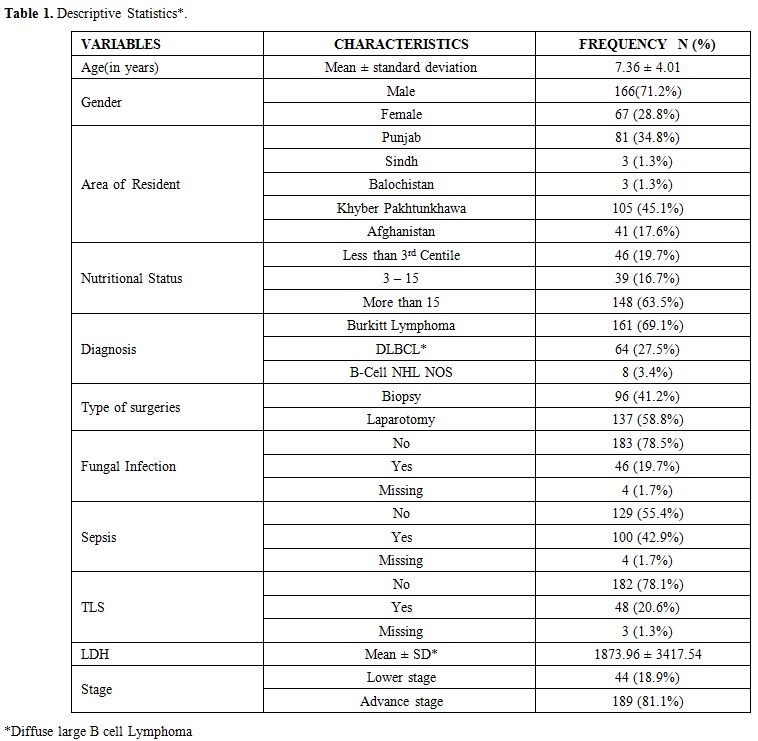
Data were collected for age, gender, residence, nutritional status, duration of symptoms, site, stage, Lactate dehydrogenase levels, treatment, complications, and outcomes. The diagnosis was made on morphology and immunohistochemistry. Patients were classified according to World Health Organization classification.
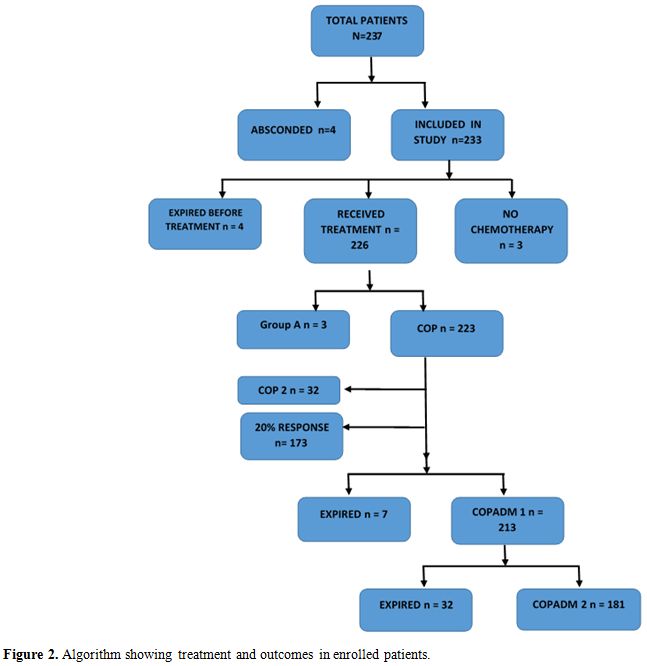
Staging done according to St. Jude Children Research Hospital staging system with CT scan.[9] Central nervous system positive disease was defined as any L3 blasts in cerebrospinal fluid, cranial nerve palsy (if not explained by extra-cranial tumor), clinical spinal cord compression, isolated intracerebral mass or parameningeal extension: cranial and/or spinal. Bone marrow with less than 25% blasts was considered positive. Stage I and II were labeled as Low stage and III and IV as Advanced stage. Patients were risk stratified in Treatment Groups A, B, and C based on staging and resection status according to United Kingdom Children’s Cancer Study Group 2003 guidelines (Figure 1).
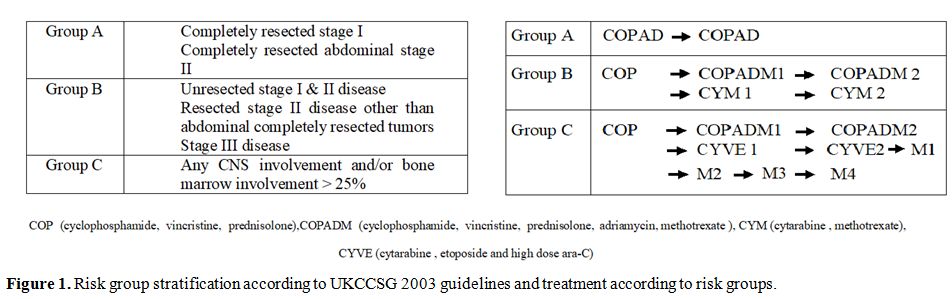 |
Figure 1. Risk group stratification according to UKCCSG 2003 guidelines and treatment according to risk groups. |
All patients were treated according to the UKCCSCG 2003 Non-Hodgkin’s lymphoma guidelines. Cytoreductive chemotherapy COP was given according to guideline recommendations. In clinically unstable patients, a second COP was also given. Reassessment scans were done on day 7 of COP in treatment groups B and C and therapy was intensified from Group B to C if there was less than 20% response. Tumor lysis syndrome preventive protocol was followed in all patients at risk.
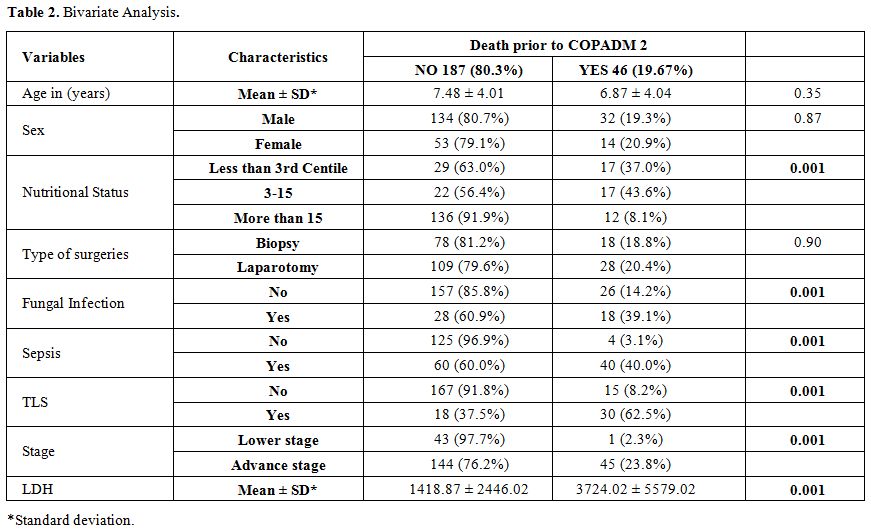
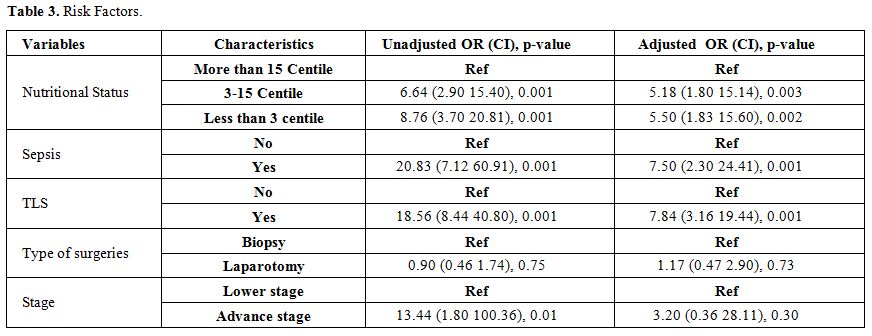
Acute mortality was defined as death before administration of the second induction course COPADM cycle 2. Risk factors studied for acute mortality were malnutrition, stage, prior surgery with open laparotomy, lactate dehydrogenase levels, tumor lysis syndrome, sepsis, and fungal infection. Malnutrition was assessed using World Health Organization weight for age percentile charts and patients were divided in three groups, less than 3rd, 3rd to 15th and more than 15th centiles.[10] Tumor lysis syndrome (TLS) was defined according to Cairo-Bishop definition as any two of the following features: Uric acid level > 8 mg/dl or 25% increase from baseline, Potassium level > 6 meq/l or 25% increase from baseline, Phosphorus > 6.5 mg/dl , Calcium level < 7 mg/dl or 25% decrease from baseline, and clinical if laboratory criteria fulfilled plus any of these three: serum creatinine > 1.5 normal upper limit for age, arrhythmias or sudden death and seizures.[11] Sepsis was considered to be present either based on the presence of positive blood cultures or labeled in clinical notes if culture is negative. Fungal infection was considered if proven histologically; biochemical markers were elevated or if the probable fungal infection was labeled and treated in clinical notes based on radiographic findings.
Statistical analysis was carried out using the SPSS software (version 20.0; SPSS, Chicago, IL, USA). Continuous variables were stated as Mean ± SD, and categorical variables were computed as frequencies and percentages. Categorical variables were compared using the chi-square test or Fisher's exact test (when necessary). The continuous variables were compared using the independent t-test. Multivariable logistic regression (MLR) model was used to identify the independent risk factors associated with mortality. Statistical significance was defined as a two-tailed p-value < 0.05.