Iron chelation therapy in the treatment
of iron overload. In: Bergeron R, Brittenham G, eds. The Development of
Iron Chelators for Clinical Use. Boca Raton, FL: CRC Press; 1994:57- 74.
- Tanno
T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH,
Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S,
Miller JL. High levels of GDF15 in thalassemia suppress expression of
the iron regulatory protein hepcidin. Nat Med. 2007;13:1096-1101. https://doi.org/10.1038/nm1629 PMid:17721544
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999; 341:1986-1995. https://doi.org/10.1056/NEJM199912233412607 PMid:10607817
- Neufeld
EJ. Oral chelators deferasirox and deferiprone for transfusional iron
overload in thalassemia major: new data, new questions. Blood.
2006;107:3436-3441. https://doi.org/10.1182/blood-2006-02-002394 PMid:16627763 PMCid:PMC1895765
- Fortin
PM, Fisher SA, Madgwick KV, Trivella M, Hopewell S, Doree C, Estcourt
LJ. Interventions for improving adherence to iron chelation therapy in
people with sickle cell disease or thalassaemia. Cochrane Database Syst
Rev. 2018 May 8;5:CD012349. https://doi.org/10.1002/14651858.CD012349.pub2
- Waldmeier
F, Bruin GJ, Glaenzel U, Hazell K, Sechaud R, Warrington S, Porter
JB.Pharmacokinetics, metabolism, and disposition of deferasirox in
beta-thalassemic patients with transfusion-dependent iron overload who
are at pharmacokinetic steady state. Drug Metab Dispos.
2010;38:808–816. https://doi.org/10.1124/dmd.109.030833 PMid:20097723
- Goldberg
SL, Giardina PJ, Chirnomas D, Esposito J, Paley C, Vichinsky E. The
palatability and tolerability of deferasirox taken with different
beverages or foods. Pediatr Blood Cancer. 2013;60:1507–1512. https://doi.org/10.1002/pbc.24561 PMid:23637051
- Chalmers
AW, Shammo JM. Evaluation of a new tablet formulation of deferasirox to
reduce chronic iron overload after long-term blood transfusions. Ther
Clin Risk Manag. 2016 Feb 15;12:201-8. https://doi.org/10.2147/TCRM.S82449
- Novartis
Pharmaceuticals Corporation . Highlights of prescribing information:
JADENU®. New Jersey, US: Novartis; 2015. [Accessed January 7, 2016].
Available from: http://www.pharma.us.novartis.com/product/pi/pdf/jadenu.pdf
- Shah NR. Advances in iron chelation therapy: transitioning to a new oral formulation. Drugs Context 2017 Jun 16;6:212502. https://doi.org/10.7573/dic.212502
- Taher
AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A, Goh AS,
Cortoos A, Huang V, Weill M, Merino Herranz R, Porter JB.New
film-coated tablet formulation of deferasirox is well tolerated in
patients with thalassemia or lower-risk MDS: Results of the randomized,
phase II ECLIPSE study. Am J Hematol. 2017;92:420- 428. https://doi.org/10.1002/ajh.24668 PMid:28142202
- Yassin
MA, Soliman AT, De Sanctis V, Abdula MA, Riaz LM, Ghori FF, Yousaf A,
Nashwan AJ, Abusamaan S, Moustafa A, Kohla S, Soliman DS. Statural
Growth and Prevalence of Endocrinopathies in Relation to Liver Iron
Content (LIC) in Adult Patients with Beta Thalassemia Major (BTM) and
Sickle Cell Disease (SCD). Acta Biomed. 2018;89(2-S):33-40.
- Hernando
D, Levin YS, Sirlin CB, Reeder SB. Quantification of Liver Iron with
MRI: State of the Art and Remaining Challenges. J Magn Reson Imaging.
2014;40:1003-1021. https://doi.org/10.1002/jmri.24584 PMid:24585403 PMCid:PMC4308740
- Fisher
SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ.
Desferrioxamine mesylate for managing transfusional iron overload in
people with transfusion-dependent thalassaemia. Cochrane Database
Syst Rev. 2013 Aug 21;(8): CD004450. https://doi.org/10.1002/14651858.CD004450.pub3
- Vichinsky
E, Pakbaz Z, Onyekwere O, Porter J, Swerdlow P, Coates T, Lane P, Files
B, Mueller BU, Coïc L, Forni GL, Fischer R, Marks P, Rofail D, Abetz L,
Baladi JF.Patient-reported outcomes of deferasirox (Exjade, ICL670)
versus deferoxamine in sickle cell disease patients with transfusional
hemosiderosis. Substudy of a randomized open-label phase II trial. Acta
Haematol. 2008;119:133– 141. https://doi.org/10.1159/000125550 PMid:18408362
- Taher
A, Al Jefri A, Elalfy MS, Al Zir K, Daar S, Rofail D, Baladi JF, Habr
D, Kriemler-Krahn U, El-Beshlawy A.Improved treatment satisfaction and
convenience with deferasirox in iron-overloaded patients with
beta-Thalassemia: Results from the ESCALATOR Trial. Acta Haematol.
2010;123:220–225. https://doi.org/10.1159/000313447 PMid:20424435
- Goldberg
SL, Giardina PJ, Chirnomas D, Esposito J, Paley C, Vichinsky E. The
palatability and tolerability of deferasirox taken with different
beverages or foods. Pediatr Blood Cancer. 2013;60:1507–1512. https://doi.org/10.1002/pbc.24561 PMid:23637051
- Porter
J, Bowden DK, Economou M, Troncy J, Ganser A, Habr D, Martin N, Gater
A, Rofail D, Abetz-Webb L, Lau H, Cappellini MD.Health-Related Quality
of Life, Treatment Satisfaction, Adherence and Persistence in
β-Thalassemia and Myelodysplastic Syndrome Patients with Iron Overload
Receiving Deferasirox: Results from the EPIC Clinical Trial. Anemia.
2012;2012:297641. https://doi.org/10.1155/2012/297641
- Angelucci
E., Urru S.A.M., Pilo F., Piperno A. Myelodysplastic syndromes and iron
chelation therapy. Mediterr J Hematol Infect Dis 2017,9 (1) : e2017021,
https://doi.org/10.4084/mjhid.2017.021
- Jensen
PD, Jensen FT, Christensen T, Eiskjaer H, Baandrup U, Nielsen JL.
Evaluation of myocardial iron by magnetic resonance imaging during iron
chelation therapy with deferrioxamine: indication of close relation
between myocardial iron content and chelatable iron pool. Blood.
2003;101:4632–4639. https://doi.org/10.1182/blood- 2002-09-2754 PMid:12576333
- Porter
JB, Shah FT. Iron overload in thalassemia and related conditions:
therapeutic goals and assessment of response to chelation therapies.
Hematol Oncol Clin North Am. 2010;24:1109-1130. https://doi.org/10.1016/j.hoc.2010.08.015 PMid:21075283
- Galanello
R, Campus S, Origa R. Deferasirox: pharmacokinetics and clinical
experience. Expert Opin Drug Metab Toxicol. 2012;8:123- 134. https://doi.org/10.1517/17425255.2012.640674 PMid:22176640
- Zamani
F, Razmjou S, Akhlaghpoor S, Eslami SM, Azarkeivan A, Amiri A. T2*
magnetic resonance imaging of the liver in thalasse¬mic patients
in Iran. China Natl J New Gastroenterol. 2011;17:522– 525.
- Majd
Z, Haghpanah S, Ajami GH, Matin S, Namazi H, Bardestani M, Karimi M.
Serum Ferritin Levels Correlation With Heart and Liver MRI and LIC in
Patients With Transfusion-Dependent Thalassemia Iran Red Crescent Med
J. 2015 Apr 25;17(4):e24959. https://doi.org/10.5812/ircmj.17(4)2015.24959
- Puliyel
M, Sposto R, Berdoukas VA, Hofstra TC, Nord A, Carson S, Wood J, Coates
TD. Ferritin trends do not predict changes in total body iron in
patients with transfusional iron overload. Am J Hematol.
2014;89:391-394. https://doi.org/10.1002/ajh.23650 PMid:24347294
[TOP]


