 |
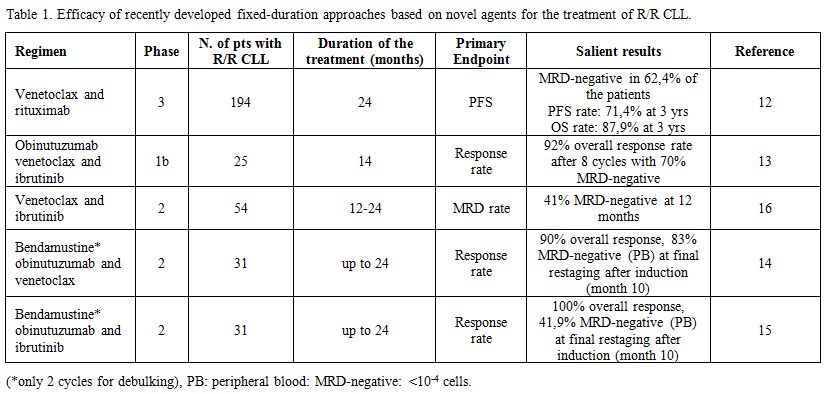
Table 1. Efficacy of recently developed fixed-duration approaches based on novel agents for the treatment of R/R CLL. |
Recently, the results of the phase-3 MURANO trial comparing venetoclax for a maximum of 24 months associated with rituximab (VR) for the first six months with the classical bendamustine and rituximab (BR) regimen given for six months in R/R CLL have been reported.
Ninety-two % of patients responded to VR, and 62% attained an undetectable MRD (uMRD) in the PB at six months, compared to 12% in the BR cohort. Sixty-four % of 130 patients who completed the two years of planned treatment with venetoclax had uMRD. Patients with uMRD or detectable MRD at low levels (10-2 to 104 residual cells) had a longer PFS than the remaining patients.[17] At a median of 9.9 months after cessation of venetoclax only 12% of 130 patients who completed the planned treatment progressed and 90% of all patients assigned to the VR arm had not undergone a further treatment for their disease at two years.[12]
In the intention to treat analysis, the VR regimen significantly improved PFS (HR: 0.16; IC 95%: 0.12-0.23; p <0.0001) and OS (HR: 0.50; 95% CI: 0.30-0.85; p = 0.0093; OS rate at 3 years: 87.9% vs 79.5%) compared to BR, which represents one of most widely employed CIT regimen in R/R CLL. Noteworthy, though crossover to venetoclax at progression in the BR arm was not pre-planned, the majority of patients received effective salvage regimen with new drugs.[17]
These findings show for the first time that a fixed-duration treatment may achieve deep and durable response and improve survival in R/R CLL, and are likely to have a significant impact in the treatment of R/R CLL in the clinical practice as the regulatory agencies FDA and EMA granted this regimen marketing authorization.