Vincenzo De Sanctis1, Ashraf T. Soliman2, Duran Canatan3, Mohamed A. Yassin4, Shahina Daar5, Heba Elsedfy6, Salvatore Di Maio7, Giuseppe Raiola8, Joan-Lluis Vives Corrons9 and Christos Kattamis10.
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
2 Departments of Pediatrics, University of Alexandria, Alexandria, Egypt.
3 Director of Thalassemia Diagnosis Center of Mediterranean Blood Diseases Foundation, Antalya, Turkey.
4 National Center for Cancer Care and Research, Medical Oncology Hematology Section HMC, Doha, Qatar.
5 Department of Haematology, College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman.
6 Department of Pediatrics, Ain Shams University, Cairo, Egypt.
7 Emeritus Director in Pediatrics, Children’s Hospital “Santobono-Pausilipon,” Naples, Italy.
8 Department of Paediatrics, Pugliese-Ciaccio Hospital, Catanzaro, Italy.
9
Red Blood Cell and Haematopoietic Disorders Unit. Institute for
Leukaemia Research Josep Carreras (IJC) and University of Barcelona,
Catalonia, Spain.
10 First Department of Paediatrics, National Kapodistrian University of Athens, Athens, Greece.
Correspondence to: Vincenzo De Sanctis MD, Pediatric and Adolescent
Outpatient Clinic, Quisisana Hospital, 44100 Ferrara, Italy; Tel: +39
0532 770243. E-mail:
vdesanctis@libero.it
Published:: May 1, 2019
Received: January 19, 2019
Accepted: March 8, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019029 DOI
10.4084/MJHID.2019.029
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Changes
in thyroid function and thyroid function tests occur in patients with
β-thalassemia major (TM). The frequency of hypothyroidism in TM
patients ranges from 4% to 29% in different reports. The wide variation
has been attributed to several factors such as patients' genotype, age,
ethnic heterogeneity, treatment protocols of transfusions and
chelation, and varying compliance to treatment. Hypothyroidism is the
result of primary gland failure or insufficient thy¬roid gland
stimulation by the hypothalamus or pituitary gland. The main laboratory
parameters of thyroid function are the assessments of serum
thyroid-stimulating hor¬mone (TSH) and serum free thyroxine (FT4). It
is of primary importance to interpret these measure¬ments within the
context of the laboratory-specific normative range for each test. An
ele¬vated serum TSH level with a standard range of serum FT4 level is
consistent with subclinical hypothyroidism. A low serum FT4 level with
a low, or inappropriately normal, serum TSH level is consistent with
secondary hypothyroidism. Doctors caring for TM patients most commonly
encounter subjects with subclinical primary hypothy¬roidism in the
second decade of life. Several aspects remain to be elucidated as the
frequency of thyroid cancer and the possible existence of a
relationship between thyroid dysfunction, on one hand, cardiovascular
diseases, components of metabolic syndrome (insulin resistance) and
hypercoagulable state, on the other hand. Further studies are needed to
explain these emerging issues. Following a brief description of thyroid
hormone regulation, production and actions, this article is
conceptually divided into two parts; the first reports the spectrum of
thyroid disease occurring in patients with TM, and the second part
focuses on the emerging issues and the open problems in TM patients
with thyroid disorders.
|
Introduction
In
recent years our knowledge of hypo-thyroidism in children, adolescents
and young adults with homozygous β-thalassemias (β-thal) has increased.
Regarding clinical phenotype β-thal are classically classified as:
major (TM), intermedia (TI), and minor (β-thal trait). Based on the
need of transfusions for survival, homozygous patients are further
characterized as Transfusion-Dependent Thalassemia (TDT), as are all
patients with TM, and Non-Transfusion Dependent Thalassemia (NTDT) as
are, with few exceptions, the TI patients. Patients with TDT require
regular, lifelong blood transfusions for survival, starting before the
age of 2 years (the "classic form" of TM).[1-4]
The
etiology of thyroid disorders in these patients is substantially
different from that of the general population because transfusional
iron overload in TDT and increased iron uptake from the
gastrointestinal tract in TI, are implicated in over 90% of morbidity
and mortality in patients with b-thal. Therefore, the knowledge of risk
factors influencing the development of hypothyroidism is a critical
component of long-term monitoring and treatment of patients affected by
TM.
Following a brief description of thyroid hormones regulation,
production, and actions, this article is conceptually divided into two
parts: the spectrum of thyroid disease occurring in patients with TM,
and the emerging issues and the open problems in TM patients with
thyroid disorders.
Regulation of Thyroid Hormones Production and Physiologic Actions
The thyroid gland is regulated by thyrotropin-releasing hormone (TRH) and thyroid stimulating hormone (TSH).[5]
Thyroid-stimulating hormone (TSH) is a glycoprotein hormone secreted by
the anterior pituitary. It usually exhibits a diurnal variation with a
peak shortly after midnight and a nadir in the late afternoon. At the
peak of this variation, the TSH can be double the value at the nadir.[6-8]
Many signals from peripheral tissues can indirectly affect TRH
secretion. These include gonadal hormones, leptin, and factors related
to feeding, cold or sleep.[9-11]
Thyroxine (T4)
and triiodothyronine (T3) are produced by the thyroid gland. The
formation of thyroid hormones depends on an exogenous supply of iodine.
About 100 µg of iodide is required daily to generate sufficient
quantities of thyroid hormone. A healthy individual produces
approximately 90 to 100 µg of T4 and 30 to 35 µg of T3 from the thyroid
gland daily. An estimated 80% of the T3 produced daily in humans is
derived from peripheral metabolism (5'-monodeiodination) of T4, with
only about 20% secreted directly from the thyroid gland itself. T4 and
T3 circulate bound primarily to carrier proteins. T4 binds strongly to
thyroxine-binding globulin (TBG, ~ 75 percent) and weakly to
thyroxine-binding prealbumin (TBPA ~ 20 percent) and albumin (~5
percent). T3 binds tightly to TBG and weakly to albumin, with little
binding to TBPA. Although T4 is produced in greater amounts, T3 is the
biologically active form.[12,13]
Thyroid
hormones are key regulators of metabolism and development and are known
to have pleiotropic effects in many different organs. Thyroid hormones
affect normal growth and development (particularly in bone and central
nervous system), help regulation of lipids (adipose tissue) and protein
breakdown in muscle, increase absorption of carbohydrates from the
intestine and increase dissociation of O2 from hemoglobin acting on RBC
2,3-diphosphoglycerate (DPG).[12,13]
Thyroid
hormone regulates virtually every anatomic and physiologic component of
the cardiovascular system. The major effects of thyroid hormones on the
heart are mediated by triiodothyronine (T3). T3 generally increases the
force and rate of systolic contraction and diastolic relaxation,[14] decreases vascular resistance, including coronary vascular tone, and increases coronary arteriolar angiogenesis.[14]
Thyroid hormones act in the liver, white adipose tissue, skeletal
muscle, and pancreas, influence plasma glucose levels, insulin
sensitivity, and carbohydrate metabolism.[15]
Prevalence of Thyroid Disorders in Thalassemia Major
1. Primary Hypothyroidism.
The advent of more precise diagnostic techniques, which enable
different aspects of thyroid function assessment, showed that
hypothyroidism is a graded phenomenon. Therefore, several definitions
have been used to define different aspects of impaired thyroid function
including in TDT patients. The following grades have been identified:
1) Sub-biochemical hypothyroidism consists of an exaggerated TSH
response to TRH test in the presence of normal TSH and FT4; 2)
Sub-clinical hypothyroidism is a combination of high TSH (> 4.2
mIU/L and <10 mIU/L) with normal FT4 levels; 3) Overt (clinical)
hypothyroidism is a combination of high TSH (TSH >10 mIU/L) with low
FT4.
The frequency of primary hypothyroidism in TDT patients, in
different reports, ranges from 4% to 29%, based on the level of FT4/T4
and TSH.[16,17] In general, subclinical
hypothyroidism is more common compared to overt hypothyroidism. This
wide variation has been attributed to several factors such as patients'
genotype age, ethnic groups, differences in treatment protocols of
transfusion and chelation with marked variations in compliance and
efficiency.[17-24]
A lower prevalence of hypothyroidism is found among patients with lower iron load, as measured by ferritin levels.[21,25] An increased frequency of hypo-hyroidism was reported by Belhoul et al. [25]
in splenectomised TM patients (26% versus 4.5% of non-splenectomised
thalassemic patients). In non-splenectomised thalassemics, the spleen
might represent a reservoir of iron excess and might have a potential
scavenging effect on free iron fractions, including non-transferrin
bound iron.[26]
However, further studies
are needed to confirm this hypothesis that should include evaluations
of factors involved in iron redistribution in TDT patients.[27]
Thyroid
failure usually starts in the second, and increases gradually in the
third and fourth decades of life in patients who started early
subcutaneous chelation therapy with desferrioxamine (Figure 1);
in patients starting late iron chelation therapy, or with poor
compliance to treatment, dysfunction of thyroid failure starts earlier.[27-31] Therefore, an assessment of thyroid function is generally recommended after the age of 10 years.[17]
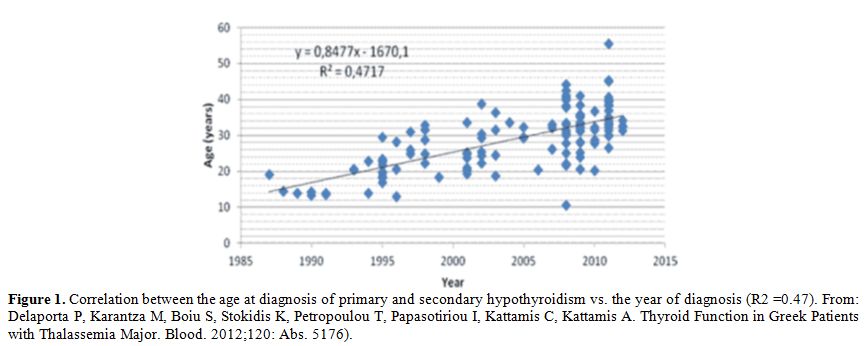 |
Figure
1. Correlation between the age at diagnosis of primary and secondary
hypothyroidism vs. the year of diagnosis (R2 =0.47). From: Delaporta P,
Karantza M, Boiu S, Stokidis K, Petropoulou T, Papasotiriou I, Kattamis
C, Kattamis A. Thyroid Function in Greek Patients with Thalassemia
Major. Blood. 2012;120: Abs. 5176). |
2. Central Hypothyroidism (CH).
The thyroid gland appears to fail before the central components of the
pituitary-thyroid axis which seems to be less susceptible to
iron-induced damage.[4,32]
The
diagnosis of central hypothyroidism (CH) is difficult from a clinical
and biochemical perspective. It is based on low circulating levels of
FT4 in the presence of low to normal TSH concentrations.
Tatò et al.[33] found an inadequate response of the free α-subunit
to TRH stimulation tests in 14 euthyroid TM patients (8 females and 6
males, aged 15-24 years), suggesting a central involvement.
De Sanctis et al.[34]
performed a cross-sectional analysis on an extensive database using the
clinical records of their TM patients to explore the prevalence of CH
in prepubertal (<11 years: 25 patients; 13 males) peripubertal
(between 11 and 16 years: 9 patients; 3 males), and pubertal TM
subjects (>16 years: 305 patients; 164 males). CH was present in 26
(7.6%) TM patients. Their mean age was 29.9 ± 8.4 years, 14 (53.8%)
were males, and 12 (46.1%) were females. The prevalence of CH,
characterized by low FT4 with low/normal TSH levels was 6% in patients
with a chronological age below 21 years and 7.9% in those above 21
years.
Similar results have been reported by Delaporta et al.[35] (Table 1),
while higher percentages were reported in Iranian[22] (16% in 114
patients, with a mean age of 20.9 ±7.8 years) and Qatari patients
(76.4% in 48 children and adolescents, up to the age of 18 years).[36]
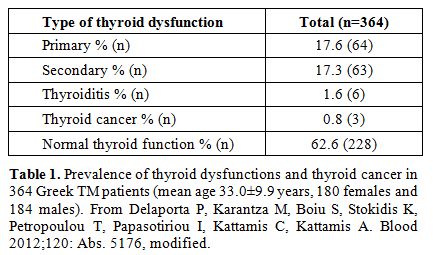 |
Table 1. Prevalence of
thyroid dysfunctions and thyroid cancer in 364 Greek TM patients (mean
age 33.0±9.9 years, 180 females and 184 males). From Delaporta P,
Karantza M, Boiu S, Stokidis K, Petropoulou T, Papasotiriou I, Kattamis
C, Kattamis A. Blood 2012;120: Abs. 5176, modified. |
In the general population, CH is about 1000-fold rarer than primary hypothyroidism.[37] In
contrast with primary hypothyroidism, low FT4 with low/normal TSH
levels are the biochemical hallmarks of overt forms of CH, while the
milder defects, characterized by FT4 levels still within the normal
range, could remain undiagnosed.[37] To support the
diagnosis of CH, a reduction of FT4 larger than 20% vs. the initial FT4
levels has been suggested in patients with different pituitary diseases
followed over several years.[38] This cut-off was set on the basis of a 10% variation over time of T4 levels in healthy individuals.[39]
In
summary, significant advancement has been made in recent years in
diagnosing CH in TM patients, thus increasing the clinical awareness of
this complication. Both the hypothalamus and pituitary gland appear to
be affected by iron overload, and this can explain the defective TSH
secretion in response to low FT4 in thalassemic patients. The
deposition of iron in the pituitary gland and its deleterious effects
on pituitary size and function has been reported in many studies and
reviews.[40-42] Nevertheless, there is a rising
impression that the frequency of CH is underestimated because only a
few studies have been reported in the current literature. Furthermore,
the presence of a mild rise of TSH levels associated with a borderline
low FT4 represents a further clinical challenge for the diagnosis and
treatment of the mixed forms of hypothyroidism (De Sanctis V, personal
observations). In patients with CH an assessment of other pituitary
hormone deficiencies may be required.
Clinical Manifestations and other Diagnostic Parameters
The
severity of the clinical manifestations gen¬erally reflects the degree
of thyroid dysfunction and time needed for the development of
hypothyroidism. The clinical presentation of patients with subclinical
hypothyroidism may be subtle, without any symptoms, and may be detected
merely during routine screening of thyroid function. Patients with
primary hypothyroidism may present with short stature, delayed puberty,
fatigue, cold intolerance, weight gain, con¬stipation, and dry skin.[43] In TM patients with clinical hypothyroidism, cardiac failure and pericardial effusion have been reported.[43]
The clinical manifestations of CH are usually milder than those observed in primary hypothyroidism.
Although
there is no significant relationship between gender and thyroid
dysfunction, a higher incidence of thyroid dysfunction has been
reported in female patients with subclinical hypothyroidism.[44]
It has been also reported that thalassemic patients with primary
hypothyroidism have more frequent endocrine complications, including
insulin dependent diabetes mellitus (79%), hypoparathyroidism (65%),
and failure of puberty (37%).[21]
In one of our
papers, the first and most common endocrine complication in TM
patients was hypogonadotropic hypogonadism (36.3%; diagnosed at
the age of 16 years in females and 18 years in males) followed by
subclinical hypothyroidism (18.1%) at a mean age of 20.2 years (range
12-32 years), insulin-dependent diabetes mellitus (36.3%) at a mean age
of 22.5 years (range 12-35 years), and secondary amenorrhea
(27.2%) at a mean age of 36.3 years (range 34-38 years).[45]
There is very little evidence for the presence of autoimmune thyroiditis. In the Delaporta et al. study[35] the reported prevalence in 364 TM patients was 1.6% (Table 1).
However, no comparison data were reported in the Greek control
population. Interestingly, the prevalence of anti-thyroid antibodies
(ATA) is significantly lower (9.2%) in TM women than that found in
age-matched euthyroid women (20.0%). This suggests that iron
overload may inhibit rather than trigger thyroid autoimmunity.[46]
In another study serum ferritin levels were found to be significantly
higher in ATA positive compared to ATA negative patients (4,870
±1,665 ng/mL versus 2,922 ± 2,773 ng/mL; p: < 0.0001), which
advocates potential iron-mediated tissue damage rather than a primary
autoimmune process of the thyroid gland.[47] Nevertheless, more well-designed studies are needed to confirm these preliminary observations.
Thyroid ultrasonography may show different echo patterns. Pitrolo et al.[48]
observed reduced echogenicity in 47% of TM patients and a diffuse
spotty echogenicity in 33% of them, indicative of thyroid dysfunction.
Filosa et al.[49] reported features of dyshomogeneity of the parenchyma with different degrees of severity.
Despite
the limitations of serum or plasma ferritin (SF) for the estimation of
iron stores in patients with iron overload, this indirect parameter
remains essential in monitoring iron overload. Assays of SF are
available worldwide, relatively well standardized, and not expensive.
In the absence of confounding factors, such as inflammation, vitamin C
deficiency, oxidative stress, hepatocyte dysfunction, and increased
cell death, SF levels correlate with the size of cellular iron stores.[50]
Currently, tissue iron can be detected by nuclear magnetic resonance
(MRI) imaging. This technique has been used to assess myocardial,
spleen, pituitary, adrenal, pancreas and liver iron content in patients
with known or suspected iron overload disorders.[51]
Pathophysiology
Thyroid
dysfunction appears to be primarily due to the toxicity of the excess
unbound iron within cells or in plasma, generating reactive oxygen
species, leading to lipid peroxidation, that under conditions of iron
overload, leads to the generation of both unsaturated (malondialdehyde
and hydroxynonenal) and saturated (hexanal) aldehydes. Both have been
implicated in cellular dysfunction, cytotoxicity and cell death.[16,17]Certain
tissues are particularly susceptible to excess iron incorporation in
the presence of Non-Transferrin-Bound Iron (NTBI). TRH stimulates TSH β
promoter activity by two distinct mechanisms involving calcium influx
through L type Ca2+ channels (LTCCs) and protein kinase C.[52,53]
The most recent evidence suggests that LTCCs are the front-runners for
mediating NTBI transport in iron overload conditions. LTCCs are
moderately abundant in thyrotropes that appear to be at the greatest
risk in iron overload. In addition, protein kinase C is regulated by
iron with the possible deleterious effect of excess iron on its
function.[53,54] Apart
from iron overload, other factors responsible for organ damage have
been recognized, including chronic hypoxia due to anaemia,[55] that may potentiate the toxicity of iron deposition in endocrine glands, and hepatitis C virus (HCV) infection.[4,16,17]Many
patients with thalassemia have been infected with hepatitis C virus
(HCV) through blood transfusion before the introduction of screening of
blood donors in 1992. HCV genotype 1b infection was the most frequent
in Italy. In cohorts of adult TDT patients epidemiological studies, the
proportion of patients with genotype 1b infection often exceeds 50%.
Chronic HCV infection is associated with a high risk of developing
cirrhosis, hepatocellular carcinoma and liver failure if left
untreated.[56] Chronic hepatitis C infection may
also lead to subclinical hypothyroidism due to the direct cytopathic
effect of HCV on thyroid cells or with the use of interferon.[57]
Liver disease is also associated with an increase in inflammatory
cytokines, which negatively affect the hypothalamus ‑pituitary‑ thyroid
axis, leading to suppressed TSH levels in some patients.[58]
In the light of eradication of HCV in the thalassemia population with
direct-acting antiviral drugs, the prevalence of thyroid dysfunctions
could be ameliorated. However long-term studies are needed to confirm
this assumption.
The Long-Term Natural History of Thyroid Function in Thalassemia
Longitudinal
studies have shown worsening of thyroid function in thalassemic
patients with advancing age. However, the progression is variable, and
it may take years to progress to overt hypothyroidism.Landau et al.[32]
studied the course of thyroid disease in TM patients in a 15-year
longitudinal study. The authors found that more than 30% of TM patients
had an abnormal response to TRH and 14% changed from normal to overt
hypothyroidism.Zervas et al.[19]
reported that approximately 1 of 5 β-thal patients with average thyroid
hormone values showed an exaggerated TSH response to the TRH test. In
another study, an exaggerated TSH response to TRH test was found in 8
out of 24 TM patients (33.3%). TSH peak values, after TRH test,
positively correlated with ferritin levels, liver enzymes (ALT), and
compliance index to chelation therapy. Three out of 8 patients (37.5%)
developed subclinical or overt hypothyroidism from 3 to 11 years later.[59]
Similar results were also observed in 25% of the patients (27 females
and 23 males, mean age 25.7 ± 1.4) by Filosa et al. during 12 years of
follow-up.[48]Soliman et al.[37]
documented a slowly progressive decrease of FT4 over a 12 year-period,
associated with a corresponding slow decrease of basal TSH. These
findings indicate a central component of hypothyroidism. In support of
these observations, Hashemi et al.[60] reported a
higher incidence of secondary hypothyroidism (12%) compared to primary
hypothyroidism (2%) in their thalassemic patients.In
conclusion, as survival rates of patients with TM are continuously
improving, there is a steadily growing need for regular follow-up and
surveillance strategies of thyroid function. TRH stimulation test may
be a useful means of early diagnosis of thyroid dysfunction. An
exaggerated TSH response to TRH test is frequently found in patients
with TM and iron overload, and may evolve into subclinical or clinical
hypothyroidism. A slowly progressive decrease of FT4 and basal TSH has
been observed in young adult subjects, indicating CH. Early diagnosis
and treatment of these complications are essential to ensure a good
quality of life and to reduce late morbidity and mortality. Risk Factors for the Development of Thyroid Disorders in Addition to Iron Overload
The
etiology of thyroid disorders in TM patients is substantially different
from that in the general population; transfusional iron overload and
increased iron uptake from the gastrointestinal tract, as a result of
ineffective erythropoiesis accompanied by anemia and hypoxia, are
implicated in over 90% of morbidity and mortality in patients with β-thal.[1-4]
Therefore, the knowledge of risk factors influencing the development of
hypothyroidism represents a critical component of long-term monitoring
and treatment of patients affected by TM.1. Iron overload. The association between iron overload and hypothyroidism was studied by Belhoul et al.[25]
in 382 TM patients treated with regular transfusions and
desferrioxamine (DFO) at the Thalassemia Center in Dubai (UAE). The
mean age of patients was 15.4 ± 7.6 years, with an equal sex
distribution. On multivariate logistic regression analysis, patients
with a serum ferritin level >2,500 ng/mL were 3.53 times (95% CI
1.09-11.40) more likely to have diabetes mellitus (DM), 3.25 times (95%
CI 1.07-10.90) more likely to have hypothyroidism, 3.27 times (95% CI
1.27-8.39) more likely to have hypoparathyroidism, and 2.75 times (95%
CI 1.38-5.49) more likely to have hypogonadism compared to patients
with a serum ferritin level ≤ 1,000 ng/mL. Splenectomized patients with
serum ferritin levels ≤ 2,500 ng/mL had comparably high rates of all
endocrinopathies as patients with serum ferritin levels > 2,500
ng/mL.In the Gamberini et al. study[21]
the main risk factors associated with endocrine complications in 273
patients with TM, were high serum ferritin levels, poor compliance with
DFO therapy, early onset of transfusion therapy (only for hypogonadism)
and splenectomy (only for hypothyroidism). Serum ferritin levels of
~2,000 ng/mL were found to correlate with hypogonadism, and levels of
3,000 ng/mL for hypothyroidism [primary hypothyroidism (80%) and
central (20%)], hypoparathyroidism and DM. A
liver iron concentration (LIC) cut-off point of ≥ 6 mg Fe/g dry
weight (d.w.) was found to be the best threshold for discriminating the
presence and absence of endocrine/bone morbidity (hypothyroidism,
osteoporosis, or hypogonadism), in NTDT patients, with a risk factor of
4.05 times higher compared to TI patients with a LIC < 6 mg Fe/g
d.w.[61]2. Amiodarone-induced hyper-hypothyroidism.
Amiodarone is an efficient antiarrhythmic agent often used in clinical
practice, despite its potentially serious side effects. Although the
mechanisms of action of amiodarone on the thyroid gland and thyroid
hormone metabolism are poorly understood, the structural similarity of
amiodarone to thyroid hormones may play a role in causing thyroid
dysfunction. A 100 mg tablet contains an amount of iodine that is
250-times higher than the recommended daily iodine requirement. Amiodarone-induced thyroid dysfunction includes amiodarone-induced thyrotoxicosis (AIT, with an incidence of ~3% to 9%)[61-66] and amiodarone-induced hypothyroidism (AIH, with an incidence of 15%-20%),[63] both of which may develop in a normal thyroid gland or in settings of a pre-existing thyroid disease.[61-65] Amiodarone-induced
thyrotoxicosis is challenging to treat, as patients are iodine
saturated and therefore cannot undergo radioiodine ablation. [61-63]
Thyroidectomy remains a valuable option for AIT management,
particularly for patients with suboptimal response to medical therapy
and high risk for cardiac complications.[67] A
higher prevalence of overt hypothyroidism (22.7%) as compared to
controls (4.1%, p: 0.02) was found in TM patients 3 months after
starting amiodarone, while the prevalence of subclinical hypothyroidism
was similar in amiodarone-treated (18.2%) and untreated (15%) TM
patients.[63]Overt
hypothyroidism resolved spontaneously after amiodarone withdrawal in
one case, while the remaining TM patients were maintained euthyroid on
amiodarone by L-thyroxine administration. After 21-47 months of
amiodarone therapy, three patients (13.6%) developed AIT (2 overt and
< 1 subclinical), which remitted shortly after amiodarone
withdrawal. No case of AIT was observed in TM controls (p= 0.012 vs.
amiodarone-treated patients).[68]In
conclusion, clinicians should keep in mind the possibility of
development of thyroid disorders in patients on treatment with
amiodarone even after several years of use. Although it is difficult to
decipher the specific factors contributing to the successful management
in patients with AIT, Kotwal et al.[67] suggested
that the outcome of these patients is most likely derived from the
coordinated efforts of endocrinologists, thyroid surgeons,
cardiologists, anesthesiologists, and members of all of collaborating
teams.
Emerging Issues
1. Thyroid cancer.
Parallel to the significant amelioration of the main clinical features
of the disease, achieved on efficient treatment, adult patients suffer
from treatment-related complications that affect the heart, liver,
bones and endocrine glands, requiring specialized health care by
specialists. The possibility of occurrence of other diseases such as
malignancies, considered rare in the past, are currently increasing
with prolonged survival opening new scenarios in oncoming years. The
most commonly reported cancers are hepatocellular carcinoma (HCC),[69] hematologic malignancies,[70.71] and thyroid cancer.[73-75]
From
2000 to 2011, in a single Thalassaemia Unit following 195 TM patients,
11 carcinomas were diagnosed: 4 of the liver, 1 of the lung, 1 of the
adrenal gland and 5 of the thyroid gland. The mean patients' age was
42.6 years.[72] A prevalence of 0.8% of thyroid cancer was reported by Delaporta et al.[35] in 364 TM patients. In a recent multicenter survey, the prevalence of thyroid papillary and follicula] carcinoma was 0.41%.[68] The highest prevalence rates were registered in Greece and Italy (1.3% and 1.57%, respectively), followed by Oman (1.0%).[75]
In
the general population, the risk of harboring thyroid cancer is highest
in women, in certain inherited genetic abnormalities (Cowden’s disease,
Gardner’s syndrome, Carney complex, type I medullary thyroid cancer, or
familial adenomatous polyposis), low iodine diets, after radiation
exposure, and to endocrine disrupters.
In thalassemia, other
factors have to be taken into consideration. Iron overload and
hepatitis C (HCV) infection have potential carcinogenic effects. Iron
overload can promote the growth of some cancer cells probably through
the activation of ribonucleotide reductase and may promote the
formation of mutagenic hydroxyl radicals. In addition, iron excess
diminishes host defenses through inhibition of the activity of CD4
lymphocytes and the suppression of the tumoricidal action of
macrophages can enhance host cell production of viral nucleic acids
which may be involved in the development of human tumors.[72]
Several clinical, epidemiologic studies have suggested the oncogenetic
role for HCV. HCV is an RNA virus that cannot be integrated into the
host genome and could exert its oncogenic potential through indirect
mechanisms, with the contribution of potential genetic or environmental
factors.[72,76] If confirmed by
further clinical and epidemiologic studies, thyroid cancer should be
included among the serious complications of iron overload and/or
chronic HCV infection.
In conclusion, the occurrence of thyroid
malignancies in adult thalassemic patients is an emerging concern for
physicians, that requires the need for an annual thyroid ultrasound
surveillance. According to the European Thyroid Association guidelines
at least one of the following thyroid ultrasound features has a high
suspicion of malignancy: irregular shape, irregular margins,
microcalcifications (<1-mm, most often round calcification), marked
hypoechogenicity.[77]
2. Hypothyroidism and the heart. The role of hormones and growth factors in modulating cardiovascular functions are well known.[78-81]
It has been reported that thyroid hormone action on cardiomyocyte
regulates myocardial contractility, diastolic, and systolic function.
Moreover, thyroid hormones also exert profound effects on the heart and
cardiovascular hemodynamics. Thyroid hormone deficiency results in low
heart rate and weakening of myocardial contraction and relaxation, with
prolonged systolic and early diastolic times.[82,83]
Typical electrocardiographic changes that can be seen in hypothyroidism
include sinus bradycardia, prolonged QTc, low voltage, and the rarely
atrioventricular block.
Ten years ago, in a long-term follow-up study, De Sanctis et al.[44]
reported that cardiac involvement was present in ~50% of TM patients
with subclinical hypothyroidism and moderate/severe iron overload.
Patients mean age was 15.7 ±
3.5 years (range 9-22 years). A positive direct correlation was
observed between the following variables: TSH and serum ferritin,
thyroglobulin and basal TSH, basal TSH and peak levels after TRH
stimulation test. During four years follow-up, 16.6% died from
heart failure and arrhythmia. In patients with hypothyroidism, the
changes in cardiovascular function responded to replacement therapy
with L-thyroxine and efficient chelation regimen.
Recently, a
retrospective cohort study was performed to evaluate, in a large
historical cohort of 957 TM patients who underwent cardiovascular
magnetic resonance (CMR) for myocardial iron overload (MIO) assessment,
whether hypothyroidism was associated with a higher risk of heart
complications (heart failure, arrhythmias, and pulmonary hypertension).[84]
The authors identified 115 (12%), hypothyroid patients. Hypothyroid and
non-hypothyroid patients had comparable MIO, but hypothyroid patients
showed significantly lower biventricular stroke volume index, ejection
fraction and left ventricular cardiac index. Accordingly, the
prevalence of overall heart dysfunction (LV, RV or both) was higher in
hypothyroid patients (43.5% vs 33.5%; p: 0.0314). Hypothyroid patients
had a significant higher frequency of heart failure (19.1% vs 9.1%; p:
0.003) and arrhythmias (11.3% vs 4.3%; p: 0.003). These data confirm
the link between thyroid function and heart diseases also in TM
patients and stress the need to prevent hypothyroidism in this
population.[84]
In conclusion, hypothyroidism
seems to increase the risk for heart failure, arrhythmias and heart
dysfunction in TM patients; thus in TM patients, a sequential
assessment of thyroid function and effective iron chelation therapy are
recommended to prevent thyroid dysfunction and significant myocardial
dysfunction. Prevention of Endocrine Complications
Efficient
treatment with iron chelating drugs of patients with TM is
considered the standard care that leads to improvement of
morbidity and of survival.[4] To date, there are
3 significant classes of iron chelators: hexadentate (Deferoxamine
[DFO], Desferal®, Novartis Pharma AG,Basel, Switzerland); bidentate
(Deferiprone, [DFP] Ferriprox®, Apotex Inc., Toronto, ON, Canada), and
tridentate (Deferasirox [DFX], Exjade® and Jadenu®, Novartis Pharma AG,
Basel, Switzerland).[85] In
2008, a longitudinal study in TM patients followed at Ferrara Centre,
showed that the incidence of hypothyroidism, diabetes mellitus, and
hypoparathyroidism declined during treatment with DFO given
subcutaneously SC (Figure 2).[21] Similar results were reported by Farmaki et al.[86-89]
The authors showed that regular and intensive combined chelation
therapy with DFO and DFP improved the thyroid function in TM patients
with iron overload. The time needed to reverse hypothyroidism with
combined chelation therapy varied according to the patient's age and
iron load status. After 6.5 consecutive years of therapy with DFX (up
to 10 years) in 86 patients with TM, no new cases of hypothyroidism or
diabetes occurred.[90]
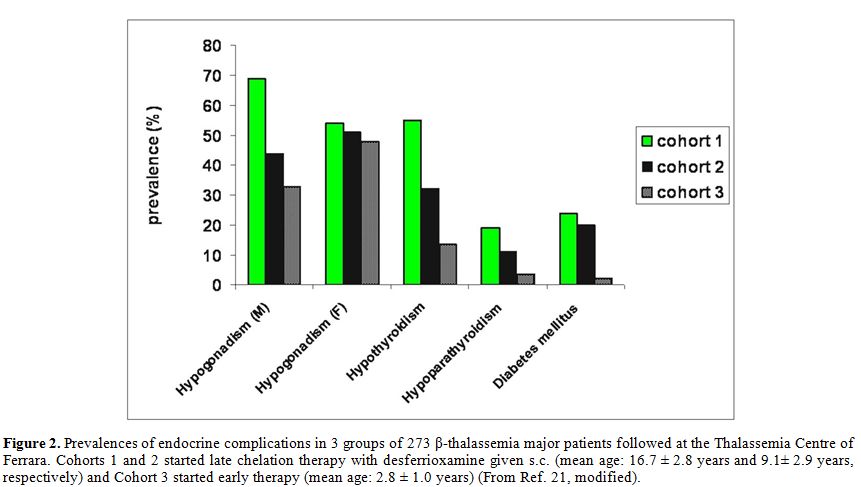 |
Figure 2. Prevalences of
endocrine complications in 3 groups of 273 β-thalassemia major patients
followed at the Thalassemia Centre of Ferrara. Cohorts 1 and 2 started
late chelation therapy with desferrioxamine given s.c. (mean age: 16.7
± 2.8 years and 9.1 ± 2.9 years, respectively) and Cohort 3 started
early therapy (mean age: 2.8 ± 1.0 years) (From Ref. 21, modified). |
In
brief, iron overload-induced hypothyroidism may respond to adequate
iron chelation therapy promoting prevention or/and reversal of the
disease and other associated comorbidities. Irrespective of iron
chelation agent, adherence to iron chelation therapy is essential in
order to achieve prevention of thyroid dysfunctions. Our experience in two groups of TM patients is reported in figure 3.
One group had very good compliance to chelation therapy with DFO, given
s.c. for 6 to 7 times a week, and another group with absent or weak
compliance (DFO therapy from 2 to 3 times a week). No endocrine
complications were observed in the first group of patients (De Sanctis
V, personal observations).
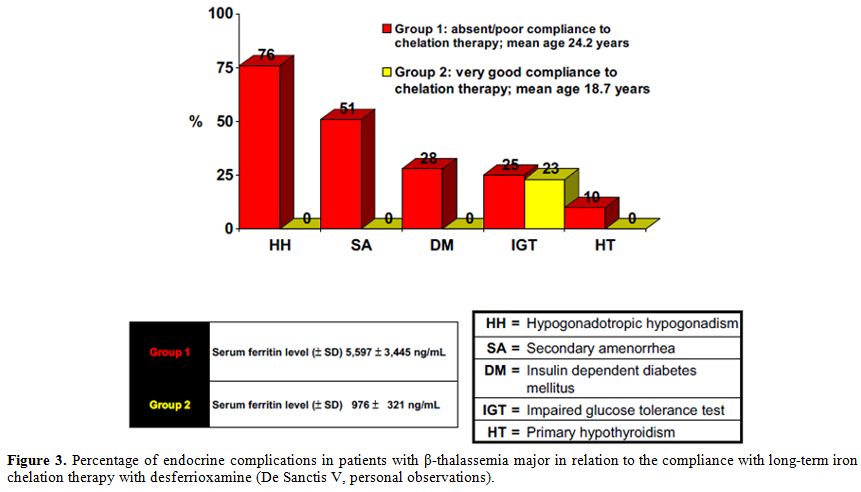 |
Figure 3. Percentage of
endocrine complications in patients with β-thalassemia major in
relation to the compliance with long-term iron chelation therapy with
desferrioxamine (De Sanctis V, personal observations). |
Treatment
1. Primary hypothyroidism.
In patients with a TSH >10 mUI/L, thyroxine therapy (L-T4) is
considered reasonable due to the systemic adverse effects of primary
hypothyroidism (Figure 4). This
is especially true in patients with iron overload. L-T4 monotherapy
remains the treatment of choice due to its long half-life and the
convenience of a single daily dose, and the assumption that T4 is
converted mainly to T3 as needed.
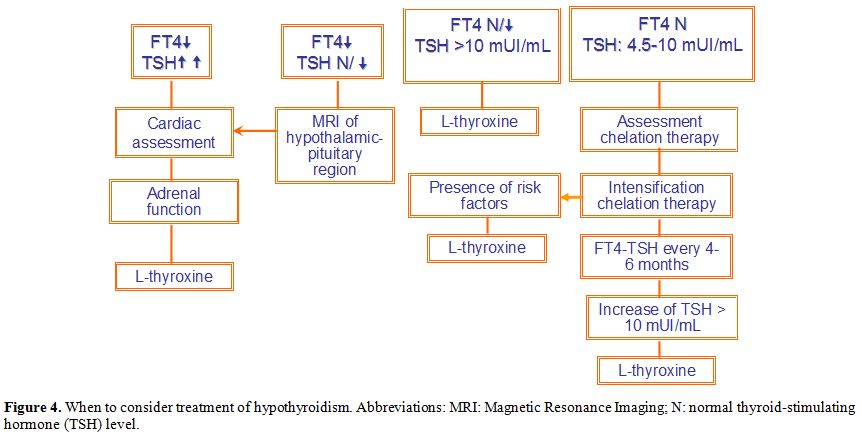 |
Figure 4. When to consider
treatment of hypothyroidism. Abbreviations: MRI: Magnetic Resonance
Imaging; N: normal thyroid-stimulating hormone (TSH) level. |
The
initial L-T4 dosage may range from 12.5 µg/daily to a full replacement
dose based on the age, weight, cardiac status and severity, and
duration of hypothyroidism of the patient. Adjustment of the dose can
be made based on clinical and laboratory data. Close monitoring is
required to avoid overtreatment because L-T4 may cause arrhythmias and
accelerated bone loss.[91,92]In
patients with CH, monitoring of therapy should be based on serum FT4
levels instead of serum TSH levels, and the sample should be collected
prior to ingesting the morning dose of thyroxine. In patients with
co-existent hypocortisolism, glucocorticoid replacement should be
initiated prior to L-T4 replacement.Certain
medications, supplements, and even some foods may affect the L-T4
absorption, such as iron supplements, alumnium hydroxide, which is
found in some antacids, and calcium supplements. Therefore, the
physician must make the appropriate adjustments in L-T4 dosage in the
face of absorption variability and drug interactions.[93]2. Subclinical hypothyroidism (average FT4 and increased TSH).
Current guidelines do not recommend routine thyroid hormone
substitution in subjects with normal FT4 levels and a TSH between 4.5
and 10 mUI/L.[94] However, the term subclinical
hypothyroidism implies that patients should be asymptomatic (although
symptoms are difficult to assess), especially in patients with chronic
disease. Thyroid function tests on a 4-6 months interval are
recommended to monitor treatment based mainly on serum TSH level (Figure 4).[94]Special
attention has to be paid to patients with clinical features or
laboratory findings of reduced growth velocity, short stature, delayed
puberty, cardiac failure, arrhythmias, or iron overload. A recovery of
subclinical hypothyroidism has been observed in some iron overloaded TM
patients after intensive iron chelation therapy.[86-89]
In individual patients, a trial of thyroid hormone substitution, for
several months, may be considered based on a combination of age,
patient's personal history, complaints, and presence of risk factors.[90]In
pregnancy or in women trying to conceive, a mildly increased serum TSH
should always be treated as mild thyroid failure which is associated
with adverse outcomes for both mother and foetus.[95]The
management of thyroid disease during pregnancy has been reviewed in the
guidelines of several societies, including the American Thyroid
Association (ATA) and the Endocrine Society and the European Thyroid
Association (ETA).[96-99] Due to the
physiologic changes in TSH levels during pregnancy, the ATA guidelines
recommend using trimester-specific reference ranges for TSH.[96]
If these reference ranges are not available in the laboratory, the
following reference ranges can be used: first trimester, 0.1 to 2.5
mIU/L; second trimester, 0.2 to 3.0 mIU/L; third trimester, 0.3 to 3.0
mIU/L.[100] Also, different reference ranges for TSH in the first trimester have been reported for different populations.[101]
Therefore, the correct interpretation of thyroid function tests
requires knowledge of a woman's gestational week and the appropriate
population-based reference interval. Open Problems
Currently
available data do not lead to definitive conclusions concerning the
treatment of subclinical thyroid disease in TM patients. In the general
population, possible consequences of subclinical hypothyroidism include
cardiac dysfunction, atherosclerosis, elevated total and LDL
cholesterol, and progression to clinical hypothyroidism.[19,32,37,59]
Several other aspects remain to be elucidated such as the frequency of
thyroid cancer and the existence of a relationship between thyroid
dysfunction, cardiovascular diseases, components of metabolic syndrome
(insulin resistance)[14,15,102] and coagulation disorders.[103,104] Therefore, further studies are needed to explain these emerging issues. Conclusions
Hypothyroidism
is a clinical disorder commonly encountered in iron-overloaded patients
with thalassemia and is defined as fail¬ure of the thyroid gland to
pro¬duce sufficient thyroid hormone to meet the metabolic demands of
the body. The etiology of thyroid disorders in thalassemia patients is
substantially different from that of the general population. Therefore,
the knowledge of risk factors influencing the development of
hypothyroidism is a critical component for long-term monitoring and
treatment of TM patients. Hypothyroidism
is the result of primary gland failure or insufficient thyroid gland
stimulation by the hypo¬thalamus or pituitary gland. The prevalence of
HT increases with age (after the second/third decade of life), although
in developing countries or in patients with severe iron overload it may
occur in the first decade of life.[28] The
identification of risk factors influencing the development of
hypothyroidism is a critical component of long-term monitoring and
treatment of TM patients, according to the international
guidelines.[94]Early
diagnosis and treatment of these complications are essential to ensure
a good quality of life and to reduce late morbidity and mortality. In
patients with CH or TSH >10 mU/L, thyroxine therapy is recommended.
A reversal of subclinical hypothyroidism or improvement of primary
hypothyroidism has been observed in some iron overloaded TM patients
after intensive iron chelation. Therefore, periodic assessment of iron
overload and follow-up to improve adherence to chelation therapy and
patients' satisfaction should be strongly considered in order to
improve the quality of life (QoL) and life expectancy of patients.The
incidence of thyroid cancer detection has increased by 4.5% per year
over the last 10 years, faster than for any other cancer.[105]
The US Preventive Services Task Force (USPSTF) does not recommend
screening for thyroid cancer in asymptomatic adult persons. It
does not apply to persons who experience hoarseness, pain, difficulty
swallowing, or other throat symptoms or persons who have lumps,
swelling, asymmetry of the neck, or other reasons for a neck
examination. It
also does not apply to persons at increased risk of thyroid cancer
because of a history of exposure to ionizing radiation (eg, medical
treatment or radiation fallout), particularly persons with a diet low
in iodine, an inherited genetic syndrome associated with thyroid cancer
(eg, familial adenomatous polyposis), first-degree relative with a
history of thyroid cancer [106] or iron
overload. The emerging issue of thyroid cancer in adult TM patients
indicates the need for preventive measures, yearly thyroid US
surveillance and careful follow-up. Recently Chen et al.,[107]
based on the Thyroid Imaging Reporting and Data System (TI-RADS), built
a new model using a combination of ultrasound patterns including
margin, shape, echogenic foci, echogenicity and nodule halo sign with
age to differentiate benign and malignant thyroid nodules, which had
high sensitivity and specificity.Finally,
additional studies are required to determine the association between
iron overload, oxidative stress, HCV infection, and thyroid carcinomas. References
- Fibach E, Rachmilewitz EA. Pathophysiology and
treatment of patients with beta-thalassemia - an update. F1000Res. 2017
Dec 20;6:2156. eCollection 2017. https://doi.org/10.12688/f1000research.12688.1
- Cappellini
MD, Motta I. New therapeutic targets in transfusion-dependent and
-independent thalassemia. Hematology Am Soc Hematol Educ Program.
2017;2017:278-283.
- Baldini M, Marcon A,
Cassin R, Ulivieri FM, Spinelli D, Cappellini MD, Graziadei G.
Beta-thalassaemia intermedia: evaluation of endocrine and bone
complications. Biomed Res Int. 2014;2014:174581. https://doi.org/10.1155/2014/174581
- De
Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Soliman NA, Elalaily R, C
Kattamis. Endocrine profile of β-thalassemia major patients followed
from childhood to advanced adulthood in a tertiary care center. Indian
J Endocr Metab. 2016;20:451-459. https://doi.org/10.4103/2230-8210.183456 PMid:27366710 PMCid:PMC4911833
- Jackson IMD. Thyrotropin-releasing hormone. N Engl J Med. 1982:306: 145-155. https://doi.org/10.1056/NEJM198201213060305 PMid:6798440
- Yamada
M, Mori M. Mechanisms related to the pathophysiology and management of
central hypothyroidism. Nat Clin Pract Endocrinol Metab. 2008;
4:683-694. https://doi.org/10.1038/ncpendmet0995 PMid:18941435
- Lania A, Persani L, Beck-Peccoz P. Central hypothyroidism. Pituitary. 2008;11:181-186. https://doi.org/10.1007/s11102-008-0122-6 PMid:18415684
- Rose SR. Cranial irradiation and central hypothyroidism.Trends Endocrinol Metab. 2001: 12:97-104. https://doi.org/10.1016/S1043-2760(00)00359-3
- Gary
KA, Winokur A, Douglas SD, Kapoor S, Zaugg L, Dinges DF. Total sleep
deprivation and the thyroid axis: effects of sleep and waking activity.
Aviat Space Environ Med. 1996;67: 513-519. PMid:8827131
- Gómez
JM.Serum leptin, insulin-like growth factor-I components and
sex-hormone binding globulin. Relationship with sex, age and body
composition in healthy population. Protein Pept Lett. 2007;14:708-811. https://doi.org/10.2174/092986607781483868
- Zhang
Z, Boelen A, Kalsbeek A, Fliers E. TRH Neurons and Thyroid Hormone
Coordinate the Hypothalamic Response to Cold. Eur Thyroid J.
2018;7:279-288. https://doi.org/10.1159/000493976 PMid:30574457
- Ortiga-Carvalho
TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE.
Hypothalamus-Pituitary-Thyroid Axis. Compr Physiol. 2016;6:1387-1428.
https://doi.org/10.1002/cphy.c150027 PMid:27347897
- Mebis
L, van den Berghe G. The hypothalamus-pituitary-thyroid axis in
critical illness. Neth J Med. 2009;67:332-340. PMid:19915227
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001; 344:501-509. https://doi.org/10.1056/NEJM200102153440707 PMid:11172193
- Crunkhorn
S, Patti ME. Links between thyroid hormone action, oxidative
metabolism, and diabetes risk? Thyroid. 2008;18: 227-237. https://doi.org/10.1089/thy.2007.0249 PMid:18279023
- De
Sanctis V, Eleftheriou A, Malaventura C, Thalassaemia International
Federation Study Group on Growth and Endocrine Complications in
Thalassaemia.Prevalence of endocrine complications and short stature in
patients with thalassaemia major: a multicenter study by the
Thalassaemia International Federation (TIF). Pediatr Endocrinol Rev.
2004;2 (Suppl 2):249-255 PMid:16462705
- De Sanctis V, Soliman A, Campisi S,M Yassin. Thyroid disorders in thalassaemia: An update. Curr Trends Endocrinol. 2012;6:17-27.
- Grundy
RG, Woods KA, Savage MO, Evans JP. Relationship of endocrinopathy to
iron chelation status in young patients with thalassaemia major.Arch
Dis Child.1994;71:128-132. https://doi.org/10.1136/adc.71.2.128 PMid:7944532 PMCid:PMC1029942
- Zervas
A, Katopodi A, Protonotariou A, Livadas S, Karagiorga M, Politis C,
Tolis G. Assessment of thyroid function in two hundred patients with
beta-thalassemia major.Thyroid.2002;12:151-154. https://doi.org/10.1089/105072502753522383 PMid:11916284
- Skordis
N, Michaelidou M, Savva SC, Ioannou Y, Rousounides A, Kleanthous M,
Skordos G, Christou S.The impact of genotype on endocrine complications
in thalassaemia major. Eur J Haematol.2006;77:150-156. https://doi.org/10.1111/j.1600-0609.2006.00681.x PMid:16800840
- Gamberini
MR, De SanctisV, Gilli G. Hypogonadism, diabetes mellitus,
hypothyroidism, hypoparathyroidism: incidence and prevalence related to
iron overload and chelation therapy in patients with thalassaemia major
followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol
Rev.2008;6 (Suppl 1):158-169. PMid:19337172
- Eshragi
P, Tamaddoni A, Zarifi K, Mohammadhasani A, Aminzadeh M. Thyroid
function in major thalassemia patients: Is it related to height and
chelation therapy? Caspian J Intern Med.2011;2:189-193. PMid:24024013
PMCid:PMC3766932
- Kurtoglu AU, Kurtoglu
E, Temizkan AK. Effect of iron overload on endocrinopathies in patients
with beta-thalassaemia major and intermedia. Endokrynol Pol.
2012;63:260-263. PMid:22933160
- Salih KM,
Al-Mosawy WF. Evaluation some consequences of thalassemia major in
splenectomized and non-splenectomized Iraqi patients. Int J Pharm Pharm
Sci.2013; 5(Suppl 4):385-358.
- Belhoul
KM, Bakir ML, Saned MS, Kadhim AM, Musallam KM, Taher AT. Serum
ferritin levels and endocrinopathy in medically treated patients with β
thalassemia major.Ann Hematol. 2012;91:1107-114. https://doi.org/10.1007/s00277-012-1412-7 PMid:22281991
- Tavazzi
D, Duca L, Graziadei G, Comino A, Fiorelli G, Cappellini MD.
Membrane-bound iron contributes to oxidative damage of
beta-thalassaemia intermedia erythrocytes. Br J Haematol.
2001;112:48-50. https://doi.org/10.1046/j.1365-2141.2001.02482.x PMid:11167782
- Malik
SA, Syed S, Ahmed N. Frequency of hypothyroidism in patients of
beta-thalassaemia. J Pak Med Assoc.2010; 60:17-20. PMid:20055273
- Rindang
CK, Batubara JRL, Amalia P, Satari H. Some aspects of thyroid
dysfunction in thalassemia major patients with severe iron overload.
Paediatr Indones. 2011;51:66-72. https://doi.org/10.14238/pi51.2.2011.66-72
- Pirinççioğlu
AG, Deniz T, Gökalp D, Beyazit N, Haspolat K, Söker M. Assessment of
thyroid function in children aged 1-13 years with Beta-thalassemia
major. Iran J Pediatr.2011;21:77-82. PMid:23056768 PMCid:PMC3446112
- Saleem
M, Ghafoor MB, Anwar J, Saleem MM. Hypothyroidism in beta thalassemia
major patients at Rahim Yar Khan. JSZMC.2016;7:1016-1019.
- Upadya
SH, Rukmini MS, Sundararajan S, Baliga BS, Kamath N. Thyroid Function
in Chronically Transfused Children with Beta Thalassemia Major: A
Cross-Sectional Hospital Based Study. Int J Pediatr. 2018 Sep
16;2018:9071213. https://doi.org/10.1155/2018/9071213
- Landau
H, Matoth I, Landau-Cordova Z, Goldfarb A, Rachmilewitz EA, Glaser B.
Cross-sectional and longitudinal study of the pituitary thyroid axis in
patients with thalassaemia major. Clin Endocrinol (Oxf).1993;38:55-61. https://doi.org/10.1111/j.1365-2265.1993.tb00973.x
- Tatò
L, Lahlou N, Zamboni G, De Sanctis V, De Luca F, Arrigo T, Antoniazzi
F, Roger M. Impaired response of free alpha-subunits after luteinizing
hormone-releasing hormone and thyrotropin-releasing hormone
stimulations in beta-thalassemia major.Horm Res. 1993;39:213-217. https://doi.org/10.1159/000182738 PMid:8314206
- De
Sanctis V, Soliman A, Candini G, Campisi S, Anastasi S, Yassin M. High
prevalence of central hypothyroidism in adult patients with
β-thalassemia major. Georgian Med News. 2013;(222):88-94. PMid:24099820
- Delaporta
P, Maria Karantza M, Sorina Boiu S, Stokidis K, Petropoulou T,
Papasotiriou I, Kattamis C, Kattamis A. Thyroid function in Greek
patients with thalassemia major. Blood 2012;120: Abs. 5176.
DOI:http//dx.doi.org.
- Soliman AT, Al
Yafei F, Al-Naimi L, Almarri N, Sabt A, Yassin M, De Sanctis V.
Longitudinal study on thyroid function in patients with thalassemia
major: High incidence of central hypothyroidism by 18 years. Indian J
Endocrinol Metab. 2013;17:1090-1095. https://doi.org/10.4103/2230-8210.122635 PMid:24381890 PMCid:PMC3872691
- Persani
L. Clinical review: Central hypothyroidism: pathogenic, diagnostic, and
therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. https://doi.org/10.1210/jc.2012-1616 PMid:22851492
- Alexopoulou
O, Beguin C, De Nayer P, Maiter D.Clinical and hormonal characteristics
of central hypothyroidism at diagnosis and during follow-up in adult
patients. Eur J Endocrinol.2004;150:1-8 https://doi.org/10.1530/eje.0.1500001 PMid:14713273
- Andersen
S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in
serum T4 and T3 in normal subjects: a clue to understanding of
subclinical thyroid disease. J Clin Endocrinol Metab. 2002;
87:1068-1072. https://doi.org/10.1210/jcem.87.3.8165 PMid:11889165
- Christoforidis
A, Haritandi A, Tsitouridis I, Tsatra I, Tsantali H, Karyda S,
Dimitriadis AS, Athanassiou-Metaxa M. Correlative study of iron
accumulation in liver, myocardium, and pituitary assessed with MRI in
young thalassemic patients. J Pediatr Hematol Oncol. 2006;28:311-315. https://doi.org/10.1097/01.mph.0000212915.22265.3b PMid:16772883
- Hekmatnia
A, Radmard AR, Rahmani AA, Adibi A, Khademi H. Magnetic resonance
imaging signal reduction may precede volume loss in the pituitary gland
of transfusion-dependent beta-thalassemic patients. Acta Radiol.
2010;5171-5177. https://doi.org/10.3109/02841850903292743
- Noetzli
LJ, Panigrahy A, Hyderi A, Dongelyan A, Coates TD, Wood JC. Pituitary
iron and volume imaging in healthy controls. AJNR Am J Neuroradiol.
2012;33:259-265. https://doi.org/10.3174/ajnr.A2788 PMid:22081683
- De
Sanctis V, Govoni MR, Sprocati M, Marsella M, Conti E. Cardiomyopathy
and pericardial effusion in a 7 year-old boy with beta-thalassaemia
major, severe primary hypothyroidism and hypoparathyroidism due to iron
overload. Pediatr Endocrinol Rev.2008;6 (Suppl 1):181-184. PMid:19337175
- De
Sanctis V, De Sanctis E, Ricchieri P, Gubellini E, Gilli G, Gamberini
MR. Mild subclinical hypothyroidism in thalassaemia major: prevalence,
multigated radionuclide test, clinical and laboratory long-term
follow-up study.Pediatr Endocrinol Rev.2008;6 (Suppl 1):174-180.
PMid:19337174
- Mariotti S, Pigliaru F,
Cocco MC, Spiga A, Vaquer S, Lai ME. β-thalassemia and thyroid failure:
is there a role for thyroid autoimmunity? Pediatr Endocrinol Rev.2011;8
(Suppl 2):307-309. PMid:21705983
- Pes GM,
Tolu F, Dore MP. Anti-Thyroid Peroxidase Antibodies and Male Gender Are
Associated with Diabetes Occurrence in Patients with Beta-Thalassemia
Major. J Diabetes Res. 2016;2016:1401829. doi: 10.1155/2016/1401829. https://doi.org/10.1155/2016/1401829
- Pitrolo
L, Malizia G, Lo Pinto C, Malizia V, Capra M. Ultrasound thyroid
evaluation in thalassemic patients: correlation between the aspects of
thyroidal stroma and function. Pediatr Endocrinol Rev. 2004;2 (Suppl
2):313-315. PMid:16462719
- Filosa A, Di
Maio S, Aloj G, Acampora C. Longitudinal study on thyroid function in
patients with thalassemia major. J Pediatr Endocrinol Metab.
2006;19:1397-1404. https://doi.org/10.1515/JPEM.2006.19.12.1397 PMid:17252692
- Sostre S, Reyes MM. Sonographic diagnosis and grading of Hashimoto's thyroiditis. J Endocrinol Invest.1991;14:115-121. https://doi.org/10.1007/BF03350281 PMid:1648115
- Krittayaphong
R, Viprakasit V, Saiviroonporn P, Wangworatrakul W, Wood JC. Serum
ferritin in the diagnosis of cardiac and liver iron overload in
thalassaemia patients' real-world practice: a multicentre study.Br J
Haematol. 2018;182:301-305. https://doi.org/10.1111/bjh.14776 PMid:28543061
- Wood JC. Estimating tissue iron burden: current status and future prospects.Br J Haematol. 2015;170:15-28. https://doi.org/10.1111/bjh.13374 PMid:25765344 PMCid:PMC4484399
- Soliman
AT, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic anemia and
thyroid function. Acta Biomed. 2017;88:119-127. PMid:28467346
- Shupnik
MA, Weck J, Hinkle PM.Thyrotropin (TSH)-releasing hormone stimulates
TSH beta promoter activity by two distinct mechanisms involving calcium
influx through L type Ca2+ channels and protein kinase C. Mol
Endocrinol. 1996;10:90-99. PMid:8838148
- Alcantara
O, Obeid L, Hannun Y, Ponka P, Boldt DH. Regulation of protein kinase C
(PKC) expression by iron: Effect of different iron compounds on
PKC-beta and PKC-alpha gene expression and role of the 5'- flanking
region of the PKC-beta gene in the response to ferric transferrin.
Blood. 1994;84:3510-3517. PMid:7949105
- Abe
H, Murao K, Imachi H, Cao WM, Yu X, Yoshida K, Wong NC, Shupnik MA,
Haefliger JA, Waeber G, Ishida T. Thyrotropin-releasing
hormone-stimulated thyrotropin expression involves islet-brain-1/c-Jun
N-terminal kinase interacting protein-1. Endocrinology.
2004;145:5623-5628. https://doi.org/10.1210/en.2004-0635 PMid:15345675
- Blackard
JT, Kong L, Huber AK, Tomer Y. Hepatitis C virus infection of a thyroid
cell line: Implications for pathogenesis of hepatitis C virus and
thyroiditis. Thyroid 2013;23:863‑70. https://doi.org/10.1089/thy.2012.0507 PMid:23259732 PMCid:PMC3704108
- Vezali
E, Elefsiniotis I, Mihas C, Konstantinou E, Saroglou G. Thyroid
dysfunction in patients with chronic hepatitis C: Virus‑ or
therapy‑related? J Gastroenterol Hepatol 2009;24:1024‑9. https://doi.org/10.1111/j.1440-1746.2009.05812.x PMid:19383078
- Al-Khabori
M, Daar S, Al-Busafi SA, Al-Dhuhli H, Alumairi AA, Hassan M, Al-Rahbi
S, Al-Ajmi U. Noninvasive assessment and risk factors of liver fibrosis
in patients with thalassemia major using shear wave elastography.
Hematology. 2019;24:183-188. https://doi.org/10.1080/10245332.2018.1540518 PMid:30453843
- De
Sanctis V, Tanas R, Gamberini MR, Sprocati M, Govoni MR, Marsella M.
Exaggerated TSH response to TRH ("sub-biochemical" hypothyroidism) in
prepubertal and adolescent thalassaemic patients with iron overload:
prevalence and 20-year natural history. Pediatr Endocrinol Rev. 2008;6
(Suppl 1):170-173. PMid:19337173
- Hashemi
A, Ordooei M, Golestan M, Akhavan Ghalibaf M, Mahmoudabadi F.
Hypothyroidism and serum ferritin level in patientswith major ß
thalassemia. Iran J Pediatr Hematol Oncol. 2012;2:12-15.
- Musallam
KM, CappelliniMD, Wood JC, Motta I, Graziadei G, Tamim H, Taher AT.
Elevated liver iron concentration is a marker of increased morbidity in
patients with β thalassemia intermedia. Haematologica. 2011;
96:1605-1612. https://doi.org/10.3324/haematol.2011.047852 PMid:21791471 PMCid:PMC3208677
- Farhan
H, Albulushi A, Taqi A, Al-Hashim A, Al-Saidi K, Al-Rasadi K,
Al-Mazroui A, Al-Zakwani I. Incidence and pattern of thyroid
dysfunction in patients on chronic amiodarone therapy: experience at a
tertiary care centre in Oman. Open Cardiovasc Med J. 2013;7:122-126. https://doi.org/10.2174/1874192401307010122 PMid:24358062 PMCid:PMC3866614
- Martino
E, Safran M, Aghini-Lombardi F, Rajatanavin R, Lenziardi M, Fay M,
Pacchiarotti A, Aronin N, Macchia E, Haffajee C. Environmental iodine
intake and thyroid dysfunction during chronic amiodarone therapy. Ann
Intern Med. 1984;101:28-34. https://doi.org/10.7326/0003-4819-101-1-28 PMid:6428291
- Martino
E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on
the thyroid. Endocr Rev. 2001;22:240-254. PMid:11294826
- Eskes SA, Wiersinga WM. Amiodarone and thyroid. Best Pract Res Clin Endocrinol Metab.2009; 23:735-751. https://doi.org/10.1016/j.beem.2009.07.001 PMid:19942150
- Cohen-Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol. 2010;6:34-641. https://doi.org/10.1038/nrendo.2009.225 PMid:19935743
- Kotwal
A, Clark J, Lyden M, McKenzie T, Thompson G, Stan MN. Thyroidectomy for
amiodarone-induced thyrotoxicosis: Mayo Clinic Experience. J Endocr
Soc. 2018;2:1226-1235. https://doi.org/10.1210/js.2018-00259 PMid:30370394 PMCid:PMC6198926
- Alexandrides
T, Georgopoulos N, Yarmenitis S, Vagenakis AG. Increased sensitivity to
the inhibitory effect of excess iodide on thyroid function in patients
with beta-thalassemia major and iron overload and the subsequent
development of hypothyroidism. Eur J Endocrinol.2000;143:319-325. https://doi.org/10.1530/eje.0.1430319 PMid:11022172
- Finianos
A, Matar CF, Taher A. Hepatocellular Carcinoma in β-Thalassemia
Patients: Review of the Literature with Molecular Insight into Liver
Carcinogenesis.Int J Mol Sci. 2018 Dec 17;19(12). pii: E4070.. https://doi.org/10.3390/ijms19124070
- Benetatos
L, Alymara V, Vassou A, Bourantas KL. Malignancies in beta-thalassemia
patients: a single-center experience and a concise review of the
literature. Int J Lab Hematol. 2008;30: 167-172. https://doi.org/10.1111/j.1751-553X.2007.00929.x PMid:18333849
- Karimi
M, Giti R, Haghpanah S, Azarkeivan A, Hoofar H, Eslami M. Malignancies
in patients with beta-thalassemia major and beta-thalassemia
intermedia: a multicenter study in Iran. Pediatr Blood Cancer.
2009;53:1064-1067. https://doi.org/10.1002/pbc.22144 PMid:19533641
- Govoni
MR, Sprocati M, Fabbri E, Zanforlin N, De Sanctis V. Papillary thyroid
cancer in thalassaemia. Pediatr Endocrinol Rev. 2011; 8 (Suppl
2):314-321. PMid:21705985
- Poggi M,
Sorrentino F, Pascucci C, Monti S, Lauri C, Bisogni V, Toscano V,
Cianciulli P. Malignancies in β-thalassemia patients: first description
of two cases of thyroid cancer and review of the literature.
Hemoglobin. 2011;35:439-446. https://doi.org/10.3109/03630269.2011.588355 PMid:21797713
- De
Sanctis V, Campisi S, Fiscina B, Soliman A. Papillary thyroid
microcarcinoma in thalassaemia: an emerging concern for physicians?
Georgian Med News. 2012; 210:71-76.
- De
Sanctis V, Soliman AT, Duran Canatan D, Tzoulis P, Daar S, Di Maio S,
Elsedfy H, Yassin MA, Filosa A, Soliman N, Karimi M, Saki F, Sobti P,
Kakkar S, Christou S, Albu A, Christodoulides C, Kilinc Y, Al Jaouni S,
Khater D, Alyaarubi SA, Lum SA, Campisi S, Anastasi S, Galati MC,
Raiola G, Wali Y, Elhakim IZ, Mariannis D, Ladis V, Kattamis C. An
ICET-A survey on occult and emerging endocrine complications in
patients with β-thalassemia major: Conclusions and recommendations.
Acta Biomed. 2019;89:481-489. PMid:30657116
- Ferri
C, La Civita L, Zignego AL, Pasero G. Viruses and cancers: possible
role of hepatitis C virus. Eur J Clin Invest.1997; 27:711-718. https://doi.org/10.1046/j.1365-2362.1997.1790728.x PMid:9352239
- Russ
G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European
Thyroid Association Guidelines for ultrasound malignancy risk
stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid
J. 2017;6:225-237. https://doi.org/10.1159/000478927 PMid:29167761 PMCid:PMC5652895
- Vargas-Uricoechea H, Sierra-Torres CH. Thyroid hormones and the heart. Horm Mol Biol Clin Investig. 2014;18:15-26. https://doi.org/10.1515/hmbci-2013-0059
- Morselli
E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of
oestrogens and their receptors on cardiometabolic health. Nat Rev
Endocrinol. 2017;13:352-364. https://doi.org/10.1038/nrendo.2017.12 PMid:28304393
- Caicedo
D, Díaz O, Devesa P, Devesa J. Growth Hormone (GH) and Cardiovascular
System. Int J Mol Sci. 2018 Jan 18;19(1). pii: E290. doi:
10.3390/ijms19010290. https://doi.org/10.3390/ijms19010290
- Bielecka-Dabrowa
A, Godoy B, Suzuki T, Banach M, von Haehling S. Subclinical
hypothyroidism and the development of heart failure: an overview of
risk and effects on cardiac function. Clin Res Cardiol. 2018 Aug 8. https://doi.org/10.1007/s00392-018-1340-1
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344 501-509. https://doi.org/10.1056/NEJM200102153440707 PMid:11172193
- Kannan
L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, Cappola TP,
Cappola AR. Thyroid Dysfunction in Heart Failure and Cardiovascular
Outcomes. Circ Heart Fail. 2018 Dec;11(12):e005266. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005266
- Gamberini
MR, Meloni A, Rossi A, Secchi G, D'Ambrosio A, Macchi S, Pulini S, De
Franceschi L, Vallone A, Lombardi M, Pepe A. Hypothyroidism and Cardiac
Complications In Thalassemia Major Patients. Blood 2013;122: abs.2254.
- Waldmeier
F, Bruin GJ, Glaenzel U, Hazell K, Sechaud R, Warrington S, Porter
JB.Pharmacokinetics, metabolism, and disposition of deferasirox in
beta-thalassemic patients with transfusion-dependent iron overload who
are at pharmacokinetic steady state. Drug Metab Dispos.
2010;38:808-816. https://doi.org/10.1124/dmd.109.030833 PMid:20097723
- Farmaki
K, Tzoumari I, Pappa Ch. Reversal of hypothyroidism in well chelated
β-thalassemia major patients. Blood. 2008;112:1323-1324.
- Farmaki
K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of
total body iron load with very intensive combined chelation reverses
cardiac and endocrine complications of thalassaemia major. Br J
Haematol. 2010;148: 466-475. https://doi.org/10.1111/j.1365-2141.2009.07970.x PMid:19912219
- Farmaki
K, Berdoukas V. Reversal of endocrinopathies in transfusional iron
overload patients - The next frontier in iron chelation. EJCMO.
2010;2:59-66.
- Farmaki K, Tzoumari I,
Pappa C. Combining oral chelators in transfusion dependents thalassemia
major patients, may prevent or reverse iron overload complications.
Blood Cell Mol Dis. 2011; 47: 33-40. https://doi.org/10.1016/j.bcmd.2011.03.007 PMid:21531154
- Casale
M, Citarella S, Filosa A, De Michele E, Palmieri F, Ragozzino A,
Amendola G, Pugliese U, Tartaglione I, Della Rocca F, Cinque P, Nobili
B, Perrotta S. Endocrine function and bone disease during long-term
chelation therapy with deferasirox in patients with β-thalassemia
major. Am J Hematol. 2014 ;89:1102-1106. https://doi.org/10.1002/ajh.23844 PMid:25197009
- Roos
A, Linn-Rasker SP, van Domburg RT, Tijssen JP,Berghout A. The starting
dose of levothyroxine in primary hypothyroidism treatment: a
prospective, randomized, double-blind trial. Arch Intern Med.
2005;165:1714-1720. https://doi.org/10.1001/archinte.165.15.1714 PMid:16087818
- Reddy
PA, Harinarayan CV, Sachan A, Suresh V, Rajagopal G. Bone disease in
thyrotoxicosis. Indian J Med Res. 2012;135:277-286. PMid:22561612
PMCid:PMC3361862
- Peeters RP. Thyroid hormones and aging. Hormones. 2008:7:28-35. https://doi.org/10.14310/horm.2002.1111035 PMid:18359742
- De
Sanctis V, Soliman AT, Elsedfy H, Skordis N, Kattamis C, Angastiniotis
M, Karimi M, Yassin MA, El Awwa A, Stoeva I, Raiola G, Galati MC,
Bedair EM, Fiscina B, El Kholy M. Growth and endocrine disorders in
thalassemia: The international network on endocrine complications in
thalassemia (I-CET) position statement and guidelines. Indian J
Endocrinol Metab. 2013;17:8-18. https://doi.org/10.4103/2230-8210.107808 PMid:23776848 PMCid:PMC3659911
- De
Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH,
Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J,
Sullivan S. Management of thyroid dysfunction during pregnancy and
postpartum: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2012;97:2543-2565. https://doi.org/10.1210/jc.2011-2803 PMid:22869843
- Stagnaro-Green
A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A,
Pearce EN, Soldin OP, Sullivan S, Wiersinga W; American Thyroid
Association Task force on Thyroid Disease During Pregnancy and Post
partum.Guidelines of the American Thyroid Association for the diagnosis
and management of thyroid disease during pregnancy and post
partum.Thyroid. 2011; 21:1081-1125. https://doi.org/10.1089/thy.2011.0087 PMid:21787128 PMCid:PMC3472679
- De
Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH,
Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J,
Sullivan S. Management of thyroid dysfunction during pregnancy and
postpartum: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2012;97:2543-2565. https://doi.org/10.1210/jc.2011-2803 PMid:22869843
- Lazarus
J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B.
European Thyroid Association guidelines for the management of
subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J.
2014;3:76-94. https://doi.org/10.1159/000362597 PMid:25114871 PMCid:PMC4109520
- Brabant
G, Peeters RP, Chan SY, Bernal J, Bouchard P, Salvatore D, Boelaert K,
Laurberg P. Management of subclinical hypothyroidism in pregnancy: are
we too simplistic? Eur J Endocrinol. 2015;173:P1-P11. https://doi.org/10.1530/EJE-14-1005 PMid:25650404
- McNeil
AR, Stanford PE. Reporting thyroid function tests in pregnancy. Clin
Biochem Rev. 2015;36:109-126. PMid:26900190 PMCid:PMC4758281
- Medici
M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in
pregnancy: what is normal? Clin Chem. 2015;61:704-713. https://doi.org/10.1373/clinchem.2014.236646 PMid:25829408
- Grande
D, Terlizzese P, Gioia MI, Parisi G, Giagulli VA, Triggiani V,
Iacoviello M. New frontiers in the therapeutic approach of patients
with cardiovascular and endocrine diseases.Endocr Metab Immune Disord
Drug Targets. 2019 Jan 1. https://doi.org/10.2174/1871530319666190101151542
- Nakova
VV, Krstevska B, Kostovska ES, Vaskova O, Ismail LG. The effect of
levothyroxine treatment on left ventricular function in subclinical
hypothyroidism.Arch Endocrinol Metab. 2018;62:392-398. PMid:30304103
- Elbers LPB, Squizzato A, Gerdes VEA. Thyroid Disorders and Hemostasis. Semin Thromb Hemost. 2018;44:676-682. https://doi.org/10.1055/s-0038-1666825 PMid:30045389
- US
Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry
SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist
AH, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M,
Simon MA, Siu AL, Tseng CW. Screening for Thyroid Cancer: US Preventive
Services Task Force Recommendation Statement. JAMA. 2017;317:1882-1887.
https://doi.org/10.1001/jama.2017.4011 PMid:28492905
- Lin
JS, Aiello Bowles EJ, Williams SB, Morrison CC. Screening for thyroid
cancer: updated evidence report and systematic review for the US
Preventive Services Task Force. JAMA. 2017;317:1888-1903. https://doi.org/10.1001/jama.2017.0562 PMid:28492904
- Chen
L, Zhang J, Meng L, Lai Y, Huang W. A new ultrasound nomogram for
differentiating benign and malignant thyroid nodules. Clin Endocrinol
(Oxf). 2019;90:351-359. https://doi.org/10.1111/cen.13898 PMid:30390403
[TOP]




