Ioannis Kotsianidis1, Sotirios G. Papageorgiou2, Vassiliki Pappa2, Athanasios G. Galanopoulos3, Nora-Athina Viniou4, Theodoros P. Vassilakopoulos5, Menelaos Papoutselis1, George Vrachiolias1, Vasileios Papadopoulos1, Panagiotis T. Diamantopoulos3, Dimitris Τsokanas4, Alexandra Kourakli6 and Argiris Symeonidis6.
1 Department of Hematology, Democritus University of Thrace Medical School; Greece.
2 Second Department of Internal Medicine, Hematology Unit, Attikon University General Hospital, Athens, Greece.
3 Department of Clinical Hematology, G. Gennimatas Hospital, Athens, Greece.
4
Hematology Unit, First Department of Internal Medicine, Laikon General
Hospital, National and Kapodistrian University of Athens, Athens,
Greece.
5 Department of Hematology, Laikon General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
6 Department of Internal Medicine, University Hospital of Patras, Rio, Greece.
Correspondence to:Ioannis Kotsianidis, MD, PhD.
Professor of Hematology, Director, Department of Hematology
Democritus University of Thrace, Medical School, Dragana,
Alexandroupolis 68100, Greece. E-mail:
ikotsian@med.duth.gr,
jankots@yahoo.gr
Published: July 01, 2019
Received: April 03, 2019
Accepted: June 10, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019045 DOI
10.4084/MJHID.2019.045
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
To the editor,
5-azacytidine (AZA) is the mainstay of treatment for high-risk MDS,[1] but both primary and secondary, i.e., after an initial response, AZA failure confers a grave prognosis.[2,3]
Allogeneic transplantation and trials with novel agents are the
preferred strategies for AZA failure, but transplant eligibility and
availability of relevant trials are key limiting factors for the vast
majority of the patients. In addition, AZA failure is currently poorly
defined, as reflected by the highly heterogeneous outcome of patients
who fail AZA,[4,5] and either lumps together pathobiologically diverse disease states,[2] or excludes treatment failure due to toxicity,[3]
a common reason of AZA discontinuation in real-life settings. Given the
limited efficacy and availability of subsequent treatment options, the
exact definition of AZA failure and the decision to discontinue AZA is
of particular importance in everyday clinical practice. Furthermore, it
is plausible to assume that the diverse prognosis of the above patients
may confuse the results of trials with novel drugs in the setting of
AZA failure.
We retrospectively analyzed data from 326 MDS,
MDS/MPN, and low blast count AML (LBC-AML) patients enrolled to
Hellenic MDS registry from July 2004 to May 2017. The data cutoff date
for the analysis was July 7, 2017, and the current analysis included
only patients who received AZA as 1st line treatment. All patients were
treated in a non-clinical trial setting at an initial dose of 75mg/m2
SC for seven days on 28-day cycles. Granulocyte colony-stimulating
factors and erythropoiesis-stimulating agents were used at the
discretion of the treating physician. Dose reductions of 25%-50% and/or
treatment delays were considered for severe myelotoxicity or
myelosuppression-related complications. Treatment response was
evaluated using the IWG 2006 criteria.[6] Survival
analysis was performed using a Kaplan-Meier estimate and Cox’s
proportional hazards model. Overall survival (OS) was defined as the
time from AZA initiation or failure to last follow up or death from any
cause. Akaike Information Criteria with correction for finite sample
sizes (AICc) was used to compare model fits.[7]
Table 1
lists the patients’ characteristics. After a median follow-up of 41.7
months, the median OS from AZA initiation for the whole cohort was 14
(95% CI: 12.6–15.4) months. Though not designed for patients with
MDS/MPN and low-risk IPSS the French Prognostic Scoring System (FPSS)[8] was the better survival discriminator compared to IPSS and IPSS-R (Figure 1A,
AICc 1958,841 vs. 2276,767 vs. 2321,742, respectively). In multivariate
analysis, FPSS (p<0.001), the best response to AZA (p<0.001) and,
marginally, disease subtype (MDS/MPN vs others, p=0.01) were all
independent predictors of OS (supplementary Table 1).
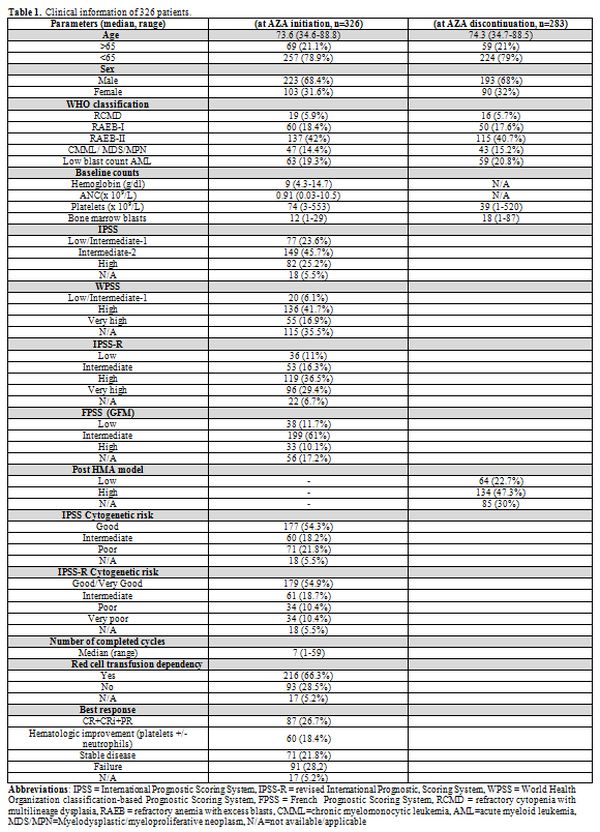 |
Table1. Clinical information of 326 patients. |
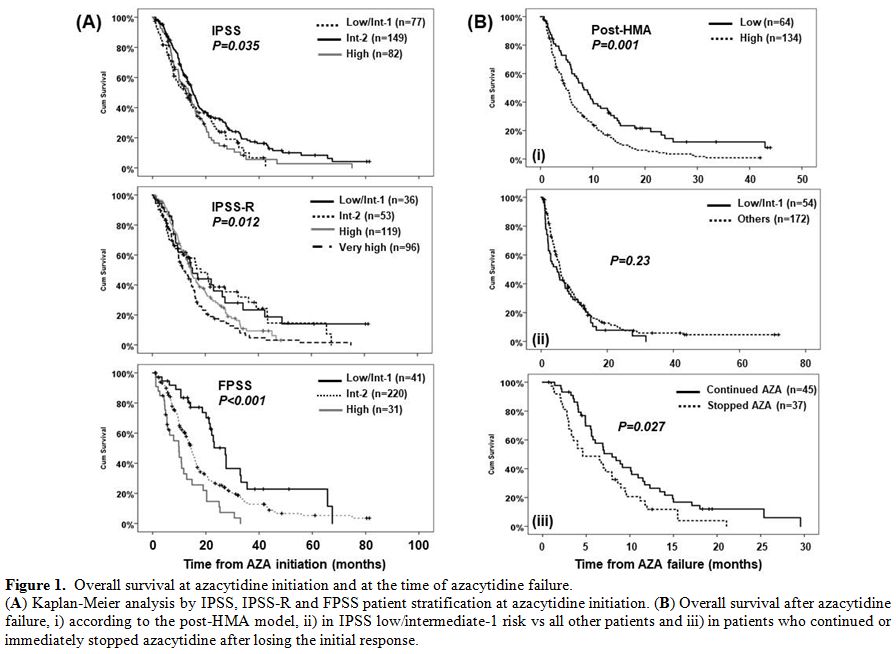 |
Figure 1. Overall survival at azacytidine initiation and at the time of azacytidine failure.
(A) Kaplan-Meier analysis by IPSS, IPSS-R and FPSS patient stratification at azacytidine initiation. (B)
Overall survival after azacytidine failure, i) according to the
post-HMA model, ii) in IPSS low/intermediate-1 risk vs all other
patients and iii) in patients who continued or immediately stopped
azacytidine after losing the initial response. |
The median time to
AZA discontinuation was 7.5 months (95% CI: 6.5–8.5) with 43 (13.2%)
patients still receiving AZA at the time of analysis. The median OS
from AZA failure (n=252), defined as AZA intolerance, no response after
at least 4 cycles along with AZA intolerance, progressive disease or
death while on treatment, and loss of response, was 5.6 (95% CI:
4.8–6.3) months, similar to the one reported in other studies.[2,3]
Progressive disease while on treatment (n=77, 28%) and AZA intolerance
(n=88, 31%) were the most common causes of AZA failure, followed by
loss of an initial response (n=41, 15%) and no response after 4 cycles
(n=25, 9%). Median OS after AZA failure was comparable for all of the
above causes (p=0.1, Figure 2),
indicating that the diverse pathobiology behind AZA failure does not
influence the outcome and rather typical clinical parameters at the
time of AZA failure, as those captured by the post-HMA model, determine
patient survival. However, the 20% rate of early discontinuation and 7%
rate of early death, i.e., occurring before cycle 4, underline the
considerable toxicity of AZA in real-life settings, highlighting the
need for early predictive factors to avoid a costly, ineffective and
potentially even harmful treatment.
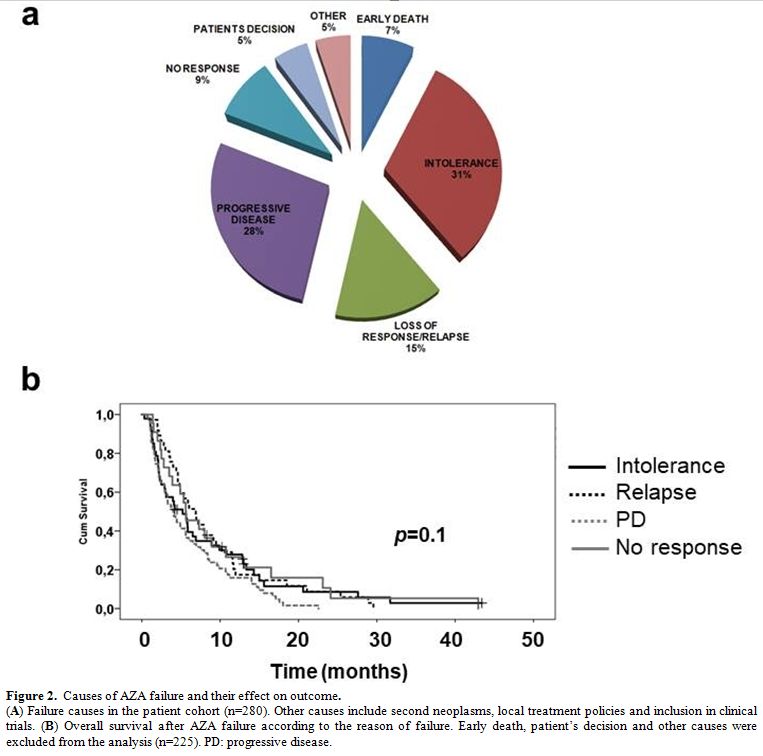 |
Figure 2. Causes of AZA failure and their effect on outcome.
(A)
Failure causes in the patient cohort (n=280). Other causes include
second neoplasms, local treatment policies and inclusion in clinical
trials. (B) Overall survival
after AZA failure according to the reason of failure. Early death,
patient’s decision and other causes were excluded from the analysis
(n=225). PD: progressive disease. |
The post-HMA model4 was applicable in 198 patients (Figure 1B);
64 patients (32.5%) were classified as low and 134 (67.5%) as high
risk, with a significant difference in median OS after AZA failure
(8.3, 95% CI: 5.8–10.8 vs. 4.8, 95% CI: 3.7–5.9 months respectively,
p<0.001). Eight out of 9 patients who proceeded to allogeneic
transplantation were classified as low and only one as high risk,
whereas even after censoring these patients at the time of
transplantation, the median OS of the two groups remained identical
(data not shown). In multivariate analysis, only the post-HMA model
retained its independent predictive value (supplementary Table 2).
Of note, our cohort included a considerable proportion of patients
categorized as a low/intermediate-1 risk by IPSS (23.6%) at AZA
initiation. The reported median OS after the failure of AZA in patients
with IPSS low/int-1-risk score is 17 months.[9,10] By
contrast, the median OS after AZA failure in our cohort of IPSS lower
risk patients (n=54) was only 4.7 (95% CI: 1.6–7.8) months similar to
one of the high-risk patients (n=172, Figure 1B).
Though not easily interpretable, this inconsistency may be attributable
to selection biases due to clinical judgment in real-life settings,
i.e., administration of AZA mainly in poor prognosis, multi-treated
patients. Indeed, at AZA initiation, only 24/75 (32%) of IPSS
lower-risk patients were categorized as low risk by IPSS-R and 13/57
(23%) by FPSS. In addition, at the time of HMA failure 31/41 (76%) of
the evaluable IPSS lower risk patients fell into the post-HMA high risk
category, although, as expected, significantly more IPSS, WPSS and
IPSS-R higher risk patients were classified as high risk by the
post-HMA model (supplementary Figure S1).
After
AZA discontinuation, most patients were treated with supportive care,
and only 9 patients proceeded to allo-SCT. Median OS after AZA failure
was identical among patients treated with best supportive care, low
dose AraC and intensive chemotherapy (p=0.22), whereas 14 patients
treated with decitabine showed significantly improved mOS compared to
all other treatments (21.1, 95% CI: 12–30.1 months, p=0.003, Supplementary Figure S2).
Regarding the above patients, 5/11 evaluable cases were low and 6/11
high risk by post-HMA, whereas 4 discontinued AZA because of relapse
after an initial response, 2 for disease progression, 2 for toxicity, 2
for failure to achieve a response after 4 cycles and one by his own
decision. The overall response rate with second-line decitabine after
AZA failure ranges from 19.4% to 63%[2,11,12]
and the median OS from 7.3 to 17.8 months, but the small patient
cohorts, the heterogeneous definitions of AZA failure and the obvious
selection biases, preclude the identification of predictive factors for
response in decitabine. Therefore, though selected patients who failed
AZA may benefit from second line decitabine, no solid recommendation
can be made, also considering the toxicity and cost of decitabine
treatment.
According to the latest International Working Group (IWG) criteria,[6]
a hemoglobin drop of ≥1.5 g/dL or ≥50% decrement of maximum response
levels of neutrophils or platelets signifies the loss of response.
However, even large fluctuations in blood counts during AZA treatment
can occasionally be observed in responding patients and, in the face of
limited subsequent therapeutic options, many physicians are reluctant
to immediately stop AZA simply only on the base of strictly defined
loss of response. In our series, 45/82 patients, who continued AZA
despite loss of response, had a significantly better OS (8.0 months,
95% CI: 5.5–10.5) compared to those (n=37) who stopped AZA immediately
at loss of response (4.6 months, 95% CI: 1.5–7.9, p=0.027, Figure 1B).
The decision to stop or not AZA when the response is lost according to
the IWG criteria is subjected to bias, of course. However, patients who
continued AZA had still significantly longer OS even when the analysis
was adjusted for gender, disease subtype at the time of AZA
discontinuation and the post-HMA model (p=0.02), though both patient
groups received comparable therapies after AZA discontinuation (supplementary Tables 3 and 4).
In line with these findings, our group has recently shown that patients
with stable disease as best response benefited significantly by the
continuation of AZA compared to the ones who stopped treatment.[13]
By contrast, prolonged survival was recently reported in patients who
stopped AZA or decitabine without disease progression, but the study
cohort consisted mainly of IPSS low-risk patients, most of whom
discontinued treatment for extra medical causes.[14]
In
summary, in our large patient dataset, we confirmed the superior
efficacy of FPSS and post-HMA models in predicting OS at AZA start and
failure, respectively. However, our data also indicate that in real
life settings, AZA failure is rather ill-defined, and physicians’
perceptions of the cause and timing of AZA discontinuation differ
widely. Retrospective data are inherently susceptible to selection and
analysis bias. Specifically for the setting of AZA failure, the highly
diverse reasons of AZA discontinuation in our series emphasize that,
since no uniform criteria for stopping AZA have been adopted,[15]
any survival comparisons should be given with caution. Failure due to
intractable toxicity and disease progression confers very poor outcome,
while patients who lose the initial response may benefit from AZA
continuation despite poor prognostic features, highlighting, on the one
hand, the diversity of the resistance mechanisms to AZA and, on the
other, the limitations of the applicability of prognostic models[16] and response criteria in everyday clinical practice.
References
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini
V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared
with that of conventional care regimens in the treatment of higher-risk
myelodysplastic syndromes: a randomised, open-label, phase III study.
The Lancet Oncology. 2009 Mar;10(3):223-32. https://doi.org/10.1016/S1470-2045(09)70003-8
- Prebet
T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome
of high-risk myelodysplastic syndrome after azacitidine treatment
failure. J Clin Oncol. 2011 Aug 20;29(24):3322-7. https://doi.org/10.1200/JCO.2011.35.8135 PMid:21788559 PMCid:PMC4859209
- Duong
VH, Lin K, Reljic T, Kumar A, Al Ali NH, Lancet JE, et al. Poor outcome
of patients with myelodysplastic syndrome after azacitidine treatment
failure. Clinical Lymphoma, Myeloma & Leukemia. 2013
Dec;13(6):711-5. https://doi.org/10.1016/j.clml.2013.07.007 PMid:24054159
- Nazha
A, Komrokji RS, Garcia-Manero G, Barnard J, Roboz GJ, Steensma DP, et
al. The efficacy of current prognostic models in predicting outcome of
patients with myelodysplastic syndromes at the time of hypomethylating
agent failure. Haematologica. 2016 Jun;101(6):e224-7. https://doi.org/10.3324/haematol.2015.140962 PMid:26992944 PMCid:PMC5013943
- Prebet
T, Fenaux P, Vey N, Groupe Francophone des M. Predicting outcome of
patients with myelodysplastic syndromes after failure of azacitidine:
validation of the North American MDS consortium scoring system.
Haematologica. 2016 Oct;101(10):e427-e8. https://doi.org/10.3324/haematol.2016.150714 PMid:27694503 PMCid:PMC5046667
- Cheson
BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et
al. Clinical application and proposal for modification of the
International Working Group (IWG) response criteria in myelodysplasia.
Blood. 2006 Jul 15;108(2):419-25. https://doi.org/10.1182/blood-2005-10-4149 PMid:16609072
- Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001 Mar;57(1):120-5. https://doi.org/10.1111/j.0006-341X.2001.00120.x PMid:11252586
- Itzykson
R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, et al.
Prognostic factors for response and overall survival in 282 patients
with higher-risk myelodysplastic syndromes treated with azacitidine.
Blood. 2011 Jan 13;117(2):403-11. https://doi.org/10.1182/blood-2010-06-289280 PMid:20940414
- Jabbour
EJ, Garcia-Manero G, Strati P, Mishra A, Al Ali NH, Padron E, et al.
Outcome of patients with low-risk and intermediate-1-risk
myelodysplastic syndrome after hypomethylating agent failure: a report
on behalf of the MDS Clinical Research Consortium. Cancer. 2015 Mar
15;121(6):876-82. https://doi.org/10.1002/cncr.29145 PMid:25410759 PMCid:PMC4378905
- Prebet
T, Thepot S, Gore SD, Dreyfus F, Fenaux P, Vey N. Outcome of patients
with low-risk myelodysplasia after azacitidine treatment failure.
Haematologica. 2013 Feb;98(2):e18-9. https://doi.org/10.3324/haematol.2012.071050 PMid:22983576 PMCid:PMC3561445
- Harel
S, Cherait A, Berthon C, Willekens C, Park S, Rigal M, et al. Outcome
of patients with high risk Myelodysplastic Syndrome (MDS) and advanced
Chronic Myelomonocytic Leukemia (CMML) treated with decitabine after
azacitidine failure. Leuk Res. 2015 May;39(5):501-4. https://doi.org/10.1016/j.leukres.2015.02.004 PMid:25735917
- Apuri
S, Al Ali N, Padron E, Lancet JE, List AF, Komrokji RS. Evidence for
Selective Benefit of Sequential Treatment With Hypomethylating Agents
in Patients With Myelodysplastic Syndrome. Clinical Lymphoma, Myeloma
& Leukemia. 2017 Apr;17(4):211-4. https://doi.org/10.1016/j.clml.2016.10.003 PMid:28185797
- Papageorgiou
SG, Kontos CK, Kotsianidis I, Vasilatou D, Symeonidis A, Galanopoulos
A, et al. The outcome of patients with high-risk MDS achieving stable
disease after treatment with 5-azacytidine: A retrospective analysis of
the Hellenic (Greek) MDS Study Group. Hematological Oncology. 2018 Aug
20. https://doi.org/10.1002/hon.2551 PMid:30129144
- Kim
DJ, Lee HS, Moon JH, Sohn SK, Kim HJ, Cheong JW, et al. Can we consider
discontinuation of hypomethylating agents in patients with
myelodysplastic syndrome: a retrospective study from The Korean Society
of Hematology AML/MDS Working Party. Oncotarget. 2017 Oct
3;8(45):79414-24. https://doi.org/10.18632/oncotarget.18258
- Komrokji
RS. Treatment of Higher-Risk Myelodysplastic Syndromes After Failure of
Hypomethylating Agents. Clinical Lymphoma, Myeloma & Leukemia. 2015
Jun;15 Suppl:S56-9. https://doi.org/10.1016/j.clml.2015.03.010 PMid:26297279
- Patel
SS, Sekeres MA, Nazha A. Prognostic models in predicting outcomes in
myelodysplastic syndromes after hypomethylating agent failure. Leukemia
& Lymphoma. 2017 Nov;58(11):2532-9. https://doi.org/10.1080/10428194.2017.1307361 PMid:28351181
Supplemental Data
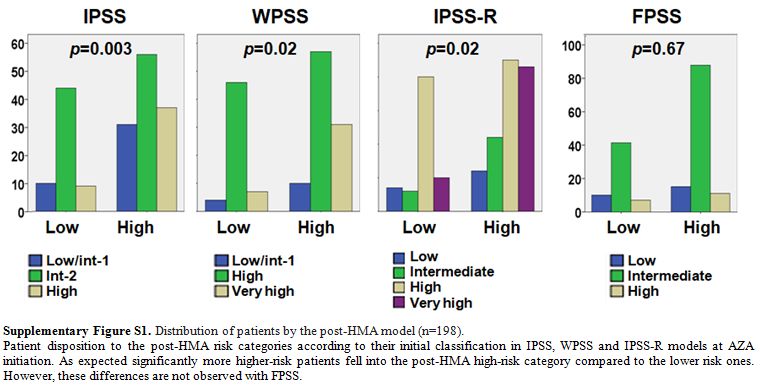 |
Supplementary Figure S1. Distribution of patients by the post-HMA model (n=198).
Patient
disposition to the post-HMA risk categories according to their initial
classification in IPSS, WPSS and IPSS-R models at AZA initiation. As
expected significantly more higher-risk patients fell into the post-HMA
high-risk category compared to the lower risk ones. However, these
differences are not observed with FPSS.
|
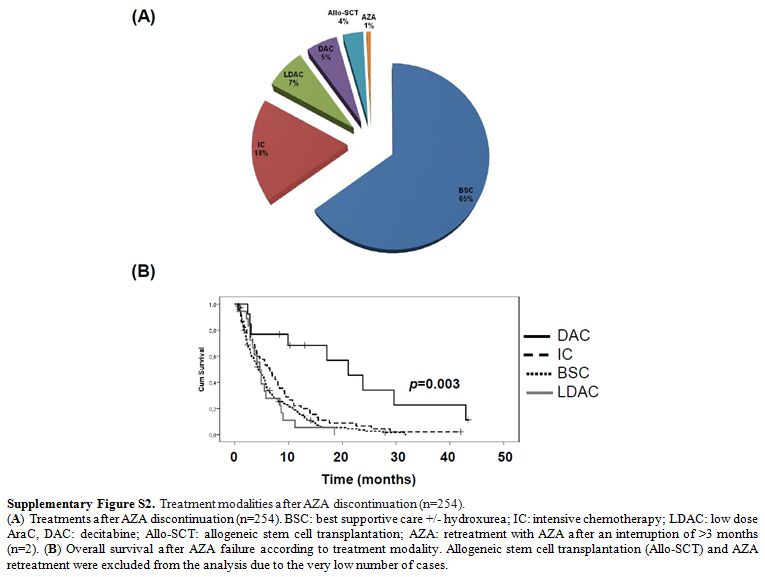 |
Supplementary Figure S2. Treatment modalities after AZA discontinuation (n=254).
(A)
Treatments after AZA discontinuation (n=254). BSC: best supportive care
+/- hydroxurea; IC: intensive chemotherapy; LDAC: low dose AraC, DAC:
decitabine; Allo-SCT: allogeneic stem cell transplantation; AZA:
retreatment with AZA after an interruption of >3 months (n=2). (B)
Overall survival after AZA failure according to treatment modality.
Allogeneic stem cell transplantation (Allo-SCT) and AZA retreatment
were excluded from the analysis due to the very low number of cases.
|
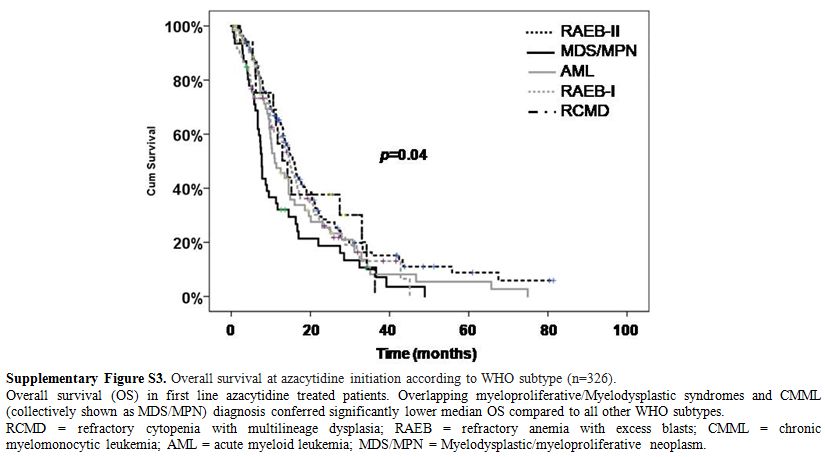 |
Supplementary Figure S3. Overall survival at azacytidine initiation according to WHO subtype (n=326).
Overall
survival (OS) in first line azacytidine treated patients. Overlapping
myeloproliferative/Myelodysplastic syndromes and CMML (collectively
shown as MDS/MPN) diagnosis conferred significantly lower median OS
compared to all other WHO subtypes.
RCMD = refractory cytopenia
with multilineage dysplasia; RAEB = refractory anemia with excess
blasts; CMML = chronic myelomonocytic leukemia; AML = acute myeloid
leukemia; MDS/MPN = Myelodysplastic/myeloproliferative neoplasm.
|
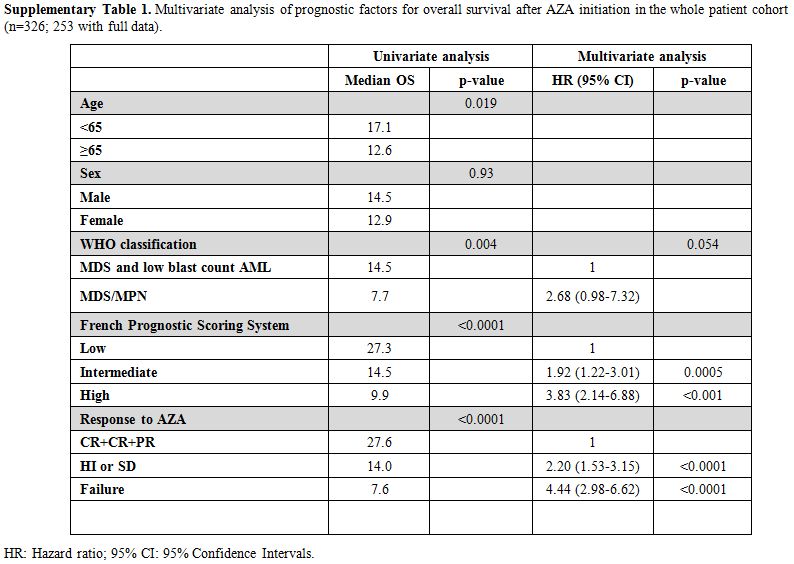 |
Supplementary Table 1. Multivariate
analysis of prognostic factors for overall survival after AZA
initiation in the whole patient cohort (n=326; 253 with full data).
|
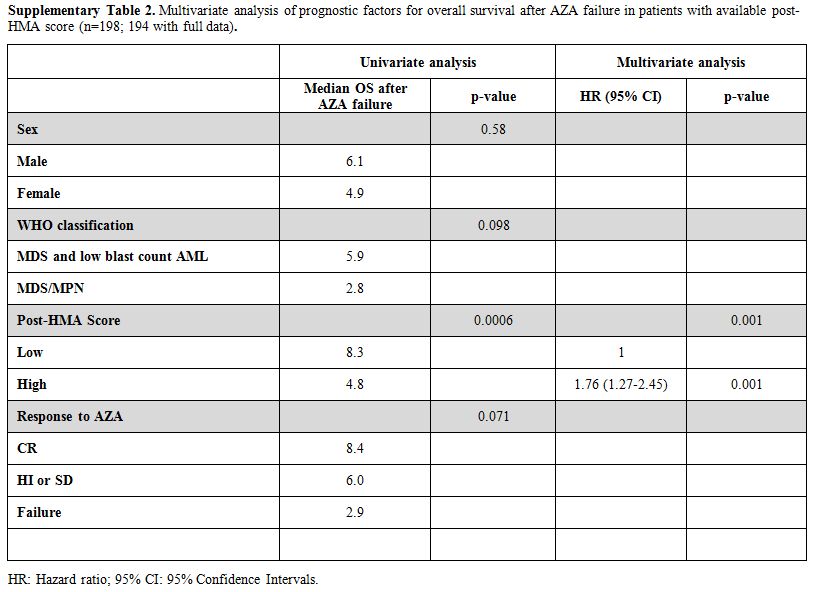 |
Supplementary Table 2. Multivariate
analysis of prognostic factors for overall survival after AZA failure
in patients with available post-HMA score (n=198; 194 with full data).
|
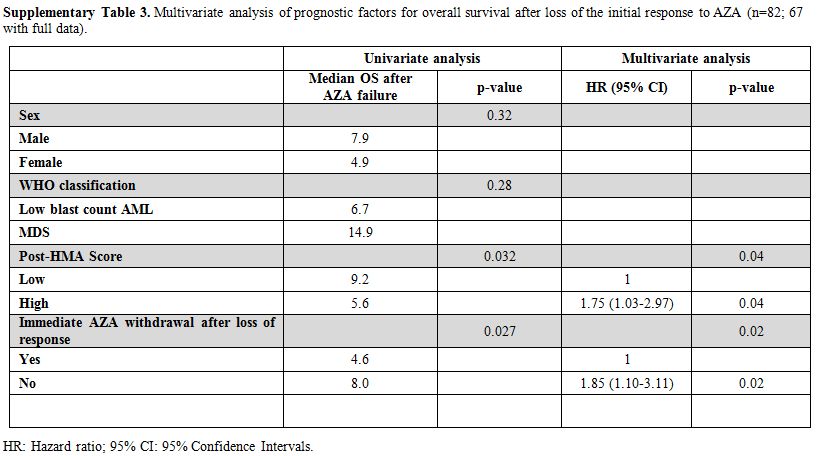 |
Supplementary Table 3. Multivariate
analysis of prognostic factors for overall survival after loss of the
initial response to AZA (n=82; 67 with full data).
|
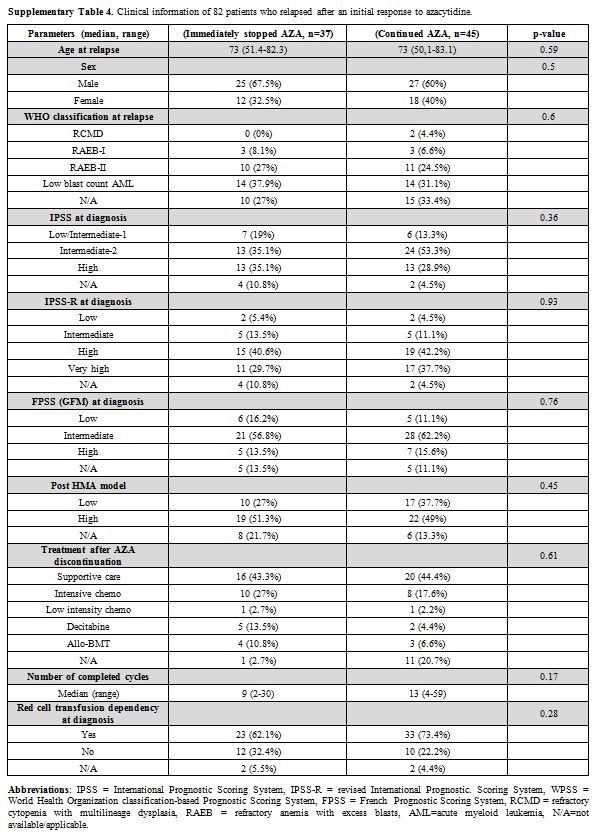 |
Supplementary Table 4. Clinical information of 82 patients who relapsed after an initial response to azacytidine. |
[TOP]









