Seung Beom Han1,2, Ju Ae Shin1, Seong koo Kim1,3, Jae Wook Lee1,3, Dong-Gun Lee2,3,4, Nack-Gyun Chung1,3, Bin Cho1,3, Dae Chul Jeong1,2 and Jin Han Kang1,2.
1 Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
2 The Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
3 Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
4
Division of Infectious Diseases, Department of Internal Medicine,
College of Medicine, The Catholic University of Korea, Seoul, Republic
of Korea.
Correspondence to: Bin Cho, MD, PhD, Professor. Department of
Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, The
Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591,
Republic of Korea. Tel.: 82 2 2258 6187, Fax.: 82 2 537 4544.
E-mail:
chobinkr@catholic.ac.kr
Published: January 1, 2019
Received: August 16, 2018
Accepted: November 3, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019006 DOI
10.4084/MJHID.2019.006
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Despite the introduction of a polymerase chain reaction (PCR) test for
the diagnosis of respiratory viral infection (RVI), guidance on the
application of this test and the management of RVI in immunocompromised
children is lacking. This study evaluated the clinical characteristics
of RVI and established strategies for the PCR test in children and
adolescents with hematological malignancies.
Methods:
This study included children and adolescents with underlying
hematological malignancies and respiratory symptoms, in whom a
multiplex PCR test was performed. Patients in whom RVI was identified
and not identified were categorized into Groups I and II, respectively.
Group I was sub-divided into patients with upper and lower respiratory
infections. The medical records of the enrolled patients were
retrospectively reviewed.
Results:
A total of 93 respiratory illnesses were included. Group I included 46
(49.5%) cases of RVI, including 31 (67.4%) upper and 15 (32.6%) lower
respiratory infections. Rhinovirus (37.0%) was the most common viral
pathogen. Significantly more patients in Group I had community-acquired
respiratory illnesses (p=0.003) and complained of rhinorrhea (p<0.001) and sputum (p=0.008)
than those in Group II. In Group I, significantly more patients with
lower respiratory infections had uncontrolled underlying malignancies (p=0.038) and received re-induction or palliative chemotherapy (p=0.006) than those with upper respiratory infections.
Conclusions:
A multiplex PCR test should be considered for RVI diagnosis in
immunocompromised children and adolescents with respiratory symptoms,
especially in those with rhinorrhea or sputum prominent over a cough.
The early application of the PCR test in patients with uncontrolled
underlying malignancies may improve outcomes.
|
Introduction
Infection is the main cause of treatment-related mortality in patients with hematological malignancies.[1]
Therefore, the early diagnosis and treatment of infection, as well as
infection prevention, are essential to improve the prognosis of immune
compromised patients. In these patients, neutropenic fever (NF) has
been the focus, and bacterial and fungal infection is emphasized.[2] Among viruses, Herpesviridae,
which maintains latency and reactivates during immune suppression
caused by anti-cancer chemotherapy and hematopoietic cell
transplantation (HCT), is considered a major pathogen. However, the
causative pathogens are not identified in 53-79% of patients with NF,[3,4] and some of which may be respiratory viruses (RVs).
Viral
culture and antigen detection methods have been used for the diagnosis
of viral infection. Because these conventional methods have low
sensitivity for detecting rhinovirus and enterovirus, which are the
most common causes of community-acquired respiratory viral infection
(RVI), RVI was identified only in 6-22% of immune compromised children
with respiratory symptoms in the past.[5-7] Also, most
previous studies were restricted to reporting RVI due to respiratory
syncytial virus (RSV), parainfluenza virus, influenza virus, and
adenovirus, which could be diagnosed by conventional methods.[8-10]
A polymerase chain reaction (PCR) test exhibiting improved sensitivity
and specificity in the diagnosis of RVI in immune compromised patients
compared to those of conventional methods has been introduced and its
use has been extended since the 2000s.[5,6,11] Recent studies using PCR tests identified RVI in 33-76% of children with NF or cancer complaining of respiratory symptoms.[5,6,12-16] RSV and influenza virus were the most frequent causes of RVI in the era of conventional methods,[8-10] whereas rhinovirus was the most frequent cause in the era of PCR tests.[5,6,12-17]
In spite of this epidemiological change accompanied by the introduction
of PCR tests, reports on the clinical characteristics and prognosis of
RVI in immune compromised children and adolescents using PCR tests are
lacking. Accordingly, guidelines on the application of a PCR test for
the diagnosis and proper management of RVI in immune compromised
children and adolescents have not been established.
This study was
performed to evaluate the clinical characteristics and outcomes of RVI
diagnosed by a multiplex PCR test for RVs and to establish strategies
for performing the PCR test in children and adolescents with
hematological malignancies, who comprise a major portion of immune
compromised children and adolescents.
Patients and Methods
Patients and study design.
Children and adolescents (<20 years of age) with underlying
hematological malignancies treated at the Department of Pediatrics,
Seoul St. Mary’s Hospital, College of Medicine, The Catholic University
of Korea, were eligible for this study. Among them, those who
complained of respiratory symptoms such as a cough, rhinorrhea, sputum,
sore throat, and dyspnea, with or without fever, and in whom a
multiplex PCR test for RVs was performed between December 2016 and
November 2017 were enrolled. Patients in whom respiratory symptoms
developed 6 months or more after the completion of anti-cancer
chemotherapy or 2 years or more after HCT were excluded. Patients in
whom hematological malignancies were newly diagnosed and anti-cancer
chemotherapy had not been administered prior to the development of
respiratory illnesses were also excluded. Patients in whom RVs were
identified and not identified were categorized into Groups I and II,
respectively. The medical records of the enrolled patients were
reviewed retrospectively and the clinical and laboratory
characteristics were compared between groups. The patients in Group I
were sub-divided into those with upper respiratory tract infections
(URIs) and lower respiratory tract infections (LRIs) and the clinical
and laboratory characteristics were also compared between the two
subgroups. This study was approved by the Institutional Review Board of
the Seoul St. Mary’s hospital with a waiver of informed consent
(Approval Number: KC18RESI0302).
Diagnosis and treatment of respiratory viral infections.
A nasopharyngeal swab was collected from patients complaining of
respiratory symptoms. The samples were sent to the Department of
Laboratory Medicine where a multiplex PCR test for RVs was performed
using a commercially available kit (AdvanSure™ RV real-time PCR kit, LG
Life Sciences Ltd., Seoul, Republic of Korea). The PCR kit tested for
influenza A and B viruses, parainfluenza virus, rhinovirus, RSV, human
metapneumovirus (HMPV), adenovirus, coronavirus, and human bocavirus.
Chest x-ray was routinely performed in patients with respiratory
symptoms, and chest computed tomography was performed based on the
attending physician’s clinical decision. Oseltamivir was administered
to all patients diagnosed with influenza. Based on the attending
physician’s decision, oral ribavirin and intravenous immunoglobulin
(IVIG) were administered. Febrile patients received empirical
antibiotic therapy.
Definitions.
An episode of respiratory illness occurring 4 or more weeks after a
previous episode was considered a separate episode if it occurred
during a separate admission and the patient did not have respiratory
complaints between the two episodes. URI was diagnosed when a patient
had respiratory symptoms that were not accompanied by hypoxemia and
abnormal findings on chest imaging studies. LRI was diagnosed when
abnormal findings were observed in chest imaging studies. Due to the
difficulty associated with obtaining sputum samples and performing
bronchoscopy in children and adolescents with underlying hematological
malignancies, as they are prone to bleed, nasopharyngeal samples were
used to diagnose LRI, although lower respiratory samples are preferred
specimens.[18] Community-acquired respiratory illness
was defined if respiratory symptoms developed before or within 2 days
of admission. Hospital-acquired respiratory illness was defined if
respiratory symptoms developed 2 or more days after admission.
Neutropenia was defined as an absolute neutrophil count <500/mm3.
Steroid use was defined if any type of glucocorticoids equivalent to 2
mg/kg/day (maximum 20 mg/day) of prednisolone or more were administered
for longer than 5 days within 1 month prior to the development of
respiratory illness. Oxygen therapy, mechanical ventilator care,
intensive care unit admission, and death that occurred within 1 month
after the development of respiratory illness were considered
complications. Co-infections were defined when any other types of
non-viral infection were identified at the same time as the patient
complaint of respiratory symptoms. Mortality within 1 month after the
development of respiratory illness was determined. Mortality due to RVI
was defined when the patient died with persisting respiratory symptoms
and signs and no other causes of death were identified.
Statistical analysis.
Categorical and continuous factors were compared using a chi-square and
Mann-Whitney tests, respectively, for comparisons between the patient
groups. Multivariate analyses to identify the independent factors for
RVI and LRI were performed for significant factors in univariate
analysis using a binary logistic regression analysis. IBM SPSS
Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA) was
used for statistical analyses, and statistical significance was defined
as a p-value <0.05.
Results
During
the study period, 74 children and adolescents
with underlying hematological malignancies who experienced 93 episodes
of respiratory illnesses were enrolled. Nine and five patients each
experienced two and three episodes, respectively. The median interval
between recurrent episodes of respiratory illnesses was 19 weeks
(range, 5-52 weeks). Group I included 46 (49.5%) episodes of RVI,
including 31 (67.4%) URIs and 15 (32.6%) LRIs. Eleven (73.3%) of the 15
LRIs were initially diagnosed with URIs that progressed to LRIs. Among
the identified RVs, rhinovirus (N=17, 37.0%) was the most frequent (Table 1),
followed by parainfluenza virus (N=14, 30.4%) and RSV (N=10, 21.7%). In
five (10.9%) episodes, two viruses were concurrently identified: two
(4.3%) episodes of rhinovirus and parainfluenza virus, two (4.3%)
episodes of rhinovirus and RSV, and one (2.2%) episode of RSV and
influenza A virus.
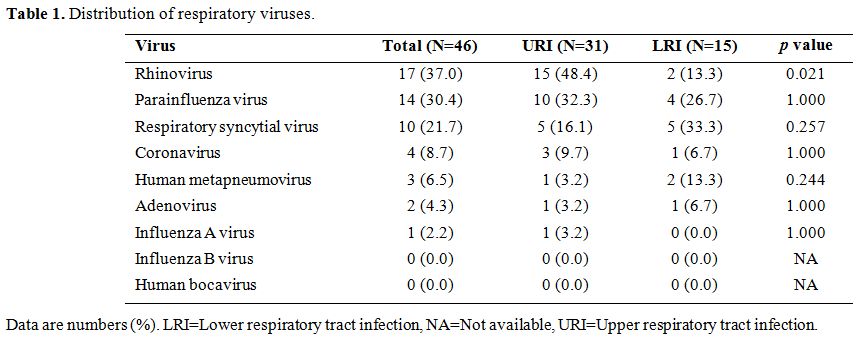 |
Table 1. Distribution of respiratory viruses. |
Co-infections were identified in 21 (22.6%) episodes. Ten (10.8%) episodes were accompanied by bacteremia (Escherichia coli in two, Pseudomonas aeruginosa in two, viridans streptococci in two, Enterococcus faecium in two, Streptococcus pneumoniae in one, and Staphylococcus epidermidis
in one). Eight (8.6%) episodes were accompanied by invasive pulmonary
aspergillosis, and two (2.2%) episodes were accompanied by herpetic
gingivostomatitis. Chickenpox, Pneumocystis jirovecii pneumonia, and Clostridium difficile infection accompanied one (1.1%) episode each.
The
rate of RV positivity was highest in autumn; however, no significant
differences were found among the four seasons (range, 31.6-61.5%, p=0.258).
Seven RSV infections occurred within a one-month interval in the same
ward, six (85.7%) of which were hospital-acquired infection.
Comparison between Group I and Group II.
The rates of different underlying hematological malignancies and
administered chemotherapies preceding respiratory illnesses were not
significantly different between the two groups. Significantly more
patients in Group II presented with hospital-acquired respiratory
illnesses (p=0.003), and accompanied neutropenia (p=0.028) than those in Group I (Table 2). Most patients overall complained of fever and cough; however, those in Group I complained of rhinorrhea (p<0.001) and sputum (p=0.008)
more frequently than those in Group II. In multivariate analysis,
rhinorrhea, sputum, and community-acquired respiratory illness were
significant factors for a diagnosis of RVI (Table 3). Significantly more complications occurred in patients in Group II compared to those in Group I (Table 2). Mortality was higher in Group II than that in Group I; however, the difference was not statistically significant.
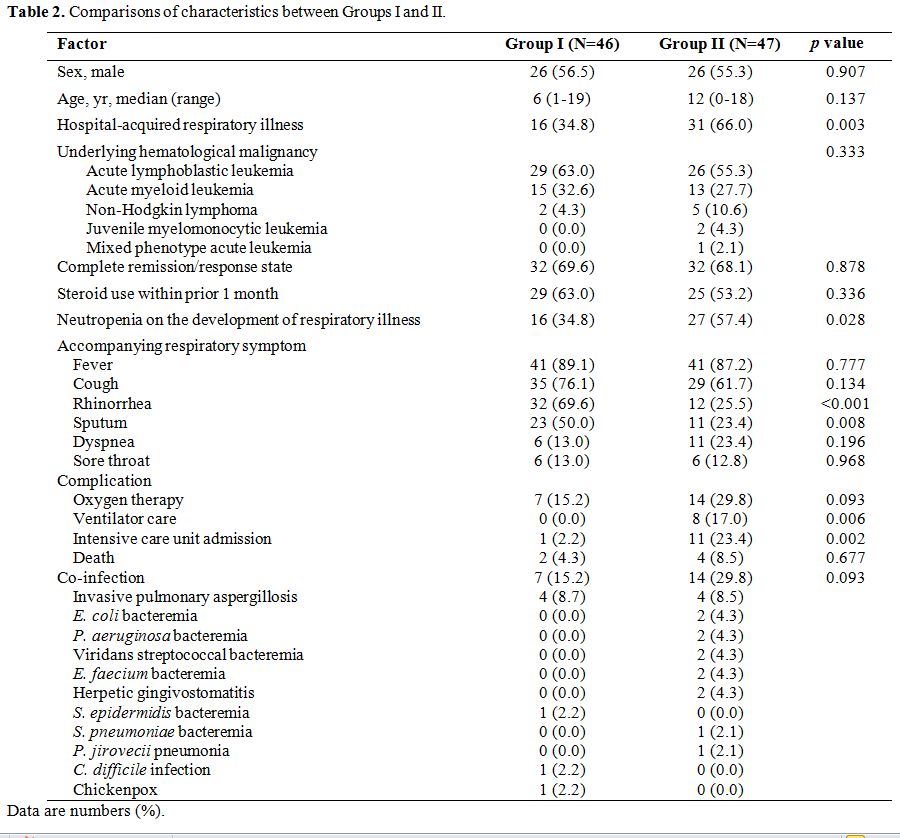 |
Table
2. Comparisons of characteristics between Groups I and II. |
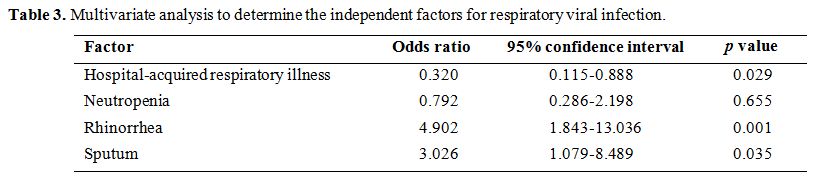 |
Table 3. Multivariate analysis to determine the independent factors for respiratory viral infection. |
Comparison between patients with upper and lower respiratory tract infections. In the patients in Group I, those with LRIs were more likely to have uncontrolled underlying malignancies (p=0.038) and receive re-induction or palliative chemotherapy (p=0.006) than those with URIs (Table 4). Among respiratory symptoms, sputum (p=0.028) and dyspnea (p=0.001) were more frequently accompanied by LRIs. More patients with LRIs experienced co-infections than those with URIs (p=0.029).
Among the five patients with LRIs and co-infections, four experienced
invasive pulmonary aspergillosis: two of whom had concomitant RSV
infection, another had concomitant adenovirus infection, and the other
had HMPV infection. The other experienced C. difficile infection
with concomitant parainfluenza virus infection. The two patients with
URIs and co-infections experienced chickenpox with concomitant
rhinovirus infection, and S. epidermidis
bacteremia with concomitant RSV infection, respectively. The rate of
rhinovirus infection was significantly higher in patients with URIs
than in those with LRIs (p=0.021). Other viral infections showed no significant association with LRIs (Table 1).
There were no independent risk factors for LRI in multivariate analysis
(data are not shown). Of 14 patients with parainfluenza virus
infection, five (35.7%) received ribavirin treatment and one (7.1%)
also received IVIG. Of 10 patients with RSV infections, eight (80.0%)
received ribavirin treatment and three (30.0%) also received IVIG.
Patients with LRIs were more likely to receive oxygen therapy (p<0.001)
than those with URIs and mortality was higher in patients with LRIs
compared to those with URIs, although the difference was not
statistically significant (p=0.101). All of the fatalities in both groups were caused by uncontrolled underlying malignancies.
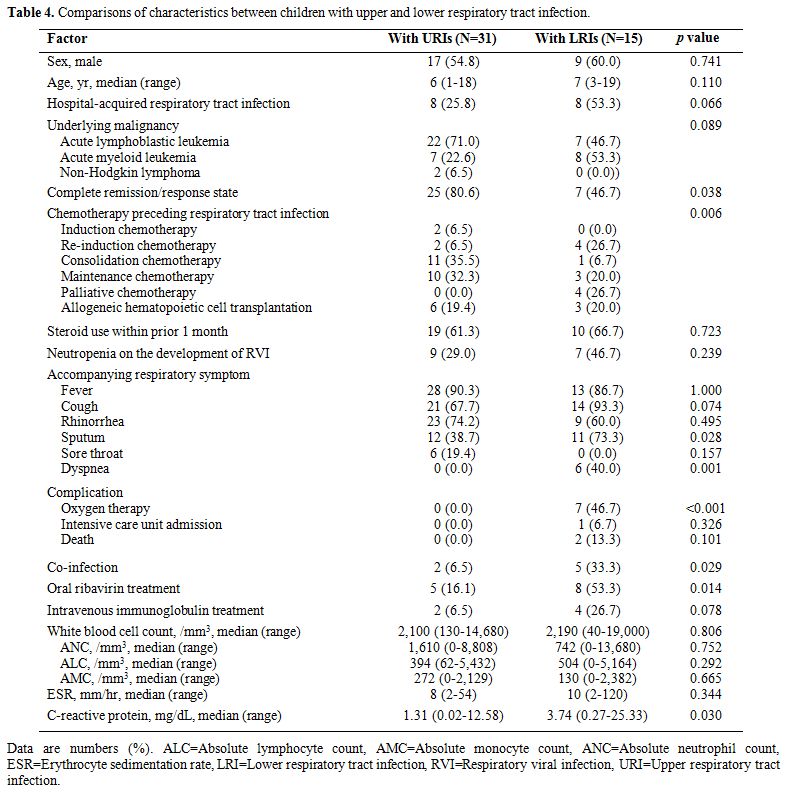 |
Table 4. Comparisons of characteristics between children with upper and lower respiratory tract infection. |
Discussion
In
this study, the clinical characteristics and outcomes of RVI were
investigated in children and adolescents with hematological
malignancies. RVI was diagnosed in about half of the enrolled patients,
consistent with the results of previous studies using PCR tests to
diagnose RVI.[5,12-15] Rhinovirus
rather than RSV and parainfluenza virus was the most frequent cause of
RVI, consistent with the results of recent studies using PCR tests.[5,6,12-16]
RVI investigation should be considered in immune compromised patients
complaining of community-acquired respiratory symptoms, preferably in
those with rhinorrhea or sputum predominant over a cough. If we
consider that early termination of empirical antibiotic therapy led to
a favorable outcome in children and adolescents with NF and RVI in a
recent report by Santolaya et al,[15] early diagnosis of RVI by a PCR test in these patients can help to avoid an unnecessary antibiotic use.
Neutropenia
was identified in only 34.8% of the patients diagnosed with RVI in this
study. Therefore, the diagnosis of RVI should not be restricted to
patients with NF. As a matter of fact, patients in Group II, in whom
the presence of RVs was not identified, were more likely to have
neutropenia, hospital-acquired and severe respiratory illnesses, and
infections with non-viral pathogens. This suggests that patients in
Group II underwent more aggressive anti-cancer chemotherapies, had a
more severe immunosuppression, and were hospitalized for longer
periods, compared to patients in Group I. Thus, pulmonary edema arising
from hyper-hydration during anti-cancer chemotherapy, pulmonary
hemorrhage due to thrombocytopenia, and bacterial and fungal pneumonias
could lead to respiratory symptoms in these patients. A negative PCR
result in patients with NF and hospital-acquired respiratory illnesses
may suggest the presence of more severe infection or treatment-related
complications.
Although community-onset respiratory illness was
significantly associated with the diagnosis of RVI in this study,
13-80% of RVI cases were hospital-acquired infection in several
studies, [6-9,19] including the
present study (34.8%). Outbreaks of RSV and parainfluenza virus
infection have been reported in an outpatient department as well as in
an inpatient ward;[20-22] we also experienced an
outbreak of RSV infection in seven patients in one month in a closed
hematology ward. Therefore, a multiplex PCR test for RVs should be
encouraged even in hospitalized patients complaining of rhinorrhea or
sputum, particularly when other patients with RVI are hospitalized in
the same ward or there is an RVI epidemic in the community. A timely
application of the PCR test can lead early diagnosis of RVI in
hospitalized patients, and to a subsequent decrease in the RVI
transmission within the hospital environment.
Recent studies on RVI in immune compromised children showed low mortality due to RVI, 0-3%.[5,2-16,19]
Fortunately, there was no death due to RVI in this study. This
favorable outcome may be attributed to a growing concern for RVI in
physicians, increasing diagnostic rates especially of mild RVI cases
using PCR tests, and improved supportive care in immune compromised
patients. Ribavirin-based anti-viral therapy can reduce progression
from URI to LRI and mortality in RSV-infected HCT recipients;[23]
however, it is not recommended in patients with hematological
malignancies who are not receiving HCT and its effect on parainfluenza
virus infection has not been confirmed.[24] In this
study, 80.0% of patients diagnosed with RSV infection received
ribavirin-based anti-viral therapy, regardless of receiving HCT, and
none of them died due to RSV infection. However, the efficacy of the
ribavirin-based therapy should be further evaluated as the anti-viral
therapy performed in this study did not rely on currently established
criteria.
The risk factors for mortality due to RVI could not be
determined in this study because there were no deaths attributable to
RVI. Most previous studies universally reported that LRI is associated
with the increased mortality.[7,10,19,23,25-27]
Even in rhinovirus infection, which causes milder respiratory illnesses
compared to those of RSV, parainfluenza virus and influenza virus,
mortality was significantly higher in patients with LRIs than that in
those with URIs.[26] Therefore, the early detection
of patients at risk of progression to LRIs and early application of
proper management for LRIs are necessary to improve the outcome of RVI
in immune compromised patients. Low absolute lymphocyte, neutrophil,
and monocyte counts; relapsed underlying malignancies; unrelated or
mismatched allogeneic-HCT; recent steroid use; oxygen need; and
co-infections are risk factors for LRI or mortality.[9,10,23,25-27] These risk factors represent the severity of immune suppression in the infected hosts.[9,25]
Accordingly, an immunodeficiency scoring system to predict outcomes and
determine the administration of anti-viral therapy has been applied to
RSV-infected HCT recipients.[28] The uncontrolled
state of underlying hematological malignancies and the presence of
co-infections were significantly associated with the development of LRI
in this study, which underscores the importance of the host’s overall
immune status in the outcome of RVI. As a result, a multiplex PCR test
for RVs should be performed preferentially in patients complaining of
rhinorrhea or sputum and with relapsed or refractory underlying
hematological malignancies, co-infections, or severe cytopenia.
Considering that 73.3% of LRI cases progressed from URIs in this study,
the early application of a multiplex PCR test during the URI period
should be emphasized.
This study had some limitations, including
biases arising from its retrospective study design. The number of
enrolled patients may not be appropriate, and we lacked a control group
including patients without respiratory symptoms. Although lower
respiratory samples, such as sputum and bronchial washing or
bronchoalveolar lavage fluids, are preferred samples for LRI diagnosis,
upper respiratory samples were used in this study. To assure an
improved diagnosis of LRI, abnormal findings in chest imaging studies
were mandatory for LRI diagnosis in this study; however, lower
respiratory samples could reveal clearer pathogenic profiles. The
seasonal distribution and epidemics of RVI in immune compromised
patients are correlated with those observed in the community.[8,29]
During the study period, the epidemics of RSV and influenza virus
infection in the community were not prominent in Korea compared to
previous years. The inclusion of more RSV and influenza virus infection
episodes may modify the outcome of RVI in this study. Future studies
should analyze data gathered for several years to include more cases of
RVI caused by a variety of RVs. A multiplex PCR test for RVs was not
routinely performed in children and adolescents with respiratory
symptoms in our hospital during the study period and was hardly
performed in the outpatient clinic. Therefore, children and adolescents
with mild respiratory symptoms and URIs could not be included in this
study. The outcomes of RVI in immune compromised children and
adolescents may be more favorable with the inclusion of mild RVI cases.
Conclusions
Considering
the confirmed RVI diagnosis in half of the immune compromised children
and adolescents with respiratory symptoms in this study, the
introduction of multiplex PCR tests for RV detection in this population
should be encouraged, especially for patients complaining of rhinorrhea
or sputum prominent over a cough. Moreover, the PCR test should address
patients with more severe immune suppression, e.g., those with relapsed
or refractory underlying malignancies and co-infections, as they are
prone to have severe RVI-related outcomes. In addition, infection
control strategies to prevent RVI transmission within the hospital
environment should be emphasized, considering the current scenario, in
which effective anti-viral therapies have not been established for most
RVI cases. Thus, early RVI detection by a PCR test may open a window of
opportunity for early intervention and infection control.
References
- O'Connor D, Bate J, Wade R, Clack R, Dhir S, Hough
R, Vora A, Goulden N, Samarasinghe S. Infection-related mortality in
children with acute lymphoblastic leukemia: an analysis of infectious
deaths on UKALL2003. Blood. 2014;124(7):1056-1061. https://doi.org/10.1182/blood-2014-03-560847 PMid:24904116
- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR; Infectious Diseases Society of
America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the
infectious diseases society of america. Clin Infect Dis.
2011;52(4):e56-93. https://doi.org/10.1093/cid/cir073 PMid:21258094
- Castagnola
E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, Garrè ML,
Moroni C, Conte M, Losurdo G, Scuderi F, Bandettini R, Tomà P, Viscoli
C, Haupt R. A prospective study on the epidemiology of febrile episodes
during chemotherapy-induced neutropenia in children with cancer or
after hemopoietic stem cell transplantation. Clin Infect Dis.
2007;45(10):1296-1304. https://doi.org/10.1086/522533 PMid:17968824
- Hakim
H, Flynn PM, Knapp KM, Srivastava DK, Gaur AH. Etiology and clinical
course of febrile neutropenia in children with cancer. J Pediatr
Hematol Oncol. 2009;31(9):623-629. https://doi.org/10.1097/MPH.0b013e3181b1edc6 PMid:19644403 PMCid:PMC2743072
- Lindblom
A, Bhadri V, Söderhäll S, Ohrmalm L, Wong M, Norbeck O, Lindau C,
Rotzén-Ostlund M, Allander T, Catchpoole D, Dalla-Pozza L, Broliden K,
Tolfvenstam T. Respiratory viruses, a common microbiological finding in
neutropenic children with fever. J Clin Virol. 2010;47(3):234-237. https://doi.org/10.1016/j.jcv.2009.11.026 PMid:20056482
- Koskenvuo
M, Möttönen M, Rahiala J, Saarinen-Pihkala UM, Riikonen P, Waris M,
Ziegler T, Uhari M, Salmi TT, Ruuskanen O. Respiratory viral infections
in children with leukemia. Pediatr Infect Dis J. 2008;27(11):974-980. https://doi.org/10.1097/INF.0b013e31817b0799 PMid:18833026
- Maeng
SH, Yoo HS, Choi SH, Yoo KH, Kim YJ, Sung KW, Lee NY, Koo HH. Impact of
parainfluenza virus infection in pediatric cancer patients. Pediatr
Blood Cancer. 2012;59(4):708-710. https://doi.org/10.1002/pbc.23390 PMid:22095941
- Raboni
SM, Nogueira MB, Tsuchiya LR, Takahashi GA, Pereira LA, Pasquini R,
Siqueira MM. Respiratory tract viral infections in bone marrow
transplant patients. Transplantation. 2003;76(1):142-146. https://doi.org/10.1097/01.TP.0000072012.26176.58 PMid:12865800
- Chemaly
RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ, Champlin RE,
Aguilera EA, Tarrand JJ, Raad II. Respiratory viral infections in
adults with hematologic malignancies and human stem cell
transplantation recipients: a retrospective study at a major cancer
center. Medicine (Baltimore). 2006;85(5):278-287. https://doi.org/10.1097/01.md.0000232560.22098.4e PMid:16974212
- Ljungman
P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, Brinch L, Brune M,
De La Camara R, Dekker A, Pauksen K, Russell N, Schwarer AP, Cordonnier
C. Respiratory virus infections after stem cell transplantation: a
prospective study from the Infectious Diseases Working Party of the
European Group for Blood and Marrow Transplantation. Bone Marrow
Transplant. 2001;28(5):479-484. https://doi.org/10.1038/sj.bmt.1703139 PMid:11593321
- Murali
S, Langston AA, Nolte FS, Banks G, Martin R, Caliendo AM. Detection of
respiratory viruses with a multiplex polymerase chain reaction assay
(MultiCode-PLx Respiratory Virus Panel) in patients with hematologic
malignancies. Leuk Lymphoma. 2009;50(4):619-624. https://doi.org/10.1080/10428190902777665 PMid:19373660
- Torres
JP, De la Maza V, Kors L, Villarroel M, Piemonte P, Izquierdo G,
Salgado C, Tordecilla J, Contardo V, Farfán MJ, Mejías A, Ramilo O,
Santolaya ME. Respiratory viral infections and coinfections in children
with cancer, fever and neutropenia: clinical outcome of infections
caused by different respiratory viruses. Pediatr Infect Dis J.
2016;35(9):949-954. https://doi.org/10.1097/INF.0000000000001209 PMid:27518750
- Srinivasan
A, Gu Z, Smith T, Morgenstern M, Sunkara A, Kang G, Srivastava DK, Gaur
AH, Leung W, Hayden RT. Prospective detection of respiratory pathogens
in symptomatic children with cancer. Pediatr Infect Dis J.
2013;32(3):e99-e104. PMid:23190778 PMCid:PMC4725698
- Benites
EC, Cabrini DP, Silva AC, Silva JC, Catalan DT, Berezin EN, Cardoso MR,
Passos SD. Acute respiratory viral infections in pediatric cancer
patients undergoing chemotherapy. J Pediatr (Rio J).
2014;90(4):370-376. https://doi.org/10.1016/j.jped.2014.01.006 PMid:24703819
- Santolaya
ME, Alvarez AM, Acu-a M, Avilés CL, Salgado C, Tordecilla J, Varas M,
Venegas M, Villarroel M, Zubieta M, Toso A, Bataszew A, Farfán MJ, de
la Maza V, Vergara A, Valenzuela R, Torres JP. Efficacy and safety of
withholding antimicrobial treatment in children with cancer, fever and
neutropenia, with a demonstrated viral respiratory infection: a
randomized clinical trial. Clin Microbiol Infect. 2017;23(3):173-178. https://doi.org/10.1016/j.cmi.2016.11.001 PMid:27856269
- Suryadevara
M, Tabarani CM, Bartholoma N, Rosenberg HF, Domachowske JB.
Nasopharyngeal detection of respiratory viruses in febrile neutropenic
children. Clin Pediatr (Phila). 2012;51(12):1164-1167. https://doi.org/10.1177/0009922812456736 PMid:22893186
- Milano
F, Campbell AP, Guthrie KA, Kuypers J, Englund JA, Corey L, Boeckh M.
Human rhinovirus and coronavirus detection among allogeneic
hematopoietic stem cell transplantation recipients. Blood.
2010;115(10):2088-2094. https://doi.org/10.1182/blood-2009-09-244152 PMid:20042728 PMCid:PMC2837322
- Miller
JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH,
Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS,
Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES,
Thomson RB Jr, Weinstein MP, Yao JD. A guide to utilization of the
microbiology laboratory for diagnosis of infectious diseases: 2018
update by the Infectious Diseases Society of America and the American
Society for Microbiology. Clin Infect Dis. 2018;67(6):e1-e94. https://doi.org/10.1093/cid/ciy381 PMid:29955859
- Lo
MS, Lee GM, Gunawardane N, Burchett SK, Lachenauer CS, Lehmann LE. The
impact of RSV, adenovirus, influenza, and parainfluenza infection in
pediatric patients receiving stem cell transplant, solid organ
transplant, or cancer chemotherapy. Pediatr Transplant.
2013;17(2):133-143. https://doi.org/10.1111/petr.12022 PMid:23228170
- Machado
AF, Sallum MA, Vilas Boas LS, Tateno AF, Machado CM. Molecular
characterization of strains of respiratory syncytial virus identified
in a hematopoietic stem cell transplant outpatient unit over 2 years:
community or nosocomial infection? Biol Blood Marrow Transplant.
2008;14(12):1348-1355. https://doi.org/10.1016/j.bbmt.2008.09.012 PMid:19041056
- Kassis
C, Champlin RE, Hachem RY, Hosing C, Tarrand JJ, Perego CA, Neumann JL,
Raad II, Chemaly RF. Detection and control of a nosocomial respiratory
syncytial virus outbreak in a stem cell transplantation unit: the role
of palivizumab. Biol Blood Marrow Transplant. 2010;16(9):1265-1271. https://doi.org/10.1016/j.bbmt.2010.03.011 PMid:20304082
- Maziarz
RT, Sridharan P, Slater S, Meyers G, Post M, Erdman DD, Peret TC,
Taplitz RA. Control of an outbreak of human parainfluenza virus 3 in
hematopoietic stem cell transplant recipients. Biol Blood Marrow
Transplant. 2010;16(2):192-198. https://doi.org/10.1016/j.bbmt.2009.09.014 PMid:19781656
- Shah
DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, Hosing C,
Rondon G, Tarrand JJ, Champlin RE, Chemaly RF. Impact of aerosolized
ribavirin on mortality in 280 allogeneic haematopoietic stem cell
transplant recipients with respiratory syncytial virus infections. J
Antimicrob Chemother. 2013;68(8):1872-1880. https://doi.org/10.1093/jac/dkt111 PMid:23572228
- Waghmare
A, Englund JA, Boeckh M. How I treat respiratory viral infections in
the setting of intensive chemotherapy or hematopoietic cell
transplantation. Blood. 2016;127(22):2682-2692. https://doi.org/10.1182/blood-2016-01-634873 PMid:26968533 PMCid:PMC4891952
- Seo
S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, Englund JA, Boeckh M.
Parainfluenza virus lower respiratory tract disease after hematopoietic
cell transplant: viral detection in the lung predicts outcome. Clin
Infect Dis. 2014;58(10):1357-1368. https://doi.org/10.1093/cid/ciu134 PMid:24599766 PMCid:PMC4001290
- Seo
S, Waghmare A, Scott EM, Xie H, Kuypers JM, Hackman RC, Campbell AP,
Choi SM, Leisenring WM, Jerome KR, Englund JA, Boeckh M. Human
rhinovirus detection in the lower respiratory tract of hematopoietic
cell transplant recipients: association with mortality. Haematologica.
2017;102(6):1120-1130. https://doi.org/10.3324/haematol.2016.153767 PMid:28183847 PMCid:PMC5451345
- Shah
DP, Shah PK, Azzi JM, Chemaly RF. Parainfluenza virus infections in
hematopoietic cell transplant recipients and hematologic malignancy
patients: A systematic review. Cancer Lett. 2016;370(2):358-364. https://doi.org/10.1016/j.canlet.2015.11.014 PMid:26582658 PMCid:PMC4684719
- Shah
DP, Ghantoji SS, Ariza-Heredia EJ, Shah JN, El Taoum KK, Shah PK,
Nesher L, Hosing C, Rondon G, Champlin RE, Chemaly RF. Immunodeficiency
scoring index to predict poor outcomes in hematopoietic cell transplant
recipients with RSV infections. Blood. 2014;123(21):3263-3268. https://doi.org/10.1182/blood-2013-12-541359 PMid:24700783 PMCid:PMC4046424
- Couch
RB, Englund JA, Whimbey E. Respiratory viral infections in
immunocompetent and immunocompromised persons. Am J Med.
1997;102(3A):2-9; discussion 25-26. https://doi.org/10.1016/S0002-9343(97)00003-X
[TOP]



