Elisabetta Abruzzese1, Alberto Bosi2, Massimo Breccia3, Mariella D'Adda4, Nicola Di Renzo5, Anna Marina Liberati6, Raffaele Porrini7, Ester Maria Orlandi8, Fabrizio Pane9, Ester Pungolino10, Federica Sorà11, Fabio Stagno12, Ginny P. Sen13, Fabiana Gentilini14, Francesco De Solda14 and Carlo Gambacorti-Passerini15.
1
S. Eugenio Hospital, Roma, Italy.
2 U.O. di Ematologia, Azienda Ospedaliera Universitaria Careggi, Firenze, Italy.
3 Azienda Policlinico Umberto I-Università Sapienza, Roma, Italy.
4 Azienda Ospedaliera Spedali Civili di Brescia, Italy.
5 U.O. di Ematologia e Trapianto di Cellule Staminali P.O "Vito Fazzi" - Lecce.
6 Università degli Studi di Perugia, - A.O. Santa Maria di Terni, Italy.
7 Ospedale Sant'Andrea Roma, Italy.
8 Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy.
9 University of Naples Federico II, Italy.
10 A.O. Ospedale Niguarda Ca' Granda, Milano, Italy.
11 UOC di Ematologia, Policlinico Universitario 'A. Gemelli', Roma, Italy.
12 Divisione Clinicizzata di Ematologia, AOU Policlinico – V. Emanuele Catania, Italy.
13 ICON Clinical Research, San Diego, California, USA.
14 Bristol-Myers Squibb, Rome, Italy.
15 Azienda Ospedaliera San Gerardo, University of Milano Bicocca, Monza, Italy.
Published: May 1, 2019
Received: October 10, 2018
Accepted: March 28, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019025 DOI
10.4084/MJHID.2019.025
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective: While
tyrosine kinase inhibitors (TKIs) have transformed CP-CML management,
limited data exist on their use in clinical practice.
Methods:
SIMPLICITY (NCT01244750) is an observational study in CP-CML patients,
exploring first line (1L) TKI use and management patterns in the US and
Europe. Over half of the patients recruited in Europe are from Italy
(n=266). This is an analysis of the Italian cohort and a comparison
with the rest of the European SIMPLICITY population. Baseline
demographic, factors influencing the choice of first-line TKI, response
monitoring patterns and predictors of monitoring, and treatment
interruptions, discontinuations and switching by index TKIs are
presented for the Italian cohort in the first year of treatment and
compared with that for the overall European SIMPLICITY cohort.
Results:
Italian patients received 1L imatinib (IM; retrospective [(n=31];
prospective [n=106]), dasatinib (DAS; n=56) or nilotinib (NIL; n=73).
Documented cytogenetic response monitoring by 12 months was lower than
expected, but almost all patients had documented molecular response
monitoring. Fewer patients discontinued first-line TKI by 12 months in
Italy compared with the rest of the European SIMPLICITY population
(p=0.003). Of those with ≥12 months follow-up since the start of 1L
TKI, only 7.1% (n=19) of Italian patients switched to a second-line
TKI, a third less than in the rest of the European SIMPLICITY
population. Of interest, intolerance as opposed to resistance, was the
main reason for switching.
Conclusions:
This analysis provides valuable insights into management and treatment
patterns in Italian patients with CML within routine clinical practice.
|
Introduction
Over
the last two decades, tyrosine kinase inhibitors (TKIs) have
transformed the management of chronic-phase chronic myeloid leukaemia
(C-P CML) from a terminal disease to a chronic illness.[1,2] Survival
rates in patients with newly-diagnosed C-P CML are thus approximating
to rates in age-adjusted general populations.[3-5] Imatinib
(Gleevec®/Glivec®, Novartis), dasatinib (Sprycel®, Bristol-Myers
Squibb) and nilotinib (Tasigna®, Novartis) are recommended as
first-line TKI therapy for C-P CML.[6-8] Once initiated, careful
monitoring of cytogenetic response (CyR) and molecular response (MR),
as well as adjustments in therapy, using time-based ’milestone’
testing, is necessary to ensure optimal outcomes.[9] While the efficacy
of TKIs in the management of CML has been demonstrated, some patients
will either experience intolerance, achieve a suboptimal response, or
fail treatment. In such patients, TKI treatment may be adjusted by dose
modification, treatment interruption, or discontinuation, followed by
switching to the next most appropriate TKI.[7,10]
European
LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN)
have published evidence-based recommendations for the management of
CML. Of particular importance for the haematological community is to
determine how closely these recommendations are followed, to identify
any influential factors that may be involved, and to understand the
impact that compliance with practice recommendations has on patient
outcomes. Insights on the rationale for TKI treatment patterns in
routine clinical practice may also better inform how treatment
decisions are made.
SIMPLICITY (NCT01244750) is an ongoing
observational study of patients with C-P CML seen in routine clinical
practice receiving first-line treatment with imatinib, dasatinib or
nilotinib. The primary objective of SIMPLICITY is to understand TKI use
and management in clinical practice. Information derived from the whole
SIMPLICITY population has shown that monitoring practices are not
entirely in accordance with the published recommendations of ELN and
NCCN. Patients may not be monitored by CyR or MR as frequently as
recommended.[11] Almost a quarter of all patients who were followed for
at least 12 months had discontinued or switched first-line TKI therapy
during the first 12 months, and intolerance or resistance was the most
common primary reason for discontinuation and switching of first-line
TKI.[12]
In addition to data reported for SIMPLICITY, there are
several other studies of patients with CML treated within routine
clinical practice;[13-25] however, most are of patients treated with
imatinib only, and of patients who are elderly with severe
comorbidities. While these studies support the use of imatinib in an
older population, the results align with those from the whole
SIMPLICITY population, where treatment and monitoring practices are not
entirely in accordance with guidelines. Importantly, studies observing
response monitoring and TKI treatment patterns in patients with CML
treated in Europe are limited. The need to follow these patterns is
crucial to identify any discord between guidelines and clinical
practice and to understand the reasons behind these discordances fully
so that the management of CML in the routine clinical practice setting
can be improved.
Here we report SIMPLICITY data for the first year
of treatment of the Italian population (data cut: September 06, 2016).
SIMPLICITY includes 241 sites (Europe, n=91; US; n=150). Of the 91
European sites included in SIMPLICITY, Italian sites make up almost a
third of these (29/91). For patients with C-P CML, the first year of
treatment – and how they respond to treatment during it – is of
particular relevance. Treatment response and tolerance are likely to
influence adherence, which ultimately has an impact on long-term
clinical outcomes.[6,7] Here, we report baseline demographic and
clinical characteristics, factors influencing the choice of first-line
TKI, response monitoring patterns (CyR and MR), and predictors of
monitoring, within the Italian population. We also report on patterns
of treatment interruptions, discontinuations and switching, stratified
by index TKI, including the reasons for discontinuation and switching
observed in these patients. These findings are compared with those for
the rest of the SIMPLICITY European population excluding Italian
patients. To our knowledge, this article is the first to report on
management patterns, and TKI use in patients with C-P CML treated in an
observational setting in Italy.
Material and Methods
Study design and patient enrolment.
The design of SIMPLICITY has been described previously.[11] It includes
three prospective cohorts of patients newly diagnosed with CP-CML, ≥18
years of age at the time of diagnosis, receiving first-line therapy
with imatinib, dasatinib or nilotinib on or after October 01, 2010, and
a retrospective imatinib cohort (January 02, 2008- September 30, 2010).
Study sites include academic and community practices in Italy.
Community practices are defined as small-size practices run by an
independent physician, or group of physicians, who offer patient care
on a local or countywide basis. Academic centres are defined as
large-size, hospital-based clinics (includes both public and private
practice), cancer centre or universities), including centres of
excellence, offering care on a regional or national basis. Patients
involved in ongoing interventional CML clinical trials were excluded.
The study protocol was reviewed and approved by the relevant
institutional review boards, and patient consent was obtained. Data
were collected using an electronic case report form (eCRF).
Demographic data collection.
Baseline demographics include data on patient comorbidities derived
from a defined checklist of 15 system organ classes, including
cardiovascular (CV), respiratory, gastrointestinal, and
endocrine/metabolic disorders. The total number of baseline
comorbidities is defined by a total count of body systems/organ classes
affected by comorbid conditions.
Physicians’ selection of first-line TKI.
Physicians were asked to record the primary reason for their choice of
first-line TKI (namely: familiarity with TKI, cost efficiency, comorbid
conditions, effectiveness, tolerability, dosing schedule or other).
Response monitoring.
Testing for CyR is based either on chromosome banding analysis (CBA) or
fluorescence in situ hybridisation (FISH). CyR monitoring is
categorised according to whether analysis was done with a date present
or not done. CyR monitoring that was done with a date present was
further classified into results available (excludes data with the
reported number of evaluated nuclei or number of examined metaphases
but missing FISH/bone marrow %Ph+ cells) or not (may include data with
known number of evaluated nuclei or number of examined metaphases). CyR
monitoring may concern FISH or bone marrow data with missing testing
dates and may include patients who were not tested due to progression.
Quantitative polymerase chain reaction (qPCR) was used for MR and was
recorded. The vast majority (94%) of patients were monitored based on
the International Scale (IS). MR monitoring is categorised according to
whether analysis was done with a date present (includes tests with
recorded dates that are available on the IS, available not on IS or
unavailable) or not done (no time reported). Patients with at least 3,
6, and 12 months of follow-up, since initiation of first-line TKI,
respectively, underwent to testing for CyR or MR with a frequency,
respectively, of 3, 6, and 12 months and to assessments performed
between ≥30 days from baseline and each respective time-point; the
reporting of ‘any test done’ includes MR or CyR assessments during the
specified timeframe. CyR and MR monitoring were analysed for the
selected population, for which there was a follow-up of ≥12 months
since initiation of index TKI, by year of TKI initiation.
Treatment patterns.
TKI treatment changes of the first year since initiating first-line
TKI, are summarised and include treatment interruptions or first-line
discontinuations, duration of treatment interruptions and the primary
reason for discontinuation of first-line TKI within the first year.
‘Treatment interruption’ was defined as a gap in treatment of >1 day
before restarting the same TKI. ‘Treatment discontinuation’ was defined
as cessation of TKI treatment that did not qualify as a treatment
interruption. Discontinuations just before data download (within 60
days) are considered treatment interruptions. TKI switch is defined as
a discontinuation of a first-line TKI within one year, followed by
initiation of a second-line TKI. Patients for whom a date of first-line
TKI discontinuation is missing but who switched to a second-line TKI
within one year of initiating first-line TKI are counted as
discontinuations. TKI treatment changes for patients who switched to a
second-line TKI within one year are summarised, including information
on the second-line TKI, days from first- to second-line TKI, and the
primary reason for switching. Intolerances leading to discontinuation
are presented for those patients who switched during the first year
since initiating first-line TKI.
Events concurrent with TKI
treatment interruptions were defined as events that occurred between
the TKI start date (and two weeks before the date of treatment
interruptions) and the end of the treatment interruption window,
signified by the start of the same TKI. The same event was summarised
once per patient and per unique event, date if concurrent with multiple
TKI treatment interruptions.
Statistical analyses.
Descriptive statistics were presented and P values calculated using a
chi-square test for categorical comparisons and Fisher’s exact test, in
the case of low cell counts; no corrections were made for multiple
comparisons. Saturated multivariable logistic regression models were
performed separately for Italy and for all other European countries
included in SIMPLICITY, to assess predictors of whether or not CyR or
MR monitoring was done among patients with at least 12 months of
follow-up since initiating a first-line TKI. The saturated model
included the following predictors: age at diagnosis, sex, practice
type, first-line TKI, Eastern Cooperative Oncology Group (ECOG)
performance status, and an indicator of whether patients were still on
their first-line TKI at the end of 12 months’ follow-up.
Saturated
multivariable logistic regression models were also performed to assess
predictors of discontinuation and switching. The following predictors
were included in the models: age at diagnosis, sex, practice type,
first-line TKI, total comorbidity counts, ECOG performance status, and
Sokal category.
Results
Study population.
1,242 patients were enrolled prospectively into the study between
October 01, 2010 and September 06, 2016 (data download) and 252
patients retrospectively. Of the 482 patients enrolled at the European
sites, 266 (55%) were recruited, at 29 sites across Italy (Supplemental
Figure S1). Most of these patients (n=249; 94%) were enrolled in the
study through academic centres. Patients received first-line imatinib
(retrospective [n=31]; median follow-up [interquartile range; IQR] 60.2
[59.4–61.1] months), prospective imatinib (n=106; median follow-up
[IQR] 54.0 [48.0–59.5] months), dasatinib (n=56; median follow-up [IQR]
39.4 [31.1–46.4] months) or nilotinib (n=73; median follow-up [IQR]
38.5 [27.0–50.9] months).
Patient demographics are shown in Table 1.
The overall median age (IQR; min., max.) of Italian patients at the
time of initiation of first-line treatment was 57.1 (44.9–69.6; 17.7,
90.0) years. Italian patients in the dasatinib cohort were older than
those in the imatinib (retrospective and prospective) and nilotinib
cohorts (P=0.01). Demographics and clinical characteristics of the
Italian population were similar to those reported for the rest of the
European SIMPLICITY population, except the fact that Italian patients
had fewer missing Sokal (20% vs. 43%, respectively) and Hasford (28%
vs. 43%, respectively) data.
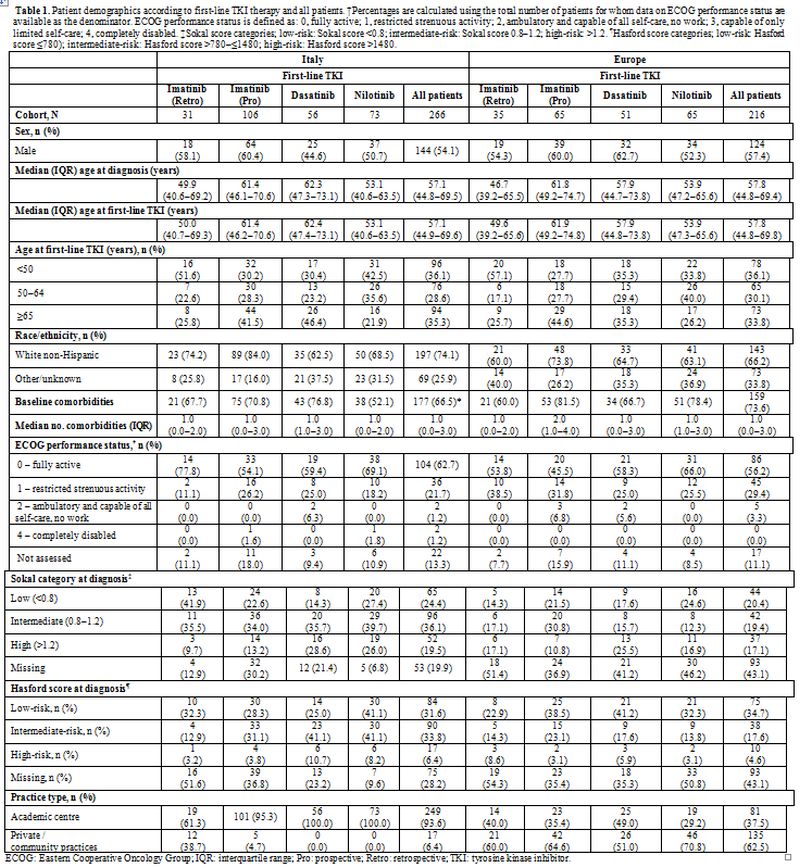 |
Table 1. Patient
demographics according to first-line TKI therapy and all patients.
†Percentages are calculated using the total number of patients for whom
data on ECOG performance status are available as the denominator. ECOG
performance status is defined as: 0, fully active; 1, restricted
strenuous activity; 2, ambulatory and capable of all self-care, no
work; 3, capable of only limited self-care; 4, completely disabled.
‡Sokal score categories; low-risk: Sokal score <0.8;
intermediate-risk: Sokal score 0.8–1.2; high-risk: >1.2. ¶Hasford
score categories; low-risk: Hasford score ≤780); intermediate-risk:
Hasford score >780–≤1480; high-risk: Hasford score >1480. |
Of
the total patients, 41% (n=110) had CV comorbidities (imatinib
retrospective: 36% [n=11]; imatinib prospective: 46% [n=49]; dasatinib:
48% [n=27]; nilotinib: 32% [n=23]). These results are similar to those
observed in Europe with some between-TKI variations (overall: 44%
[n=94]; imatinib retrospective: 34% [n=12]; imatinib prospective: 55%
[n=36]; dasatinib: 35% [n=18]; nilotinib: 43% [n=28]).
Physicians’ selection of first-line TKI.
The primary reason cited by the treating physician for selecting the
first-line TKI was perceived ’effectiveness’ in both Italy and the rest
of the European SIMPLICITY population (35% and 46%, respectively).
Other reasons that were primary drivers for treatment choice in Italy
and the rest of the European SIMPLICITY population included familiarity
with TKI (15% and 13%, respectively), cost efficiency (19% and 14%,
respectively) and the presence of comorbidities (18% and 10%,
respectively).
Response monitoring patterns.
Among patients followed for at least 12 months, the median (IQR) time
from initiation of first-line TKI to the end of follow-up was 50.5
(36.1–59.1) months and was comparable with the other European countries
(47.2 [34.7–58.2] months). All Italian patients (100%), and 97% of the
rest of the European SIMPLICITY population had documentation of
monitoring for either CyR or MR by 12 months.
CyR monitoring patterns. The proportion of patients with documentation of CyR monitoring increased, as expected, with longer patient follow-up (Table 2).
By 3 months, the percentage of patients who had documentation of CyR
was low in both Italy and the rest of the European SIMPLICITY
population (25% and 16%, respectively By 12 months, a greater
proportion of Italian patients had documentation of CyR compared with
the European populations (80% vs. 53%; P<0.001) and the proportion
of patients with ‘not done/recorded’ status decreased for both
populations. Of those patients with documentation of CyR, similar
proportions were classified with ‘results available’ in the Italian and
European populations (95% and 89%).
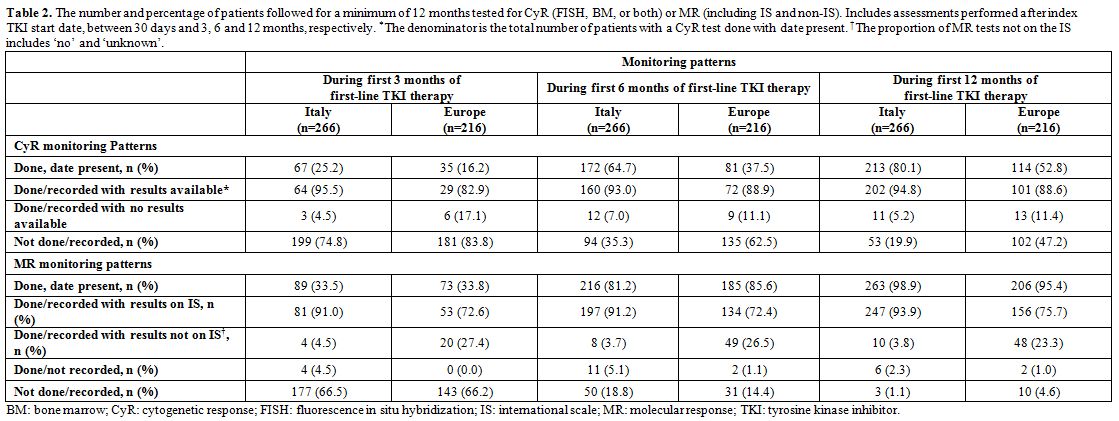 |
Table 2. The number and
percentage of patients followed for a minimum of 12 months tested for
CyR (FISH, BM, or both) or MR (including IS and non-IS). Includes
assessments performed after index TKI start date, between 30 days and
3, 6 and 12 months, respectively. *The denominator is the total number
of patients with a CyR test done with date present. †The proportion of
MR tests not on the IS includes ‘no’ and ‘unknown’. |
MR monitoring patterns. The proportion of patients with documentation of MR monitoring increased, as expected, with longer patient follow-up (Table 2).
By three months, the proportion of patients who had documentation of MR
was low in both Italy and Europe (34% for both). By 12 months, most
patients in the Italian and European populations had MR monitoring (99%
and 95%, respectively) and the proportion of patients with ‘not
done/recorded’ status decreased for both populations. Among patients
tested for MR by 12 months, a greater proportion of Italian patients
had MR assessments on the IS, compared with those in the rest of Europe
(94% vs 76%; P<0.001). This was most likely due to haematological
centres in Italy having better access to referral labs through the
LabNet network than other centres in Europe.
CyR and MR monitoring stratified by year of first-line TKI initiation in SIMPLICITY. Figure 1
shows the proportion of Italian patients from SIMPLICITY with
documented response monitoring throughout the study. Documentation of
CyR monitoring decreased somewhat, while that for MR monitoring on the
IS remained steady overall (90–100%) between 2008 and 2015, except in
2011, when the rate was lower.
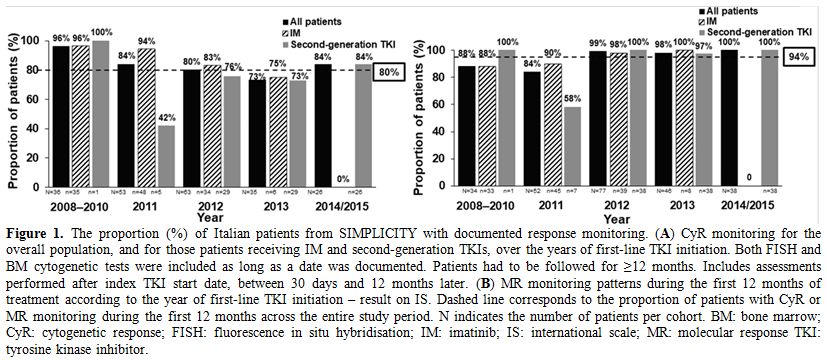 |
Figure 1. The proportion
(%) of Italian patients from SIMPLICITY with documented response
monitoring. (A) CyR monitoring for the overall population, and for
those patients receiving IM and second-generation TKIs, over the years
of first-line TKI initiation. Both FISH and BM cytogenetic tests were
included as long as a date was documented. Patients had to be followed
for ≥12 months. Includes assessments performed after index TKI start
date, between 30 days and 12 months later. (B) MR monitoring patterns
during the first 12 months of treatment according to the year of
first-line TKI initiation – result on IS. Dashed line corresponds to
the proportion of patients with CyR or MR monitoring during the first
12 months across the entire study period. N indicates the number of
patients per cohort. BM: bone marrow; CyR: cytogenetic response; FISH:
fluorescence in situ hybridisation; IM: imatinib; IS: international
scale; MR: molecular response TKI: tyrosine kinase inhibitor. |
Predictors of monitoring.
Logistic regression analysis could not be performed for the Italian
population because all patients in the cohort had documentation of
monitoring for either CyR or MR by 12 months. In the rest of the
European SIMPLICITY population, the model showed that there were no
statistically significant predictors of monitoring.
Treatment Interruptions.
Differences in treatment interruptions were observed between the
Italian and the rest of the SIMPLICITY European population, as well as
between first-line TKIs. Of the patients in Italy who had ≥12 months of
follow-up since initiating first-line TKI, 16.2% (n=43) had a treatment
interruption within 1 year of initiating first-line TKI, compared with
a slightly lower proportion (11.1%; n=24) in the rest of the SIMPLICITY
European population. For both Italian and the rest of the SIMPLICITY
European population, the proportion of patients interrupting first-line
TKI treatment was greatest in the imatinib prospective cohort (22.6%
[n=24] and 13.8% [n=9], respectively) vs. other cohorts (imatinib
retrospective: 16.1% [n=5] and 8.6% [n=3], respectively; dasatinib:
12.5% [n=7] and 9.8% [n=5]; nilotinib: 9.6% [n=7] and 10.8% [n=7]).
For
patients in Italy, the median duration of treatment interruption (IQR)
was 24.0 (14.0–118.0) days and was longer in comparison with the rest
of the SIMPLICITY European population (14.0 [10.0–36.5] days). Patients
in Italy receiving first-line imatinib (prospective) had the shortest
median duration of treatment interruption (16.5 [12.0–52.5] days),
whilst those receiving first-line dasatinib had the longest median
duration of treatment interruption (124.0 [28.0–209.0] days); the
results were different in comparison with the results for the rest of
the SIMPLICITY European population (imatinib prospective: 12.0
[10.0–31.0] days; dasatinib: 12.0 [9.0–14.0] days). Similarly, there
were disparities between Italy and the rest of the SIMPLICITY European
population in regard to the median duration of treatment interruption
for imatinib (retrospective; 18.0 [7.0–32.0] vs. 50.0 [21.0–270.0]
days, respectively) and nilotinib (65.0 [20.0–152.0] vs. 20.0
[13.0–30.0] days, respectively) cohorts.
A total of 91 events were
recorded as concurrent with TKI treatment interruption in the first
year of treatment in Italy, the most common of which were
thrombocytopenia (16 events) and neutropenia (11 events). In the rest
of the SIMPLICITY European population, more than half the number of
events reported in Italian patients (41 events) were recorded as
concurrent with TKI treatment interruption, the most common being
thrombocytopenia (6 events).
Treatment discontinuations. Treatment discontinuations are presented in Figure 2.
A smaller proportion of patients in Italy discontinued their first-line
TKI during the first year since initiating first-line TKI compared with
those in the rest of the European SIMPLICITY population (13.2% vs.
23.6%; P=0.003). In Italy, most patients remained on first-line TKI for
≥12 months (86.8%; n=231); results were similar for the rest of the
European SIMPLICITY population (76.4%; n=165). In the first year of
treatment in Italy, the median time to discontinuation of first-line
TKI (IQR) was 3.9 (1.6–7.0) months. For imatinib (retrospective),
imatinib (prospective), dasatinib and nilotinib, median times (IQR)
were, respectively, 2.3 (1.7–3.6), 4.6 (1.8–7.8), 4.6 (1.6–5.7) and 5.5
(1.3–7.0) months. In the rest of the European SIMPLICITY population, in
the first year of treatment, the median time to discontinuation of
first-line TKI (IQR) was 3.7 (1.6–8.4) months. For imatinib
(retrospective), imatinib (prospective), dasatinib and nilotinib,
median times to discontinuation (IQR) were, respectively, 7.6
(4.6–10.5), 3.6 (2.1–8.5), 4.4 (1.7–7.2) and 1.4 (0.9–4.1) months.
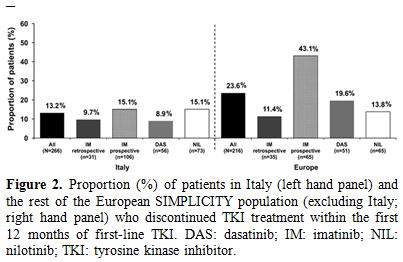 |
Figure 2. Proportion (%)
of patients in Italy (left hand panel) and the rest of the European
SIMPLICITY population (excluding Italy; right hand panel) who
discontinued TKI treatment within the first 12 months of first-line
TKI. DAS: dasatinib; IM: imatinib; NIL: nilotinib; TKI: tyrosine kinase
inhibitor. |
Intolerance was
the most common primary reason for discontinuation of first-line
TKI, reported in 70.4% (n=19; imatinib retrospective: 100% [n=1];
imatinib prospective: 53.3% [n=8]; dasatinib: 66.7% [n=2]; nilotinib:
100.0% [n=8]) of all patients who discontinued; this was slightly lower
than in the rest of the European SIMPLICITY population (75.6% [n=31];
imatinib retrospective: 100.0% [n=2]; imatinib prospective: 66.7%
[n=16]; dasatinib: 100.0% [n=8]; nilotinib: 71.4% [n=5]). Primary
resistance was the second most common primary reason for
discontinuation of first-line TKI, reported in 14.8% (n=4) of all
patients who discontinued: all four patients were from the imatinib
prospective cohort. Results were similar observations for the rest of
the European SIMPLICITY population, in which 7.3% (n=3) of all patients
discontinued first-line TKI because of primary resistance: all three
patients were from the imatinib prospective cohort. Other reasons for
discontinuation in the Italian population included acquired resistance
(7.4% [n=2]), insurance/financial reasons (3.7% [n=1]) and unrelated
medical conditions (3.7% [n=1]).
Predictors of discontinuation.
Logistic regression analysis showed that there were no statistically
significant predictors of first-line TKI discontinuation in Italian
patients. However, in the rest of the European SIMPLICITY population,
In Europe, however, female vs. male patients were more likely to
discontinue first-line TKI treatment (odds ratio [OR; 95% CI] 2.60
[1.26, 5.36]; P=0.01), as were patients on prospective imatinib vs.
dasatinib (OR [95% CI] 3.04 [1.21, 7.62]; P=0.018).
TKI switching patterns.
Of the Italian patients with ≥12 months of follow-up since initiating
first-line TKI, 7.1% (n=19) switched to a second-line TKI – a smaller
proportion than in the rest of the European SIMPLICITY population,
where almost three times as many patients switched to a second-line TKI
(20.4% [n=44]). In Italy, a greater proportion of patients initiating
prospective imatinib as a first-line TKI switched to a second-line TKI
within 12 months, compared with the imatinib retrospective, dasatinib
and nilotinib cohorts (13.2% [n=14] vs. 0% [n=0] vs. 3.6% [n=2] vs.
4.1% [n=3], respectively). Of those who switched from imatinib
prospective, six (42.9%) patients switched within the first 3 months
and six (42.8%) switched between 6 and 12 months of first-line TKI
initiation. Patients on first-line dasatinib switched either between 3
and 6 months (50.0% [n=1]) or 6 and 9 months (50.0% [n=1]). Of those
who switched from nilotinib, one patient (33.3%) switched within the
first 3 months and two patients (66.7%) switched between 6 and 9 months
of initiating a first-line TKI.
The median time (IQR) to switch
from first-line TKI in Italy was 172.0 (73.0–239.0) days. Between-TKI
differences were noted for the median time (IQR) to switch from
first-line TKI: this was longest in the nilotinib cohort (239.0
[61.0–255.0] days), followed by the dasatinib cohort (163.5
[140.0–187.0] days), and finally the imatinib prospective cohort (149.0
[73.0–214.0] days). In the rest of the European SIMPLICITY population,
the median time (IQR) to switch from first-line TKI was 135.5
(65.5–265.0) days. Between-TKI differences were also noted: the longest
time was for the imatinib retrospective cohort (292.5 [235.0–350.0]
days), followed by the dasatinib (181.0 [134.0–281.0] days), imatinib
prospective (131.5 [73.0–262.0] days) and nilotinib (44.0 [19.0–137.0]
days) cohorts.
The switching patterns (Figure 3)
were largely comparable between Italy and the rest of the European
SIMPLICITY population. In Italy, intolerance was the most common
primary reason for discontinuation of a first-line TKI and switching to
a second-line TKI, reported in 56.3% (n=9; imatinib retrospective: 0%;
imatinib prospective: 46.2% [n=6]; dasatinib: 100.0% [n=1]; nilotinib:
100.0% [n=2]) of all patients who discontinued first-line TKI; this was
lower in comparison with the rest of the European SIMPLICITY population
(76.3% [n=29]; imatinib retrospective: 100.0% [n=2]; imatinib
prospective: 63.6% [n=14]; dasatinib: 100.0% [n=8]; nilotinib: 83.3%
[n=5]). Table 3 shows the
events concurrent with treatment discontinuation and switching in
Italy. Primary resistance was the second most common primary reason for
discontinuation of first-line TKI and switching to a second-line TKI in
Italy, reported in 25.0% (n=4) of all patients who discontinued: all
four patients were from the imatinib prospective cohort.
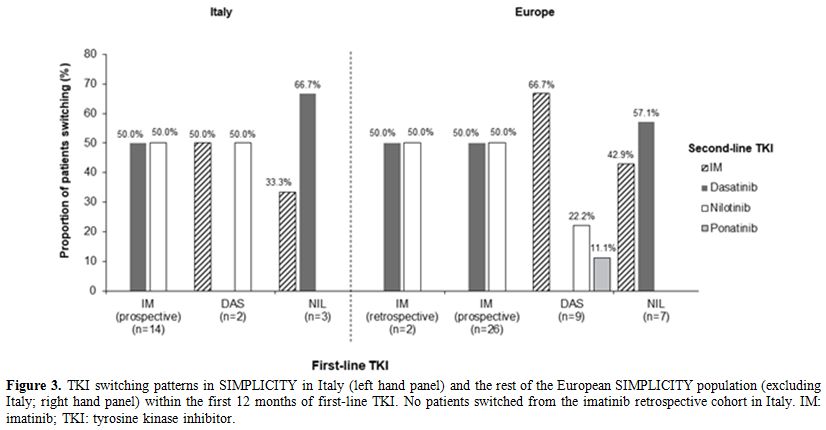 |
Figure 3. TKI switching patterns in
SIMPLICITY in Italy (left hand panel) and the rest of the European
SIMPLICITY population (excluding Italy; right hand panel) within the
first 12 months of first-line TKI. No patients switched from the
imatinib retrospective cohort in Italy. IM: imatinib; TKI: tyrosine
kinase inhibitor. |
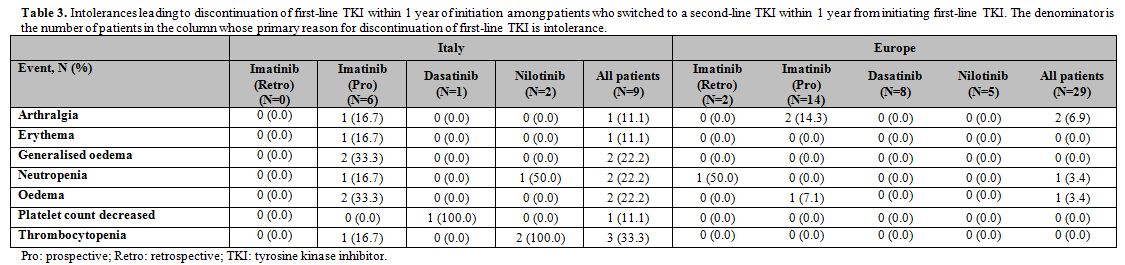 |
Table 3. Intolerances leading to
discontinuation of first-line TKI within 1 year of initiation among
patients who switched to a second-line TKI within 1 year from
initiating first-line TKI. The denominator is the number of patients in
the column whose primary reason for discontinuation of first-line TKI
is intolerance. |
Predictors of Switching.
Logistic regression analysis showed that patients who were on imatinib
prospective were more likely to switch from a first-line TKI than those
on dasatinib (OR=5.62, P=0.036). Patients who had three or more
comorbidities were less likely to switch from first-line TKI than those
who had no comorbidities (OR=0.17, P=0.048). In the rest of the
European SIMPLICITY population, female vs. male patients (OR=2.11,
P=0.060) and those prospectively treated with imatinib vs dasatinib
(OR=2.97, P=0.025) were more likely to switch from a first-line
TKI..
Discussion
Here,
we report response monitoring and TKI treatment patterns in patients
with C-P CML treated in sites in Italy from the SIMPLICITY study and
make detailed comparisons with the rest of the European SIMPLICITY
population.
This manuscript furthers our knowledge by focusing
on an analysis of the Italian cohort of 266 patients, which is the
largest European cohort within the SIMPLICITY population and,
importantly, is the first to report on management practices of the
first year of TKI therapy. These data, from a country with high
first-line usage of imatinib, dasatinib and nilotinib, reveals valuable
insights into treatment and monitoring patterns in CP-CML patients by
exploring TKI choice, switching pattern and reason for the switching in
the first 12 months of TKI therapy.
As with the SIMPLICITY
population as a whole,[11] SIMPLICITY patients in Italy are older
(median 57.1 years) than the CML patients studied in the three pivotal
clinical trials of the TKIs under investigation in newly diagnosed C-P
CML (median 46.0–50.0 years), and in the three investigator-initiated
randomised controlled trials evaluating use of imatinib (51.0–53.0
years).[6,26-31] Unsurprisingly,
given the interdependent nature of age and comorbidity, two-thirds of
the Italian population had baseline comorbidities, indicating that
Sokal and Hasford prognostic measurements are frequently carried out in
Italy. Prognostic scores are of crucial importance, and guidelines
recommend determining a patient’s score before making any first-line
treatment decisions.[7] While risk score was not an
individual category on the eCRF when capturing the rationale of
treating physicians for TKI selection; it may be considered under the
wider category of perceived effectiveness, which was the primary reason
for treatment selection.
Management of C-P CML requires early
and routine monitoring of CyR and MR and it is essential to identify
whether or not patients are responding to treatment.[6,7]
In this analysis, the proportion of patients who were monitored by CyR
and MR by 3 months was low. It is possible that, for a proportion of
patients, testing had been carried out but with no date recorded and
thus falling into the ‘not done’ category. Similarly, for some
patients, testing may not have been possible for reasons of disease
progression, or initial temporary drug interruption/reduced dosage, or
other patient-related factors so that testing may have occurred outside
of the strict 3-month timeframe.
Standardised MR assessments are gaining increased recognition for their importance;[32,33]
in SIMPLICITY this was particularly evident for the Italian population,
demonstrating accordance with the ELN recommendations, regarding MR
testing on the IS. Most patients were enrolled in the study through
academic centres – a factor that may have influenced adherence to
treatment recommendations on MR testing on the IS. A network of more
than 50 standardised laboratories (LabNet) performs BCR-ABL analysis
for hematologic clinics, and the availability of this resource may have
also contributed to greater adherence to MR testing on the IS.
For
patients not responding to their first-line TKI, and those who
experience intolerance to treatment, guidelines recommend several
options: dose modification, treatment interruption, or discontinuation
followed by switching to the next most appropriate treatment.[6,7]
In Italy and Europe, the proportion of patients who interrupted their
first-line TKI was generally low, although a lower proportion of
patients discontinued first-line TKI in Italy, compared with the rest
of the European SIMPLICITY population. This finding may reflect
differences in management between sites in Italy and the rest of
Europe. In Italy, CML care is centralised: almost all haematologists
operate within hospitals, and TKIs can be dispensed only by hospital
pharmacies.[34] The significant variations observed
in duration of treatment interruptions between TKIs may result from
patients’ variability in speed of recovery, following any concurrent
adverse events.
Intolerance was the most common primary reason for
discontinuation of first-line TKI. The ELN and NCCN recommendations
suggest a clinical interpretation of BCR-ABL levels >10% at 3 months
before changing TKIs as result of resistance,[6,7]
since there is currently no evidence to demonstrate any advantage for
patients switching their TKI by 3 months because of BCR-ABL levels
>10%. The adherence to this “careful” approach, paired with a low
rate of early monitoring, could explain the higher percentage of
discontinuations due to intolerance compared with resistance. The
primary resistance observed in the imatinib (prospective) cohort is not
surprising, given that primary resistance to imatinib is generally seen
in 15–25% of patients.[7] In one instance, it was
reported that the discontinuation was due to the patient’s
insurance/financial reasons, and it is important to highlight that the
Italian healthcare system is a regionally based National Health Service
that provides universal coverage, mainly free of charge.[35]
Plausible
explanations for imatinib (prospective) being a predictor of switching,
in addition to primary resistance, include that patients who achieve a
suboptimal response, therefore, switch to a second-line TKI.
Additionally, as second-generation TKIs became more widely available,
and clinicians gained experience with them, further treatment options
were then available for patients who were imatinib-intolerant, or who
had suboptimal outcomes, increasing the likelihood of patients
switching from first-line imatinib. Interestingly, primary resistance
was not observed in the imatinib (retrospective) cohort, and this could
be a result of selection bias associated with the retrospective nature
of this cohort. Interpretation of switching patterns is not possible
from this study, due to low numbers of patients.
While
observational studies can capture the management of patients within the
routine clinical practice setting; they are associated with inherent
limitations, which need to be considered when interpreting results.[36]
Such limitations, regarding this study, include selection bias related
to the method of patient enrolment, as well as bias related to the year
of enrolment and choice of TKI. Results should be interpreted in the
context of shifting practices that may ultimately be influenced by
evolving treatment recommendations. An artefact of observational
studies is the capture of management practices over time. The update to
the ELN recommendations in 2013, which specifically concerned routine
response monitoring by MR,[6] could only influence
monitoring practices after that date, so this might explain the pattern
of observations reported. It is also worth noting that the European
SIMPLICITY population is not representative of Europe as a whole, with
the majority of patients enrolled in either Italy or Germany. Finally,
the numbers in the patients who switched from first-line TKI within the
first 12 months of treatment was small, which might be considered a
positive result, but caution needs to be taken when making such
inferences from the results, for the reasons stated above.
Monitoring
practices in Italy, and the rest of the European SIMPLICITY population,
are not in full accordance with treatment recommendations. These
results are consistent with those reported previously for the whole
SIMPLICITY population.[11,12] The detailed
information regarding the switching patterns and the reason for
switching during the first year of CML therapy are presented here for
the first time. These data provide insight into the "dynamic" real-life
picture of the CML population during the most important time-frame,
where patients are characterised and stabilised on the most appropriate
therapy according to their results and tolerability to current TKI
therapy. Future analyses will assess the relationship between response
monitoring patterns, TKI switching patterns and clinical response in
the SIMPLICITY population.
Acknowledgements
We
thank all SIMPLICITY study investigators, the patients who consented to
be part of the study and LATITUDE (AXON Communications) who provided
medical writing services on behalf of the authors and Bristol-Myers
Squibb Pharmaceuticals Ltd.
References
- Hochhaus, A, Educational session: managing chronic
myeloid leukemia as a chronic disease. Hematology Am Soc Hematol Educ
Program. 2011;2011:128-35. https://doi.org/10.1182/asheducation-2011.1.128 PMid:22160024
- Jabbour,
E, G Saglio, J Radich, H Kantarjian, Adherence to BCR-ABL inhibitors:
issues for CML therapy. Clin Lymphoma Myeloma Leuk. 2012;12(4):223-9. https://doi.org/10.1016/j.clml.2012.04.002 PMid:22633166 PMCid:PMC4428159
- Gambacorti-Passerini,
C, L Antolini, F-X Mahon, F Guilhot, M Deininger, C Fava, A Nagler, C
Della Casa, E Morra, E Abruzzese, A D'Emilio, F Stagno, P le Coutre, R
Hurtado-Monroy, V Santini, B Martino, F Pane, A Piccin, P Giraldo, S
Assouline, M Durosinmi, O Leeksma, E Pogliani, M Puttini, E Jang, J
Reiffers, M Valsecchi, D-W Kim, Multicenter Independent Assessment of
Outcomes in Chronic Myeloid Leukemia Patients Treated With Imatinib.
Journal of the National Cancer Institute. 2011;103(7):553-561. https://doi.org/10.1093/jnci/djr060 PMid:21422402
- Huang,
X, J Cortes, H Kantarjian, Estimations of the increasing prevalence and
plateau prevalence of chronic myeloid leukemia in the era of tyrosine
kinase inhibitor therapy. Cancer. 2012;118(12):3123-3127. https://doi.org/10.1002/cncr.26679 PMid:22294282 PMCid:PMC3342429
- Sasaki,
K, SS Strom, S O'Brien, E Jabbour, F Ravandi, M Konopleva, G Borthakur,
N Pemmaraju, N Daver, P Jain, S Pierce, H Kantarjian, JE Cortes,
Relative survival in patients with chronic-phase chronic myeloid
leukaemia in the tyrosine-kinase inhibitor era: analysis of patient
data from six prospective clinical trials. The Lancet Haematology.
2015;2(5):e186-e193. https://doi.org/10.1016/S2352-3026(15)00048-4
- Baccarani,
M, MW Deininger, G Rosti, A Hochhaus, S Soverini, JF Apperley, F
Cervantes, RE Clark, JE Cortes, F Guilhot, H Hjorth-Hansen, TP Hughes,
HM Kantarjian, DW Kim, RA Larson, JH Lipton, FX Mahon, G Martinelli, J
Mayer, MC Muller, D Niederwieser, F Pane, JP Radich, P Rousselot, G
Saglio, S Saussele, C Schiffer, R Silver, B Simonsson, JL Steegmann, JM
Goldman, R Hehlmann, European LeukemiaNet recommendations for the
management of chronic myeloid leukemia: 2013. Blood.
2013;122(6):872-84. https://doi.org/10.1182/blood-2013-05-501569 PMid:23803709 PMCid:PMC4915804
- National
Comprehensive Cancer Network (NCCN). NCCN Guidelines Chronic
Myelogenous Leukemia Version 1.2016. 2016 [cited 2017 May]; Available
from: http://www.nccn.org/professionals/physician_gls/pdf/cml.pdf.
- Abruzzese,
E, M Breccia, R Latagliata, Second-generation tyrosine kinase
inhibitors in first-line treatment of chronic myeloid leukaemia (CML).
BioDrugs. 2014;28(1):17-26. https://doi.org/10.1007/s40259-013-0056-z PMid:24043361
- Baccarani,
M, G Saglio, J Goldman, A Hochhaus, B Simonsson, F Appelbaum, J
Apperley, F Cervantes, J Cortes, M Deininger, A Gratwohl, F Guilhot, M
Horowitz, T Hughes, H Kantarjian, R Larson, D Niederwieser, R Silver, R
Hehlmann, Evolving concepts in the management of chronic myeloid
leukemia: recommendations from an expert panel on behalf of the
European LeukemiaNet. Blood. 2006;108(6):1809-20. https://doi.org/10.1182/blood-2006-02-005686 PMid:16709930
- Sundar,
HJ Radich, Optimizing Patient Care in Chronic Phase Chronic Myelogenous
Leukemia: A Multidsciplinary Approach. Journal of the National
Comprehensive Cancer Network: JNCCN. 2016;14 (Supplement 1):S1-S6. https://doi.org/10.6004/jnccn.2016.0197
- Goldberg,
SL, J Cortes, C Gambacorti-Passerini, R Hehlmann, HJ Khoury, M
Michallet, R Paquette, B Simonsson, T Zyczynski, A Foreman, E
Abruzzese, D Andorsky, A Beeker, P Cony-Makhoul, R Hansen, E Lomaia, E
Olavarria, M Mauro, First-line treatment selection and early monitoring
patterns in chronic phase-chronic myeloid leukemia in routine clinical
practice: SIMPLICITY. Am J Hematol. 2017;92(11):1214-1223. https://doi.org/10.1002/ajh.24887 PMid:28815757 PMCid:PMC5659133
- Hehlmann,
R, J Cortes, C Gambacorti-Passerini, SL Goldberg, HJ Khoury, M Mauro, M
Michallet, H Mohamed, R Paquette, B Simonsson, M Subar, M Turner, T
Zyczynski. Tyrosine kinase inhibitor (tki) switching: Experience from
simplicity, a prospective observational study of chronic-phase chronic
myeloid leukemia (cp-cml) patients in clinical practice. in 19th
Congress of the European Hematology Association (EHA). 2014. Milan,
Italy.
- Bollu, V, A Quintas-Cardama, M
Flamm, M Lill, M Thirman, F Ravandi-Kashani, LP Akard, M Talpaz, PCN12
Resource utilization and perceptions of major molecular response in
chronic myeloid leukemia (CML): results of a Delphi panel study. Value
in Health. 2011;14:A156-A157. https://doi.org/10.1016/j.jval.2011.02.870
- Chen,
L, A Guérin, J Xie, EQ Wu, AP Yu, SG Ericson, E Jabbour, Monitoring and
switching patterns of patients with chronic myeloid leukemia treated
with imatinib in community settings: a chart review analysis. Curr Med
Res Opin. 2012;28(11):1831-9. https://doi.org/10.1185/03007995.2012.741577 PMid:23127201
- Goldberg,
SL, L Chen, A Guerin, AR Macalalad, N Liu, M Kaminsky, SG Ericson, EQ
Wu, Association between molecular monitoring and long-term outcomes in
chronic myelogenous leukemia patients treated with first line imatinib.
Curr Med Res Opin. 2013;29(9):1075-82. https://doi.org/10.1185/03007995.2013.812034 PMid:23738923
- Hanfstein,
B, MC Müller, R Hehlmann, P Erben, M Lauseker, A Fabarius, S
Schnittger, C Haferlach, G Gohring, U Proetel, HJ Kolb, SW Krause, WK
Hofmann, J Schubert, H Einsele, J Dengler, M Hanel, C Falge, L Kanz, A
Neubauer, M Kneba, F Stegelmann, M Pfreundschuh, CF Waller, S Branford,
TP Hughes, K Spiekermann, GM Baerlocher, M Pfirrmann, J Hasford, S
Saussele, A Hochhaus, Early molecular and cytogenetic response is
predictive for long-term progression-free and overall survival in
chronic myeloid leukemia (CML). Leukemia. 2012;26(9):2096-102. https://doi.org/10.1038/leu.2012.85 PMid:22446502
- Marin,
D, AR Ibrahim, C Lucas, G Gerrard, L Wang, RM Szydlo, RE Clark, JF
Apperley, D Milojkovic, M Bua, J Pavlu, C Paliompeis, A Reid, K
Rezvani, JM Goldman, L Foroni, Assessment of BCR-ABL1 transcript levels
at 3 months is the only requirement for predicting outcome for patients
with chronic myeloid leukemia treated with tyrosine kinase inhibitors.
J Clin Oncol. 2012;30(3):232-8. https://doi.org/10.1200/JCO.2011.38.6565 PMid:22067393 PMCid:PMC6366954
- Stanek,
E, RE Aubert, C Sanders, FW Frueh, J Yao, RS EpsteinI Medco Health
Solutions, Franklin Lakes, NJ, Inadequate BCR-ABL monitoring in
imatinib-treated patients with chronic myelogenous leukemia. Journal of
Clinical Oncology. 2009;27(Suppl)(15s):7077.
- Henk,
HJ, M Woloj, M Shapiro, J Whiteley, Real-world analysis of tyrosine
kinase inhibitor treatment patterns among patients with chronic myeloid
leukemia in the United States. Clin Ther. 2015;37(1):124-33. https://doi.org/10.1016/j.clinthera.2014.10.019 PMid:25467191
- Rashid,
N, HA Koh, K Lin, C Dimaano, E Felber, Real World Treatment Patterns in
Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase
Inhibitors in First Line in an Integrated Healthcare System. Blood.
2015;126:5157.
- Sail, KR, L Chen, J
Jackson, SG Ericson, S Haislip, T Ibison, J Gilmore, MN Saleh,
Treatment Patterns Among Patients with Philadelphia Chromosome Positive
Chronic Myeloid Leukemia (Ph+ CML) Treated with Imatinib in a Community
Setting. Blood. 2012;120(21):3179.
- Vander
Velde, N, L Chen, A Guo, H Sharma, M Marynchenko, EQ Wu, J Liu, H Yang,
L Shi, Study of imatinib treatment patterns and outcomes among US
veteran patients with Philadelphia chromosome-positive chronic myeloid
leukemia. J Oncol Pract. 2013;9(5):e212-9. https://doi.org/10.1200/JOP.2012.000822 PMid:23943889
- Chen,
L, D Latremouille-Viau, A Guerin, R Nitulescu, P Gagnon-Sanschagrin.
Treatment Patterns and Healthcare Costs in Newly Diagnosed Patients
with Chronic Myeloid Leukemia Receiving Dasatinib or Nilotinib As
First-Line Therapy in the United States: A Retrospective Claims Databse
Analysis. in ASH 57th Annual Meeting & Exposition. 2015. Orlando, Florida.
- Latagliata,
R, D Ferrero, A Iurlo, F Cavazzini, F Castagnetti, E Abruzzese, C Fava,
M Breccia, M Annunziata, F Stagno, M Tiribelli, G Binotto, G Mansueto,
A Gozzini, S Russo, L Cavalli, E Montefusco, G Gugliotta, M Cedrone, A
Russo Rossi, P Avanzini, P Pregno, E Mauro, A Spadea, F Celesti, G
Giglio, A Isidori, M Crugnola, E Calistri, F Sora, S Storti, A
D'Addosio, G Rege-Cambrin, L Luciano, G Alimena, Imatinib in very
elderly patients with chronic myeloid leukemia in chronic phase: a
retrospective study. Drugs Aging. 2013;30(8):629-37. https://doi.org/10.1007/s40266-013-0088-6 PMid:23681399
- Rosti,
G, I Iacobucci, S Bassi, F Castagnetti, M Amabile, D Cilloni, A Poerio,
S Soverini, F Palandri, G Rege Cambrin, F Iuliano, G Alimena, R
Latagliata, N Testoni, F Pane, G Saglio, M Baccarani, G Martinelli,
Impact of age on the outcome of patients with chronic myeloid leukemia
in late chronic phase: results of a phase II study of the GIMEMA CML
Working Party. Haematologica. 2007;92(1):101-5. https://doi.org/10.3324/haematol.10239
- Hehlmann,
R, MC Müller, M Lauseker, B Hanfstein, A Fabarius, A Schreiber, U
Proetel, N Pletsch, M Pfirrmann, C Haferlach, S Schnittger, H Einsele,
J Dengler, C Falge, L Kanz, A Neubauer, M Kneba, F Stegelmann, M
Pfreundschuh, CF Waller, K Spiekermann, GM Baerlocher, G Ehninger, D
Heim, H Heimpel, C Nerl, SW Krause, DK Hossfeld, HJ Kolb, J Hasford, S
Saussele, A Hochhaus, Deep molecular response is reached by the
majority of patients treated with imatinib, predicts survival, and is
achieved more quickly by optimized high-dose imatinib: results from the
randomized CML-study IV. J Clin Oncol. 2014;32(5):415-23. https://doi.org/10.1200/JCO.2013.49.9020 PMid:24297946
- Kantarjian,
H, NP Shah, A Hochhaus, J Cortes, S Shah, M Ayala, B Moiraghi, Z Shen,
J Mayer, R Pasquini, H Nakamae, F Huguet, C Boque, C Chuah, E
Bleickardt, MB Bradley-Garelik, C Zhu, T Szatrowski, D Shapiro, M
Baccarani, Dasatinib versus imatinib in newly diagnosed chronic-phase
chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260-70. https://doi.org/10.1056/NEJMoa1002315 PMid:20525995
- O'Brien,
SG, F Guilhot, RA Larson, I Gathmann, M Baccarani, F Cervantes, J
Cornelissen, T Fischer, A Hochhaus, T Hughes, K Lechner, J Nielsen, P
Rousselot, J Reiffers, G Saglio, J Shepherd, B Simonsson, A Gratwohl, J
Goldman, H Kantarjian, K Taylor, G Verhoef, A Bolton, R Capdeville, B
DrukerI Investigators., Imatinib compared with interferon and low-dose
cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia.
The New England Journal of Medicine. 2003;348(11):994-1004. https://doi.org/10.1056/NEJMoa022457 PMid:12637609
- Preudhomme,
C, J Guilhot, FE Nicolini, A Guerci-Bresler, F Rigal-Huguet, F
Maloisel, V Coiteux, M Gardembas, C Berthou, A Vekhoff, D Rea, E
Jourdan, C Allard, A Delmer, P Rousselot, L Legros, M Berger, S Corm, G
Etienne, C Roche-Lestienne, V Eclache, FX Mahon, F Guilhot, S
Investigators C France Intergroupe des Leucemies Myeloides, Imatinib
plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med.
2010;363(26):2511-21. https://doi.org/10.1056/NEJMoa1004095 PMid:21175313
- Saglio,
G, DW Kim, S Issaragrisil, P le Coutre, G Etienne, C Lobo, R Pasquini,
RE Clark, A Hochhaus, TP Hughes, N Gallagher, A Hoenekopp, M Dong, A
Haque, RA Larson, HM Kantarjian, Nilotinib versus imatinib for newly
diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251-9. https://doi.org/10.1056/NEJMoa0912614 PMid:20525993
- Simonsson,
B, T Gedde-Dahl, B Markevarn, K Remes, J Stentoft, A Almqvist, M
Bjoreman, M Flogegard, P Koskenvesa, A Lindblom, C Malm, S Mustjoki, K
Myhr-Eriksson, L Ohm, A Rasanen, M Sinisalo, A Sjalander, U Stromberg,
OW Bjerrum, H Ehrencrona, F Gruber, V Kairisto, K Olsson, F Sandin, A
Nagler, JL Nielsen, H Hjorth-Hansen, K Porkka, CMLSG Nordic,
Combination of pegylated IFN-alpha2b with imatinib increases molecular
response rates in patients with low- or intermediate-risk chronic
myeloid leukemia. Blood. 2011;118(12):3228-35. https://doi.org/10.1182/blood-2011-02-336685 PMid:21685374
- Branford,
S, L Fletcher, NC Cross, MC Muller, A Hochhaus, DW Kim, JP Radich, G
Saglio, F Pane, S Kamel-Reid, YL Wang, RD Press, K Lynch, Z Rudzki, JM
Goldman, T Hughes, Desirable performance characteristics for BCR-ABL
measurement on an international reporting scale to allow consistent
interpretation of individual patient response and comparison of
response rates between clinical trials. Blood. 2008;112(8):3330-8. https://doi.org/10.1182/blood-2008-04-150680 PMid:18684859
- Cross,
NC, HE White, MC Müller, G Saglio, A Hochhaus, Standardized definitions
of molecular response in chronic myeloid leukemia. Leukemia.
2012;26(10):2172-5. https://doi.org/10.1038/leu.2012.104 PMid:22504141
- monitor, Thsap. Health systems in transition (HiT) profile of Italy. January 2018]; Available from: http://www.hspm.org/countries/italy25062012/livinghit.aspx?Section=5.6%20Pharmaceutical%20care&Type=Section.
- Ferré,
F, A Giulio de Belvis, L Valerio, S Longhi, A Lazzari, G Fattore, W
Ricciard, iA Maresso. Italy Health system review. 2014 January 2018];
Available from: http://www.euro.who.int/data/assets/pdffile/0003/263253/HiT-italy.pdf
- Mauro,
MJ, C Davis, T Zyczynski, HJ Khoury, The role of observational studies
in optimizing the clinical management of chronic myeloid leukemia.
Therapeutic Advances in Hematology. 2015;6(1):3-14. https://doi.org/10.1177/2040620714560305 PMid:25642311 PMCid:PMC4298489
[TOP]