Alessandra Serrao, Benedetta
Lucani, Davide Mansour, Antonietta Ferretti, Erminia Baldacci, Cristina
Santoro, Robin Foà and Antonio Chistolini.
Hematology, Department of Translational and Precision Medicine, Sapienza University, Rome, Italy
Correspondence to:Antonio Chistolini. Hematology, Department of Translational and Precision Medicine, Sapienza University, Rome, Italy. E-mail:
antonio.chistolini@uniroma1.it
Published: July 01, 2019
Received: April 17, 2019
Accepted: June 10, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019044 DOI
10.4084/MJHID.2019.044
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Thrombophilia is a condition that predisposes to a higher incidence of
venous thromboembolisms (VTE), some also in atypical sites. Direct oral
anticoagulants (DOACs) have proven to be effective in the treatment of
deep vein thrombosis (DVT). However, their use can be sometimes
challenging in particular settings of patients such as those with major
thrombophilia - antithrombin, protein C and protein S deficiency,
homozygous mutation of Factor V Leiden, homozygous mutation of Factor
II G20210A, combined heterozygous mutation of factor V Leiden and
Factor II G20210A – carrying a high thrombotic risk.
Patients and Methods:
At our Center, 45 patients with major thrombophilia were treated with
DOACs: 33 after an initial treatment with vitamin K antagonists (VKA)
and 12 as first-line therapy for VTE. The median follow-up of DOACs
treatment was 29 months.
Conclusions:
No patient presented hemorrhagic or thrombotic complications during
DOAC therapy. DOACs have proven to be effective and safe in this
real-life series of patients with major thrombophilia.
|
Introduction
Thrombophilia
is defined as a predisposition condition towards thrombosis, in
particular, venous thrombosis. This condition increases the risk and
the recurrence of venous thromboembolism (VTE).[1] A thrombophilic phenotype occurs in approximately 4% of patients with idiopathic VTE.[2]
Inherited thrombophilia includes physiologic coagulation inhibitors
deficiency: antithrombin (AT), protein C (PC), protein S (PS), F V
Leiden mutation, and prothrombin G20210A mutation. Major thrombophilia
(physiologic inhibitors deficiency, homozygous F V Leiden,
homozygous F II G20210A, combined defects) differs from minor
thrombophilia (FV Leiden or F II G20210A heterozygous) because it
exposes the affected patients to a higher risk of VTE complication.[3,4]
Treatment
of VTE is represented by anti-vitamin K antagonists (VKA) or direct
oral anticoagulants (DOACs). Clinical studies evaluating the use of
DOACs in congenital thrombophilia include case reports[5-8] and post-hoc analysis of clinical trials;[9-11]
these studies have analyzed minor and major thrombophilic patients. Few
data are available to support the use of DOACs in the treatment of VTE
in patients with major thrombophilia. We hereby report our experience
on the use of DOACs for the treatment of VTE in patients affected by
major thrombophilia. Aim of our study was to evaluate the efficacy -
prevention of recurrent VTE - and safety - the absence of bleeding
complications - in the above-mentioned population.
Methods
Study population.
We studied 45 patients affected by major thrombophilia: 5 patients with
AT deficiency, 5 with PC deficiency, 18 with PS deficiency, 12 with
homozygous mutation of Factor V Leiden, 1 with homozygous mutation of
Factor II G20210A and 4 with a combined heterozygous mutation of Factor
V Leiden and Factor II G20210A. Twenty-four were male and 21 female
with an average age of 40.3 years (16-73) at the start of anticoagulant
therapy. Patients were affected by VTE: 32 had a diagnosis of deep
venous thrombosis (DVT), 13 presented a DVT and pulmonary embolism (PE)
(Table 1). DOACs were
administered front-line or after VKA. The patients were switched from
VKA to DOACs because of a fluctuating international normalized ratio
(INR), difficulty in carrying out a regular monitoring or patient
request. The patients were treated with the following DOACs:
rivaroxaban, dabigatran, apixaban, edoxaban (Table 2).
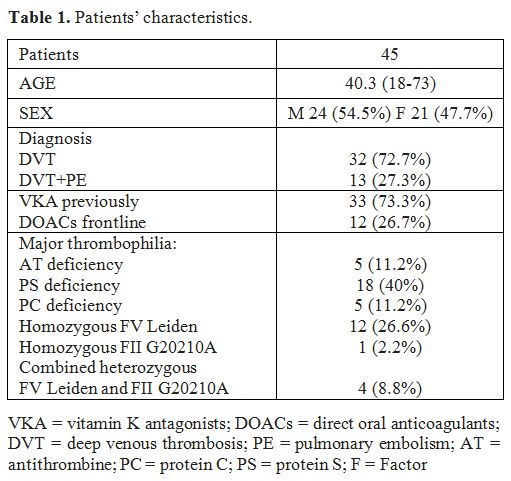 |
Table1. Patients’ characteristics. |
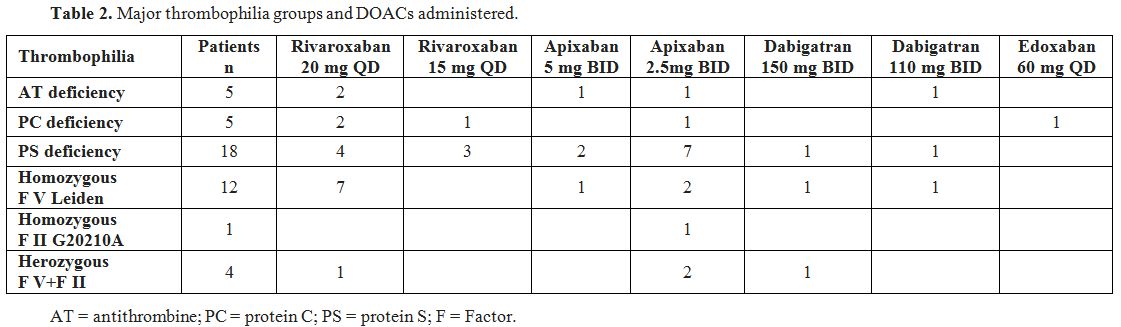 |
Table
2. Major thrombophilia groups and DOACs administered. |
Results
Twelve
patients were treated with DOACs front-line, 33 patients switched from
VKA: 13 for a fluctuating INR with time in therapeutic range (TTR)
lower than 50%, 12 patients for poor compliance and eight following
their request. The median VKA treatment follow-up was 60 months (range
6-180); the median DOACs treatment follow-up was 29 months (6-66).
Rivaroxaban was administered to 20 patients: front-line in 6 and after
previous VKA treatment in 14. Dabigatran was administered to 6 patients
(front-line 2, after VKA treatment 4). Apixaban was administered to 18
patients: 5 front-line and 13 after VKA. Edoxaban was administered to 1
patient front-line at the standard dose of 60 mg QD. During VKA
treatment, we observed three hemorrhagic complications with an
incidence rate of 1.82% patient-years and two thrombotic events with an
incidence rate of 1.21% patient-years. The bleeding events were: an
episode of mild gum bleeding; epistaxis in a patient with a PS
deficiency who was also taking clopidogrel, hematuria. The two
thrombotic events were: central retinal vein thrombosis and
DVT recurrence in a patient with PS deficiency and TTR 26%. During
treatment with DOACs, none of the 45 patients presented hemorrhagic or
thromboembolic complications (Table 3).
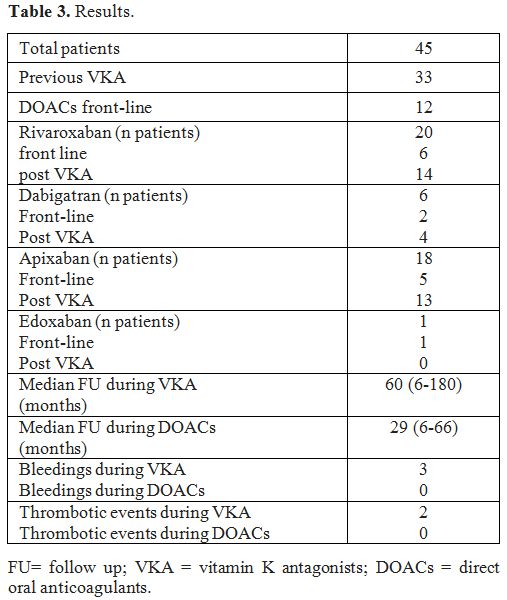 |
Table 3. Results. |
Discussion
Patients
affected by inherited thrombophilia present a high risk of DVT
complicated by PE or thrombosis in atypical sites at a young age. These
patients need to start anticoagulant therapy. The role of DOACs in the
treatment of VTE complications in thrombophilic patients remains
unclear. The prevalence of known thrombophilia in VTE trials with DOACs
ranges from 2 to 18%.[12] RE-COVER, RE-COVER II and RE-MEDY studies compared dabigatran with warfarin,[9] Einstein studies compared rivaroxaban with warfarin,[13,14] Amplify and Hokusai studies compared warfarin with apixaban and edoxaban, respectively.[15,16]
The post-hoc analysis of these studies shows no differences in the
efficacy and safety of DOACs regardless of the presence or absence of
thrombophilia. However, these clinical studies included patients
affected by minor and major thrombophilia; in addition, the patients
included were not routinely screened for congenital thrombophilia, and
tests were not performed centrally. There are few data on the real-life
use of DOACs in patients diagnosed with severe inherited thrombophilia.
The
aim of our study was to evaluate the efficacy and safety of DOACs in
the treatment and prevention of thromboembolic events in patients
affected by major congenital thrombophilia. We studied the role of
DOACS front-line and in patients who switched from VKA. The tests for
the thrombophilic status were all performed in our dedicated laboratory.
The
majority of our patients (73%) switched from VKA to DOACs. During VKA
treatment, we observed three mild hemorrhagic complications and two
thrombotic events. No adverse events have been reported in patients
during DOACs therapy. Probably this result is influenced by the
different length of the two treatments follow-up. We did not observe
differences in the efficacy and tolerability in the 4 DOACs regardless
of the type of thrombophilia. Conflicting reports have been published
regarding the efficacy of DOACs in preventing recurrent VTE in patients
with PC and PS deficiency.[6,8] Undas et al. reported VTE recurrence in 2 of 3 patients affected by PS deficiency during DOACs treatment.[10]
We studied 18 patients with PS deficiency: 4 patients treated with
DOACs front-line, 14 patients who switched from VKA. We did not observe
any thrombotic complications. Regarding PC deficiency; a case report
described treatment failure during DOACs treatment in a rare
heterozygous mutation of the protein C gene.[17] In our cohort of PC deficiency patients (5 patients), DOACs have shown efficacy in treating VTE.
Another
not negligible aspect is the quality of life of patients who switch
from a treatment that requires periodic controls of INR to a less
demanding regimen with fewer drug interactions.
Conclusions
Although
the poor casuistry (partially due to the rarity of major thrombophilia)
with a brief follow-up and the limitations of a retrospective study,
our evidence suggests that DOACs are a promising therapeutic option for
the treatment of acute VTE in the presence of major thrombophilia, in
terms of efficacy, safety and quality of life.
References
- Christiansen SC, Cannegieter SC, Koster T,
Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and
recurrent venous thrombotic events. Jama. 2005; 293: 2352-61. https://doi.org/10.1001/jama.293.19.2352 PMid:15900005
- Garcia
-Horton A, Kovacs MJ, Abdulrehman J, Taylor JE, Sharma S, Lazo-Langner
A. Impact of thrombophilia screening on venous thromboembolism
management practices. Thrombosis Research. 2017; 149: 76 -80. https://doi.org/10.1016/j.thromres.2016.11.023 PMid:27931012
- Lijfering
WM, Brouwer JL, Veeger NJ, Bank I, Coppens M, Middeldorp S, Hamulyak K,
Prins MH, Buller HR, van der Meer J. Selective testing for
thrombophilia in patients with first venous thrombosis: results from a
retrospective family cohort study on absolute thrombotic risk for
currently known thrombophilic defects in 2479 relatives. Blood.
2009113: 5314-22. 10.1182/blood - 2008 -10 -184879. https://doi.org/10.1182/blood-2008-10-184879 PMid:19139080
- Crowther
MA, Kelton JG. Congenital thrombophilic states associated with venous
thrombosis: a qualitative overview and proposed classification system.
Annals of Internal Medicine. 2003; 138: 128-34. https://doi.org/10.7326/0003-4819-138-2-200301210-00014
- Kawai
H, Matsushita H, Kawada H, Ogawa Y, Ando K. The Successful Prevention
of Thromboembolism Using Rivaroxaban in a Patient with Antithrombin
Deficiency during the Perioperative Period. Intern Med. 2017 Sep
1;56(17):2339-2342. https://doi.org/10.2169/internalmedicine.8487-16 PMid:28794370 PMCid:PMC5635311
- Wypasek
E, Potaczek DP, Alhenc-Gelas M, Undas A. PROS1 mutations associated
with protein S deficiency in Polish patients with residual vein
obstruction on rivaroxaban therapy. Thromb Res. 2014 Jul;134(1):199-201
https://doi.org/10.1016/j.thromres.2014.01.023 PMid:24507871
- Martinelli
I, Mannucci PM, De Stefano V, Taioli E, Rossi V, Crosti F, Paciaroni K,
Leone G, Faioni EM. Different risks of thrombosis in four coagulation
defects associated with inherited thrombophilia: a study of 150
families. Blood. 1998 Oct 1;92(7):2353-8
- Hermans
C, Eeckhoudt S, Lambert C. Dabigatran etexilate (Pradaxa®) for
preventing warfarin-induced skin necrosis in a patient with severe
protein C deficiency. Thromb Haemost. 2012 Jun;107(6):1189-91 https://doi.org/10.1160/TH11-11-0788 PMid:22398431
- Goldhaber
SZ, Eriksson H, Kakkar A, Schellong S, Feuring M, Fraessdorf M, Kreuzer
J, Schueler E, Schulman S. Efficacy of dabigatran versus warfarin in
patients with acute venous thromboembolism in the presence of
thrombophilia: Findings from RE-COVER®, RE-COVER™ II, and RE-MEDY™.
Vasc Med. 2016 Dec;21(6):506-514 https://doi.org/10.1177/1358863X16668588 PMid:27807306
- Undas
A, Góralczyk T. Direct Oral Anticoagulants in Patients with
Thrombophilia: Challenges in Diagnostic Evaluation and Treatment. Adv
Clin Exp Med. 2016 Nov-Dec;25(6):1321-1330 https://doi.org/10.17219/acem/65853 PMid:28028988
- Elsebaie
MA, van Es N, Langston A, Büller HR, Gaddh M. Direct Oral
Anticoagulants in Patients with Venous Thromboembolism and
Thrombophilia: A Systematic Review and Meta-Analysis. J Thromb Haemost.
2019 Jan 28. https://doi.org/10.1111/jth.14398 PMid:30690830
- Sciascia
S, Lopez-Pedrera C, Cecchi I, Pecoraro C, Roccatello D, Cuadrado MJ.
Non-vitamin K antagonist oral anticoagulants and antiphospholipid
syndrome. Rheumatology (Oxford). 2016 Oct;55(10):1726-35 https://doi.org/10.1093/rheumatology/kev445 PMid:26843482
- Bauersachs
R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing
AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P,
Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S.
Oral rivaroxaban for symptomatic venous thromboembolism. The New
England Journal of Medicine. 2010; 363: 2499 -510. https://doi.org/10.1056/NEJMoa1007903
- Buller
HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob
GE, Schellong SM, Schwocho L, Segers A, Shi M, Verhamme P, Wells P.
Edoxaban versus warfarin for the treatment of symptomatic venous
thromboembolism. The New England Journal of Medicine. 2013; 369: 1406
-15.
- Agnelli
G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U,
Pak R, Thompson J, Raskob GE, Weitz JI. Oral apixaban for the treatment
of acute venous thromboembolism. The New England Journal of Medicine.
2013; 369: 799 -808. https://doi.org/10.1056/NEJMoa1302507 PMid:23808982
- Buller
HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob
GE, Schellong SM, Schwocho L, Segers A, Shi M, Verhamme P, Wells P.
Edoxaban versus warfarin for the treatment of symptomatic venous
thromboembolism. The New England Journal of Medicine. 2013; 369: 1406
-15.
- Boey
JP, Jolley A, Nicholls C, Lerda N, Duncan E, Gallus A, Ross DM,
Sobieraj -Teague M. Novel protein C gene mutation in a compound
heterozygote resulting in catastrophic thrombosis in early adulthood:
diagnosis and long -term treatment with subcutaneous protein C
concentrate. British Journal of Haematology. 2016; 172: 811-3. https://doi.org/10.1111/bjh.13538 PMid:26103879
[TOP]




