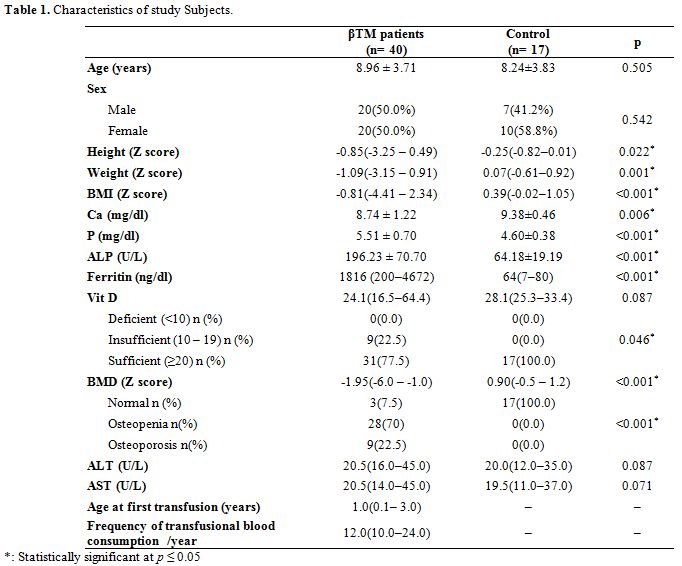
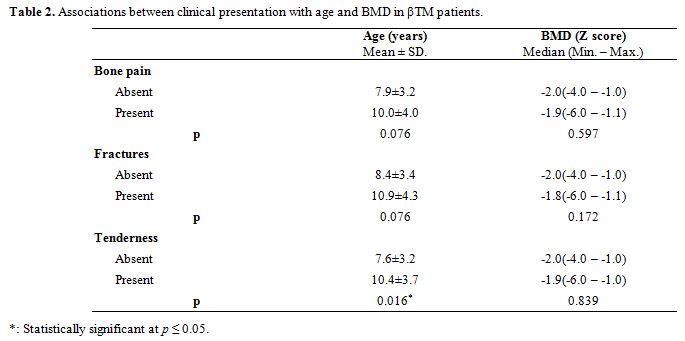
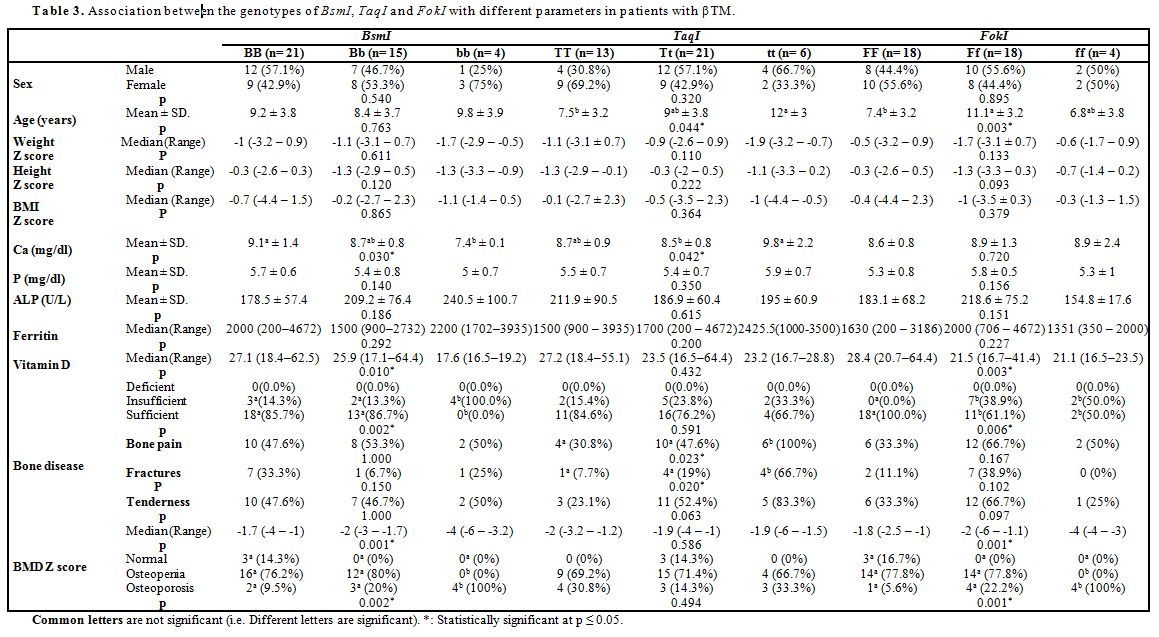
All patients were treated conventionally with regular blood transfusions in order to maintain pre-transfusion Hb levels above 9 g/dl, and adequate iron-chelation therapy with deferiprone, deferasirox or combined therapy with desferrioxamine. All patients were on oral vitamin D supplementation dose of 1000 IU daily.
Data collection. A full history was taken from all patients including duration of illness, the frequency of blood transfusion, iron chelation therapy: type and compliance, the presence of complications of thalassemia e.g. cardiac disease, liver disease, diabetes, the presence of complications that could be caused by vitamin D deficiency e.g. bone pain, or pathological fractures. All patients underwent thorough clinical examination including anthropometric measurements of weight and height with body mass index (BMI), height Z score and BMI Z score calculation, abdominal examination to detect hepatomegaly, tender liver, and splenomegaly, and musculoskeletal system examination to detect bone tenderness.
Blood sample collection. Peripheral blood samples were obtained by venipuncture using a sterile aseptic technique. About 5 milliliters of venous blood was withdrawn and divided into three vacutainer tubes. Three ml were delivered into a plain tube, for chemical investigation and vitamin D measurement. Two ml were divided equally into 2 EDTA tube for complete blood count (CBC) and DNA extraction for genotyping respectively.
Chemical analysis. Complete blood count was carried out for all study subjects on ADVIA 2120i Hematology System (Siemens Healthcare GmbH, Germany). All patients were evaluated biochemically for renal function, liver function tests, electrolytes including serum calcium (Ca), phosphorus (P) and alkaline phosphatase (ALP) on the Dimension® RxL Max® Integrated Chemistry System (Siemens Healthcare GmbH, Germany). Ferritin was measured on ADVIA Centaur XP Immunoassay System (Siemens Healthcare GmbH, Germany). Serum vitamin D (25(OH)D3) levels were measured by Enzyme-linked Fluorescent Assay (ELFA) technique on VIDAS (bioMérieux Clinical Diagnostics, France). A patient was considered to have vitamin D sufficiency if 25(OH)D3 (≥20) ng/ml, insufficiency if from 10 to 19 ng/ml and vitamin D deficiency if < 10 ng/ml.[13]
VDR genotyping. Total genomic DNA was purified according to the manufacturer protocol using the QIAamp DNA Blood Mini Kit, (QIAGEN, Germany). The quantity and purity of DNA were assayed using a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). A260: A230 ratio greater than 1.6 and A260:280 ratios greater than 1.8 were considered indicators for highly pure DNA.
Vitamin D receptor genotyping regarding BsmI (rs1544410), TaqI (rs731236), FokI (rs2228570) single nucleotide polymorphisms was carried out by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. The PCR reaction was carried out on the SimpliAmp Thermal Cycler (Applied Biosystems, USA). Two sequence-specific primers were used for each targeted fragment of VDR gene including intron 9 TaqI restriction site, intron 8 BsmI restriction site and intron 2 FokI restriction site as previously described.[14] The PCR primers were:
 |
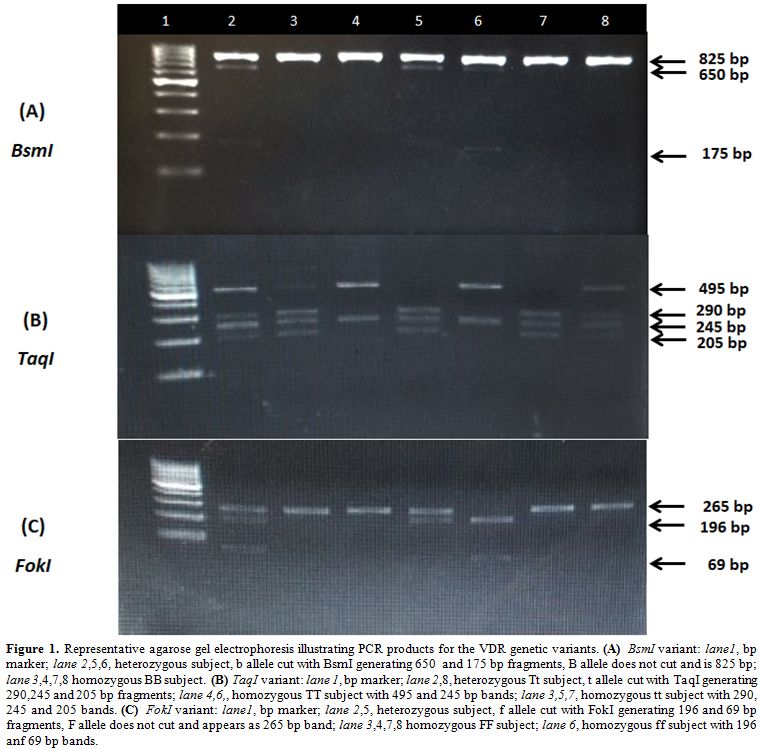
One hundred ng DNA was added to 12.5 ul of MyTaq Red Mix (Bioline, London, United Kingdom) and ten pmol of each primer in a total volume of 25 uL reaction mix. The thermal profile was 95°C for 1 minute as initial denaturation step, followed by 35 cycles each of 95°C for 15 seconds, 63°C for 15 seconds, and 72°C for 10 seconds for TaqI and BsmI. The annealing temperature for FokI was 85°C. The amplified products were digested by the New England Biolabs BsmI, TaqI, and FokI restriction endonucleases. BsmI and TaqI restriction endonucleases were incubated at 65ºC for 15 minutes then inactivated at 80ºC for 20 minutes. The FokI restriction endonuclease was incubated at 37ºC for 15 minutes then inactivated at 65ºC for 20 minutes. Digested products were electrophoretically separated electrophoresed through 2% agarose gels and 0.5X TBE buffer and visualized under UV light with ethidium bromide staining using Dolphin Doc gel documentation system (figure 1).
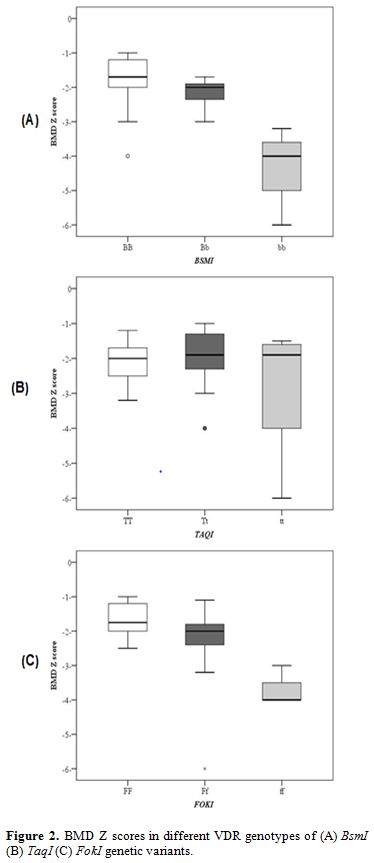
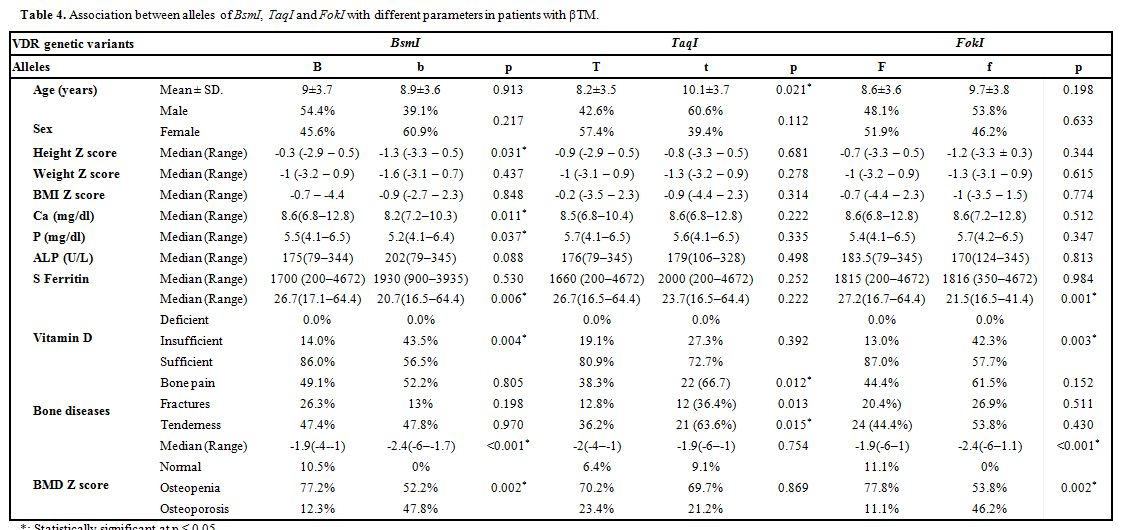
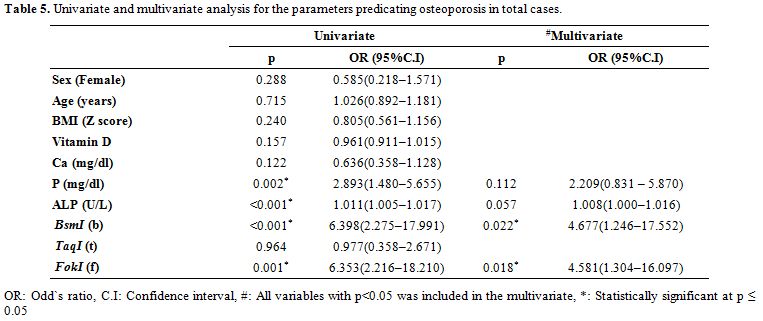
Bone mineral density measurement. Every patient underwent dual-energy X-ray absorptiometry (DEXA) scan using GE Lunar DPX Duo Bone Densitometer (GE Healthcare GmbH, Germany) of the lumbar spine (L1-L4). The BMD results were converted to age and gender-specific Z scores based on the normative reference data for BMD in Egyptian children. Patients were considered to be normal (Z score of -1 or higher), osteopenic (Z score between -1 and -2.5) or osteoporotic (Z score -2.5 or lower) based on WHO criteria.[15]
Statistical analysis of the data. Data analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp).The Kolmogorov-Smirnov, Shapiro and D’agstino tests were used to verify the normality of distribution of variables. Comparisons between groups for categorical variables were assessed using the Chi-square test (Fisher or Monte Carlo). Student t-test was used to compare two groups for normally distributed quantitative variables while ANOVA was used for comparing between more than two groups and followed by Post Hoc test (Tukey) for pairwise comparison. Mann Whitney test was used to compare between two groups for not normally distributed quantitative variables while Kruskal Wallis test was used to compare more than two groups for not normally distributed quantitative variables and followed by Post Hoc test (Dunn's) for pairwise comparison. Spearman coefficient was used to correlate between quantitative variables. Multivariable models were fit using logistic hazards models. The significance of the obtained results was judged at the 5% level.