Sema Arayici, Gulsum Kadioglu Şimşek, Fuat Emre Canpolat, Mehmet Yekta Oncel, Nurdan Uras and Serife Suna Oguz.
Division of Neonatology, Zekai Tahir Burak Maternity Teaching Hospital, Ankara, 06230, Turkey.
Correspondence to: Sema Arayici MD, Division of Neonatology, Zekai
Tahir Burak Maternity Teaching Hospital, Altındağ, 06230, Ankara,
Turkey. Phone +90 505 8314170. Fax +90 312 3114645. E-mail:
semadr@hotmail.com
Published: March 1, 2019
Received: August 16, 2018
Accepted: January 19, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019014 DOI
10.4084/MJHID.2019.014
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Neonatal sepsis remains an important and potentially life-threatening
clinical syndrome and a major cause of neonatal mortality and
morbidity. The aim of this study to investigate whether values of base
excess before the onset of clinical signs and symptoms of sepsis
indicate infection in the early diagnosis of neonatal sepsis.
Methods:
In this study, a total of 118 infants were enrolled. The infants were
classified into two groups: group 1 (sepsis, n=49) and group 2
(control, n=69). Blood gas analysis investigated for the screening of
neonatal sepsis.
Results:
A total of 49 newborns with neonatal sepsis and 69 healthy controls
were enrolled. Comparison of markers of sepsis revealed C-reactive
protein, interleukin-6 level to be significantly higher and pH, pCO2, HCO3,
and base excess values to be significantly lower in newborns with
sepsis compared healthy controls (p<0.01). The optimum cut-off value
in the diagnosis of neonatal sepsis was found to be -5 mmol/L for base
excess. Sensitivity, specificity, positive predictive value and
negative predictive value of this base excess cut-off for neonatal
sepsis were 75, 91, 86 and 84% respectively.
Conclusion:
This is the first study to determine the relationship between the
decreased value of the base excess and early stage of neonatal sepsis.
If the value of base excess <-5 mmol/L without an underlying another
reason, may need close follow up of infants for neonatal sepsis and it
may help early diagnosis.
|
Introduction
Neonatal
sepsis (NS) remains an important and potentially life-threatening
clinical syndrome and a major cause of neonatal mortality and
morbidity, particularly in preterm infants. The risk of sepsis
increases with decreasing birth weight and gestational age.[1,2]
Early diagnosis and adequate antibiotic treatment are required because
of the high rates of mortality and morbidity. The spectrum of NS
symptoms in preterm infants ranges from nonspecific or subtle findings
to fulminant septic shock. Due to subtle or nonspecific signs and
symptoms, NS is difficult to diagnose in the early period.[2,3]
Additionally,
clinical signs associated with normal physiological disturbances and
those of sepsis can overlap. Diagnosis is made by clinical and
laboratory findings. Blood culture is the gold standard laboratory
technique for diagnosis, but results may take 48-72 h, and
false-negative results may occur. Several markers such as C-reactive
protein (CRP), interleukins (ILs), procalcitonin (PCT), and
immunoglobulins, have been used to diagnose sepsis.[4,5] However, there is no suitable marker for diagnosis of NS, particularly in the early period.
Sepsis
is associated with many clinical features, including acidosis.
Metabolic acidosis results from a variety of common etiologies,
including lactic acidosis, hyperchloremic acidosis, renal failure, and
ketoacidosis. Anaerobic respiration begins, and metabolic acidosis
develops when an imbalance between oxygen supply and demand. Acidosis
can be determined from direct blood gas analysis by examining base
excess. Although there are a variety of other causes of metabolic
acidosis, the early identification of those infants with tissue dysoxia
may facilitate the early diagnosis of NS.[6-10]
This
study aimed to investigate whether base excess values before the onset
of clinical signs and symptoms of sepsis indicate infection in the
early diagnosis of NS.
Materials and Methods
The
study was conducted in the NICU at Zekai Tahir Burak Maternity Teaching
Hospital, Turkey. This unit has 150 incubators and serves as a referral
Level III NICU, with approximately 3000 newborn admissions per year.
This single-center, retrospective study was conducted between June 2013
and November 2013 after approval from the local ethics committee.
Participants and definitions. Cases with a gestational age ≤ 32 weeks and/or a birth weight ≤ 1,250 g were included in the study.
A
diagnosis of clinical sepsis required the presence of at least three of
the following: bradycardia (<100/min), tachycardia (>200/min),
hypotension, hypotonia, seizure, apnea, tachypnea, cyanosis,
respiratory distress, poor skin color and perfusion, feeding
difficulty, irritability, and lethargy in addition to laboratory
results showing elevated levels of CRP or interleukin-6 (IL-6).
Late-onset sepsis (LOS) was defined as sepsis that occurred after the
first 72 h of age. Patients with culture positivity were considered to
have proven sepsis.[11]
Patients with LOS (group
1) were further divided into two subgroups based on whether they had
proven (group 1a: newborns with positive blood cultures, clinical
findings in agreement with the diagnosis, and elevated IL-6 and/or CRP
levels during the clinical course) or clinical sepsis (group 1b:
newborns with clinical findings of infection, plus a significant rise
in IL-6 and/or CRP levels during the clinical course, but with negative
blood cultures). The control group (group 2) consisted of healthy
newborns without sepsis. Infants in the control group had normal
physical examination findings and were matched as much as possible in
demographic characteristics to those in the proven and clinical sepsis
groups.
Methods.
Blood gas analysis values in the sepsis group taken 12-24 h before the
onset of signs and symptoms of sepsis were evaluated. Hemodynamic
findings (heart rate, mean arterial pressure, urine output), actual
weight, serum sodium level, and total fluid intake were recorded
simultaneously. Hematocrit, complete white blood count (WBC), platelet,
CRP, and IL-6 levels as well as blood cultures, which were performed
after the appearance of symptoms and signs of sepsis, were also
recorded.
Blood gas analysis values and hemodynamic and laboratory
findings were recorded at similar times in the control group and
compared with those in the sepsis group.
Exclusion criteria were
defined as congenital heart disease, heart failure, renal failure,
inborn errors of metabolism, chromosomal aberrations, patients with
respiratory acidosis, and the presence of definite causes resulting in
lactic acidosis such as seizure.
Blood samples for culture were
taken from patients with a diagnosis of sepsis before antibiotic
therapy. Urine and cerebrospinal fluid cultures were taken when
clinically indicated. Blood culture was not taken from healthy
controls. The Bactec microbial detection system (Becton-Dickinson,
Sparks, MD) was used to detect positive blood cultures. Two-blood
culture positivity was required to confirm Staphylococcus epidermidis sepsis.
All
capillary blood samples were analyzed using in a RAPIDlab 1265 (Siemens
Diagnostic Product Corporation, Los Angeles, CA) blood gas analyzer.
Serum
concentrations of CRP were measured by a Tinaquant CRP (Latex) high
sensitive immune turbidimetric assay on a Roche Modular P analyzer
(Roche kit, Roche Diagnostics, Mannheim, Germany) according to
manufacturer instructions. Plasma levels of IL-6 were determined by
IL-6 solid phase, enzyme labeled, chemiluminescent sequential
immunometric assay on an IMMULITE 2000 analyzer (Siemens Diagnostic
Product Corporation, Los Angeles, CA) as per manufacturer instructions.
Statistical Analyses.
Statistical analyses were performed using the SPSS software version 20.
Categorical variables between groups were analyzed using the
chi-squared test. Comparison of mean between two groups was examined
using a t-test where the data fit a normal distribution, and the
Mann–Whitney U test where the data was non-normal. ROC analysis was
used to determine the power of variables to differentiate groups, and
the area under the curve (AUC) was calculated; significant cut-off
levels were calculated using a Youden index. A p-value of less than
0.05 was considered indicative of statistical significance.
Results
The
study group included 118 patients, 49 with sepsis and 69 controls. The
demographic characteristics of the study population are summarized in Table 1. Gestational age, birth weight, gender, and mode of delivery were similar in groups 1 and 2 (p > 0.05).
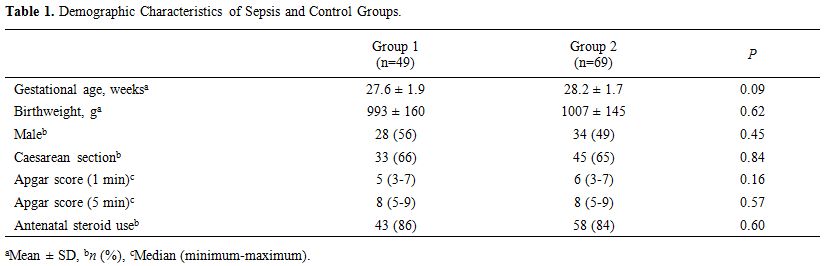 |
Table
1. Demographic Characteristics of Sepsis and Control Groups. |
The most frequently isolated microorganisms were Staphylococcus epidermidis (50%), Staphylococcus aureus (12%), Enterobacter cloacae (10%), Klebsiella pneumoniae (6%), Escherichia coli (6%), Pseudomonas aeruginosa (2%).
Table 2 shows
the hemodynamic and metabolic status of the infants in each group. No
significant differences in hemodynamic findings, total fluid intake,
serum sodium levels, hematocrit, or platelet levels were observed
between the sepsis and control groups (p > 0.05). However,
significant differences were observed for WBC, pH, HCO3, base excess, CRP, and IL-6 levels between the sepsis and control groups (p < 0.05).
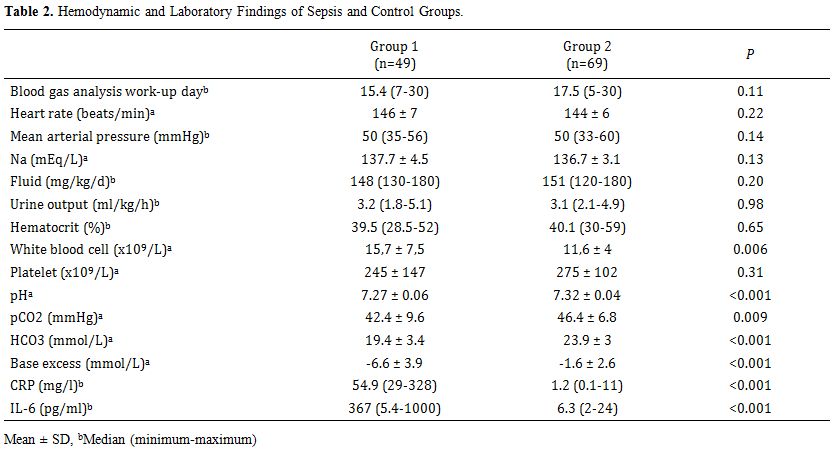 |
Table 2. Hemodynamic and Laboratory Findings of Sepsis and Control Groups. |
No
significant differences were noted between the proven and clinical
sepsis subgroups in any of the laboratory parameters (p > 0.05). No
significant difference in any variable was noted between infants with
gram-negative and -positive culture positivity in the proven sepsis
group (p > 0.05).
The optimal cut-off levels for base excess
between the sepsis and control groups and between the sepsis subgroups
and the control group were calculated by drawing receiver operating
characteristic curves. Table 3
shows the cut-off levels. The sepsis group and sepsis subgroups had
similar cutoff levels vs. the control group. The optimal base excess
cut-off level between groups 1 and 2 was −5 mmol/L. Sensitivity,
specificity, positive predictive value (PPV) and negative predictive
value (NPV) values for base excess were 75, 91, 86, and 84%,
respectively (Table 3).
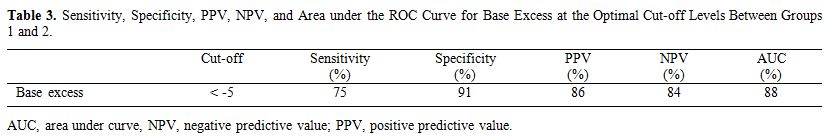 |
Table 3. Sensitivity,
Specificity, PPV, NPV, and Area under the ROC Curve for Base Excess at
the Optimal Cut-off Levels Between Groups 1 and 2. |
Discussion
Bacterial
sepsis is an important problem in very low birth weight (VLBW) infants
despite advances in neonatal intensive care and continues to be an
important cause of morbidity and mortality.[1-3] Blood
culture is the gold standard for diagnosing NS; however, 48-72 h are
required to obtain results. It is important to identify infected
neonates as early as possible, but the nonspecific clinical signs and
the absence of good diagnostic tests are obstacles to an early
diagnosis. Diagnostic markers are useful indicators of NS. Serial
measurements of infection markers can improve diagnostic sensitivity,
and the use of multiple markers can enhance diagnostic accuracy. Many
studies have evaluated various markers.[12,13]
However, sensitivity and specificity of hematological criteria such as
absolute neutrophil count, platelet counts, and immature to total
neutrophil ratio vary widely among studies.[4,14-16] Celik et al.[17]
reported the sensitivity, specificity, PPV, and NPV for CRP of 67, 97,
99, and 39% respectively, and for IL-6 of 72, 84, 95, and 42%,
respectively, with cut-off values of 4.82 mg/l and 24.65 pg/ml. Elawady
et al.[18] reported a sensitivity of 96%, specificity
of 100%, PPV of 96.2%, and NPV of 100% for neutrophil CD64, with
cut-off values of 45.8% and 46.0% in proven and clinical sepsis groups,
respectively. Dilli et al.[19] reported a sensitivity of 88.6% and NPV of 94%. Cetinkaya et al.[20] found sensitivities for CRP, PCT, and serum amyloid A of 72.3, 74.8, and 76.4%, respectively. Abdollahi et al.[21]
reported the simultaneous measurement of PCT, IL-6 and high
sensitive-CRP (hs-CRP) is more sensitive in the diagnosis of neonate
infections. They found that the combination of PCT and IL-6 had a
sensitivity of 88%, PCT and hs-CRP had a sensitivity of 82%. Patel et
al.[22] evaluated the role of serum PCT as a
biomarker of bacterial infection in acute sickle cell vaso-occlusive
crisis and they found that PCT value of >2ng/mL is indicative of
bacterial infection necessitating early antimicrobial therapy.
Presepsin, is another biomarker of sepsis, was also studied in the
diagnosis of NS; Poggi et al.[23] reported that its sensitivity, specificity, and AUC were 94%, 100%, and 0.972 respectively. Ozdemir AA et al.[24]
evaluated the efficacy of presepsin in the diagnosis of early onset NS
by comparing this with CPR and PCT. They found that CRP, PCT, and
presepsin had a sensitivity of 83, 67, and 80%, and a specificity of
75, 67, 75%, respectively, with cut-off values for presepsin of 539
pg/mL with an AUC of 0.772. Recently, Bellos et al.[25]
reported a meta-analysis about the diagnostic accuracy of presepsin in
NS. The findings of this meta-analysis suggest that presepsin may serve
as a promising biomarker in NS, given its sensitivity 0.91, specificity
0.97 (AUC 0.99). Our findings indicate that base excess values
decreased before the emergence of signs and symptoms of sepsis. We
found that the sensitivity, specificity, PPV, and NPV values for base
excess were 75, 91, 86, and 84%, respectively, with a cut-off value of
−5 mmol/L. We found no differences between the proven and clinical
sepsis and the gram-positive and -negative sepsis subgroups. Base
excess to predict sepsis in preterm newborns has not been reported.
This study is the first to show the base excess levels as evaluative of
early diagnosis of NS.
The development of metabolic acidosis
during NS has been attributed to progressive tissue ischemia resulting
from reduced oxygen delivery. Sepsis causes hemodynamic instability
through several processes, resulting in tissue hypoperfusion. Some
studies have shown that base excess is a prognostic factor in patients
who develop sepsis. Acidosis is a powerful marker of poor prognosis in
critically ill patients.[6-10,26] However, base excess for diagnosis of sepsis has not been investigated previously.
Several
routine blood gas analysis strategies are used in neonatal intensive
care units, and, so, an evaluation of blood gases should be made every
morning or at round visits. Blood gas analysis is performed using 0.2
ml of blood, which is also used for respiratory function analysis,
particularly in ventilated newborns. Several sepsis biomarkers have
been used for the early diagnosis of sepsis, but the use of more than
two markers simultaneously in the same infant is not possible due to
excessive blood loss. Therefore analysis of blood gases for the
diagnosis of sepsis may be a useful, simple and cost-effective method.
Our
study had several limitations due to its retrospective and
observational nature. Additionally, serum lactate levels were not
evaluated to confirm the diagnosis of acidosis.
Conclusion
The
results of this trial suggest that base excess < −5 mmol/L has 75%
predictability for diagnosing NS. Therefore, NS could be predicted
through a simple capillary blood gas analysis test. The clinician may
be alerted to an earlier evaluation for possible neonatal infection
prior to the development of sepsis. Capillary blood gas analysis is a
method commonly practiced in neonatal intensive care units. Because the
analysis requires a small amount of blood, it is superior to other
laboratory tests. If the base excess value is < −5 mmol/L with no
known underlying reason, close follow-up of the infant for NS may be
needed for an early diagnosis. Our findings should be confirmed by more
comprehensive controlled and prospective trials.
References
- Stoll BJ, Hansen N. Infections in VLBW infants:
studies from the NICHD Neonatal Research Network. Semin Perinatol
2003;27:293-301. https://doi.org/10.1016/S0146-0005(03)00046-6
- Stoll
BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth
weight neonates: the experience of the NICHD Neonatal Research Network.
Pediatrics 2002;110:285-91. https://doi.org/10.1542/peds.110.2.285 PMid:12165580
- Polin
RA, Denson S, Brady MT. Committee on Fetus and Newborn; Committee on
Infectious Diseases. Epidemiology and diagnosis of health
care-associated infections in the NICU. Pediatrics 2012;129:e1104-9. https://doi.org/10.1542/peds.2012-0147 PMid:22451708
- Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed 2004;89:F229-35. https://doi.org/10.1136/adc.2002.023838 PMid:15102726 PMCid:PMC1721679
- Døllner
H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis:
comparing C-reactive protein, interleukin-6, soluble tumour necrosis
factor receptors and soluble adhesion molecules. J Clin Epidemiol
2001;54:1251-7. https://doi.org/10.1016/S0895-4356(01)00400-0
- Kellum
JA. Metabolic acidosis in patients with sepsis: epiphenomenon or part
of the pathophysiology? Crit Care Resusc 2004;6:197-203. PMid:16556122
- Noritomi
DT, Soriano FG, Kellum JA, et al. Metabolic acidosis in patients with
severe sepsis and septic shock: a longitudinal quantitative study. Crit
Care Med 2009;37:2733-9. https://doi.org/10.1097/CCM.0b013e3181a59165 PMid:19885998
- Park
M, Azevedo LC, Maciel AT, et al. Evolutive standard base excess and
serum lactate level in severe sepsis and septic shock patients
resuscitated with early goal-directed therapy: still outcome markers?
Clinics (Sao Paulo) 2006;61:47-52. https://doi.org/10.1590/S1807-59322006000100009
- Smith
I, Kumar P, Molloy S, et al. Base excess and lactate as prognostic
indicators for patients admitted to intensive care. Intensive Care Med
2001;27:74-83. https://doi.org/10.1007/s001340051352 PMid:11280677
- Couto-Alves
A, Wright VJ, Perumal K, et al. A new scoring system derived from base
excess and platelet count at presentation predicts mortality in
paediatric meningococcal sepsis. Crit Care 2013;11;17:R68.
- Haque KN. Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med 2005;6:45-9. https://doi.org/10.1097/01.PCC.0000161946.73305.0A PMid:15857558
- Oncel MY, Dilmen U, Erdeve O, et al. Proadrenomedullin as a prognostic marker in neonatal sepsis. Pediatr Res 2012;72:507-12. https://doi.org/10.1038/pr.2012.106 PMid:22885414
- Oncel MY, Ozdemir R, Yurttutan S, et al. Mean platelet volume in neonatal sepsis. J Clin Lab Anal 2012;26:493-6. https://doi.org/10.1002/jcla.21552 PMid:23143634
- Da
Silva O, Ohlsson A, Kenyon C. Accuracy of leukocyte indices and
C-reactive protein for diagnosis of neonatal sepsis: a critical review.
Pediatr Infect Dis J 1995;14:362–6. https://doi.org/10.1097/00006454-199505000-00005 PMid:7638010
- Waliullah
SM, Islam MN, Siddika M, et al. Evaluation of simple hematological
screen for early diagnosis of neonatal sepsis. Mymensingh Med J
2010;19:41-7. PMid:20046170
- Manucha V,
Rusia U, Sikka M, et al. Utility of haematological parameters and
C-reactive protein in the detection of neonatal sepsis. J Paediatr
Child Health 2002;38:459-64. https://doi.org/10.1046/j.1440-1754.2002.00018.x PMid:12354261
- Celik
IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and
CRP in neonatal sepsis? J Clin Lab Anal 2010;24:407-12. https://doi.org/10.1002/jcla.20420 PMid:21089127
- Elawady
S, Botros SK, Sorour AE, et al. Neutrophil CD64 as a Diagnostic Marker
of Sepsis in Neonates. J Investig Med 2014;62:644-9. https://doi.org/10.2310/JIM.0000000000000060 PMid:24463977
- Dilli
D, Oğuz ŞS, Dilmen U, et al. Predictive values of neutrophil CD64
expression compared with interleukin-6 and C-reactive protein in early
diagnosis of neonatal sepsis. J Clin Lab Anal 2010;24:363-70. https://doi.org/10.1002/jcla.20370 PMid:21089165
- Cetinkaya
M, Ozkan H, Köksal N, et al. Comparison of serum amyloid A
concentrations with those of C-reactive protein and procalcitonin in
diagnosis and follow-up of neonatal sepsis in premature infants. J
Perinatol 2009;29:225-31. https://doi.org/10.1038/jp.2008.207 PMid:19078972
- Abdollahi
A, Shoar S, Nayyeri F, Shariat M. Diagnostic Value of Simultaneous
Measurement of Procalcitonin, Interleukin-6 and hs-CRP in Prediction of
Early-Onset Neonatal Sepsis. Mediterr J Hematol Infect Dis.
2012;4:e2012028. https://doi.org/10.4084/mjhid.2012.028 PMid:22708043 PMCid:PMC3375671
- Patel
DK, Mohapatra MK, Thomas AG, Patel S, Purohit P. Procalcitonin as a
biomarker of bacterial infection in sickle cell vaso-occlusive crisis.
Mediterr J Hematol Infect Dis. 2014;6:e2014018. https://doi.org/10.4084/mjhid.2014.018
- Poggi
C, Bianconi T, Gozzini E, Generoso M, Dani C. Presepsin for the
detection of late-onset sepsis in preterm newborns. Pediatrics.
2015;135:68-75. https://doi.org/10.1542/peds.2014-1755 PMid:25511124
- Ozdemir
AA, Elgormus Y. Diagnostic Value of Presepsin in Detection of
Early-Onset Neonatal Sepsis. Am J Perinatol. 2017;34:550-556. https://doi.org/10.1055/s-0036-1593851 PMid:27825177
- Bellos
I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The
diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis.
Eur J Pediatr. 2018;177:625-632. https://doi.org/10.1007/s00431-018-3114-1 PMid:29476345
- Parry
G, Tucker J, Tarnow-Mordi W. UK Neonatal Staffing Study Collaborative
Group. CRIB II: an update of the clinical risk index for babies score.
Lancet 2003;24;361:1789-91. https://doi.org/10.1016/S0140-6736(03)13397-1
P]


