Irena Kostic1,
Carmela Gurrieri1, Elisa Piva2, Gianpietro
Semenzato1, Mario Plebani2, Ilaria Caputo1
and Fabrizio Vianello1.
1 Hematology
and Clinical Immunology Unit, Department of Medicine, University of
Padua School of Medicine, Padova, Italy.
2 Department of Laboratory Medicine, Department of Medicine,
University of Padua School of Medicine, Padova, Italy.
Published: September 1, 2019
Received: March 22, 2019
Accepted: August 2, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019047 DOI
10.4084/MJHID.2019.047
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Bacterial
infections represent life-threatening complications in patients with
febrile neutropenia (FN). Diagnostic biomarkers of infections may help
to differentiate bacteraemia from non-bacteraemia FN. We aimed to
evaluate the utility of procalcitonin (PCT), presepsin (PS), C-reactive
protein (CRP) and interleukin-8 (IL-8) as biomarkers of bacteraemia in
adult FN patients with haematological malignancies.
Concentrations
of PCT, PS, CRP and IL-8 were prospectively measured in 36 FN episodes
experienced by 28 oncohaematological patients.
11 out of 36
episodes were classified as bacteraemia. PCT was the best biomarker to
predict bacteraemia with the area under the curve (AUC) ROC of 0,9;
specificity 100% and positive predictive value 100%, while the most
sensitive was IL-8 (90,9%) with AUC ROC of 0,88 and negative predictive
value 95,2%. All patients with PCT concentrations above 1,6 μg/l had
bacteraemia. Patients with IL-8 concentrations superior to 170 pg/ml
had a 40 times higher risk for bacteraemia than the ones with lower
levels. Patients with PS concentrations superior to 410 pg/ml had 24
times higher risk for bacteraemia than the patients with lower
levels.
PCT has higher accuracy than CRP, IL-8 and PS in predicting bacteraemia
in adult hematologic patients with FN.
|
Introduction
Febrile neutropenia (FN)
refers to the occurrence of fever during a period of severe neutropenia
(neutrophils <0.5x109/L).
Particularly in the setting of chemotherapy-induced FN, these patients
are very prone to bacterial infections.[1,2] Only
20% of FN episodes have a microbiologically-proven infection. In most
cases, the therapeutic approach is based on the clinical picture and
the laboratory evaluation of surrogate markers. Among them, CRP, PCT
and IL - 8 have been extensively evaluated as diagnostic biomarkers of
infection.[3,4] Soluble CD14 subtype, also known as
PS, is a novel biomarker of microbial infection.[5]
PS is a surface marker in monocytes/macrophages that binds to the
lipopolysaccharide (LPS)-LPS binding protein. Following the infection
and the phagocytosis of the CD14-pathogen complex, PS is generated and
released.[6] An increasing number of studies have
shown the ability of PS to serve as a valuable marker in sepsis
diagnosis.[3]
However, there is no clear evidence that PS may have a role in
identifying infections in adult oncohaematological patients with FN.[7]
The
aim of the present study was to analyse the utility of PCT, PS, CRP and
IL-8 as biomarkers of bacteraemia during febrile episodes that occurred
in neutropenic patients with haematological malignancies, with the goal
to define reliable tools that predict bacteraemia.
Methods and Patients
Study design.
This prospective observational study has been performed at the
Haematology and Clinical Immunology Unit of the University of Padua,
Italy over ten months (from April 2017 to January 2018).
Participants.
Consecutive patients were considered eligible for the study if they met
the following criteria:
-
admission to our Unit for high dose chemotherapy alone or followed by
autologous bone marrow stem cell transplantation
- the occurrence of FN as defined according to the
Infectious Diseases Society of America (IDSA) guidelines.[2]
- patients
under 18 years old, those with documented viral or fungal infections,
patients with fever before neutropenia onset and patients who received
antibiotic treatment before neutropenia onset were excluded from the
study.
Episodes of FN were classified according to the International
Immunocompromised Host Society into three groups:[8]
Group 1 – microbiologically documented infection or bacteraemia,
defined as the presence of live bacteria in the bloodstream,
Group
2 – local infection – focal signs, defined as localized, clinically
documented infection of one organ or organ system without bacteraemia,
Group
3 – fever of unknown origin (FUO), defined as an episode of fever
without a recognizable cause of infection (clinically documented site
of infection and microbiologically isolation).
All patients
included in the study received antibiotic prophylaxis with levofloxacin
and G-CSF at neutropenia onset. AML patients were also started on
posaconazole prophylaxis, all patients with ALL and those undergoing
autologous bone marrow transplantation received co-trimoxazole.
Fluconazole was administered to ALL patients during induction
therapy.
All subjects underwent central venous catheter
(CVC) or peripheral intravenous central catheter (PICC) placement for
chemotherapy. The study was approved by the Hospital Ethical Board, and
written consent was obtained from each patient.
Test
methods.
Complete white blood cell count and CRP concentration were checked
daily. As index tests, we considered CRP, PCT, PS and IL-8. PCT, PS and
IL-8 were measured at the onset of neutropenia (baseline level), and
after a single oral temperature measurement of ≥38.3° C or a
temperature of ≥38.0°C sustained over a 1-h period. All subjects had
blood samples collected for PCT, PS and IL-8 measurement between 90 and
120 minutes from fever onset, before any broad-spectrum empirical
antibiotic therapy. PCT and PS were measured at the same time, while
IL-8 was quantified on the following day. PCT, PS and IL-8
concentrations were determined by standardized assays (Liaison Brahms
PCT II GEN, Roche Diagnostics, Germany, determination range 0,02-100
μg/l; Pathfast Presepsin test, Mitsubishi Chemical Europe, Germany,
determination range 20-20000 pg/ml; Immulite 1000, Siemens, Germany,
determination range 2-7500 pg/ml, respectively). CRP was determined by
the particle enhanced immunonephelometry provided by the Dimension
VistaTM System (Siemens Healthcare Diagnostics Inc, Marburg, Germany).
Blood culture was considered as a reference standard for diagnosing
bacteraemia. Isolation of pathogens from blood cultures was performed
using a BacT/Alert three-dimensional (3D) (bioMérieux Inc., Marcy
l’Etoile, France) automated blood culture system. Each blood culture
consisted of a set of four (FA Plus aerobic, FN Plus anaerobic, SA, and
SN) bottles. Antibiotic sensitivity was studied with the VITEK 2 system
by Biomerieux (Marcy l'Etoile, France). Only results of CRP and PCT
were available to the clinicians during febrile neutropenia periods.
Common
skin contaminants were considered significant only if they could be
found in two consecutive BC samples or if there were concurrent skin,
soft tissue, or catheter-related infections.
In subjects with a
suspect of viral infection (like an influenza-like illness), a
nasopharyngeal swab for PCR influenza testing was collected, as well as
PCR or RT-PCR were performed to rule out respiratory syncytial virus,
adenovirus, parainfluenza virus or human metapneumovirus
infections. A suspect of fungal infections was evaluated in
high-risk patients (not on posaconazole prophylaxis) by checking
Aspergillus galactomannan antigen and the beta-D-glucan assay starting
with the onset of neutropenia and continued until neutrophil recovery.
High-resolution
chest CT was performed in all patients with respiratory symptoms and
febrile neutropenia. CT scanning of other sites (head, sinuses,
abdomen/pelvis) was performed at the discretion of the clinician.
Analysis.
Data were analysed for sensitivity, specificity, positive predictive
value (PPV), and negative predictive value (NPV) derived from the
receiver operating characteristic (ROC) curves. To establish the
optimal cut-off values of index tests, authors constructed receiver
operating characteristic (ROC) curves, and the areas under the curve
(AUC) were determined. The ROC curve is a plot of the true-positive
values (sensitivity) vs the false-positive values (1-specificity) for
distinct index test cut-off values. The more the curve is located in
the top left-hand corner of the graph, the higher the AUC and the
higher the accuracy of the diagnostic test is. We performed several ROC
analyses to evaluate the accuracy of each index test.
Cut-off
values for each parameter determining bacteraemia were calculated from
the areas under the ROC curves (AUC). The comparison between groups was
made by the Mann-Whitney test, and the proportions of patients were
compared by the chi-square test. The non-parametric analysis of
variance was made with the Kruskal-Wallis test. Differences at
the level of p<0,05 were considered statistically significant. The
diagnostic accuracy was expressed as a proportion of correctly
classified mucosal impedance measurements (true negative and true
positive measures) among all measures. We considered P ≤ 0.05 to be
significant.
Statistical analysis was performed using IBM SPSS Statistics (version
19, SPSS Inc., IBM Company, Chicago, IL, USA).
Results
Ninety-eight
subjects were admitted to our Unit for high dose chemotherapy over the
ten months of the study. Fifteen subjects had a fever and/or evidence
of viral infections (n= 13) or a previous diagnosis of probable
invasive aspergillosis (n=2) and therefore, were not considered
eligible for the study. Fifty subjects did not experience fever during
neutropenia. Full results were unavailable for five patients due to
inappropriate sampling time or incomplete biomarkers
analysis. Therefore 28 (29%) patients were analysed in this study (Figure 1).
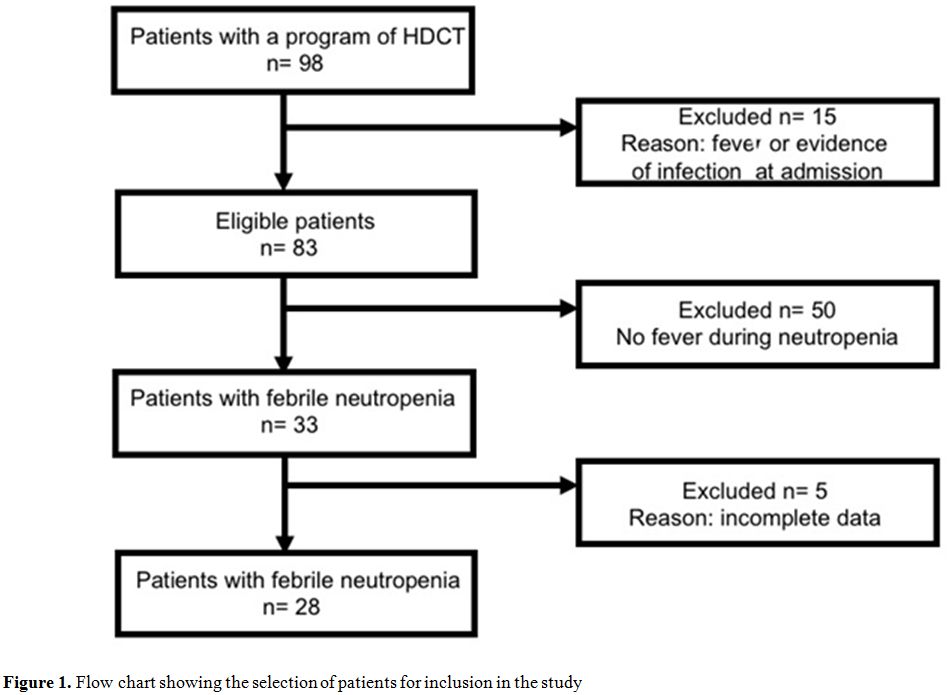 |
Figure 1. Flow chart
showing the selection of patients for inclusion in the study |
CRP,
IL-8, PCT and PS were measured in 36 episodes of FN occurred in 28
patients (17 males and 11 females). All subjects received high-dose
chemotherapy. Induction or consolidation chemotherapy for acute
leukaemia was the reason for treatment in 17 patients (13 acute myeloid
leukaemias, AML- and four acute lymphoblastic leukaemias, ALL). Four
patients with multiple myeloma and one patient with non-Hodgkin
lymphoma (NHL) received autologous stem cell transplantation. In 6
patients, salvage chemotherapy for NHL was administered.
There
were no differences between groups of hematologic diseases in terms of
age and sex (ANOVA p=0,9 and p=0,7, respectively). Ten subjects were
neutropenic at diagnosis before starting chemotherapy. Biomarkers at
onset of neutropenia were as follow: PCT 0.03 ± 0.01 µg/l; PS 125 ± 97 pg/ml; CRP 4.4 ± 2.6 µg/l; IL-8 44 ± 27 pg/ml. Distribution
of FN subtypes was as follows:
Group
1 – Among 11 episodes of bacteraemia, 7/11 showed growth of
gram-negative bacteria (Klebsiella pneumoniae in 4 cases, Escherichia
coli in 2 cases, Klebsiella oxytoca in 1 case). In 4 out of 11
episodes, cultures grew gram-positive bacteria (Enterococcus faecalis
in 2 cases, Propionibacterium and Staphylococcus hominis in the other
cases)
Group 2 –Local infections were diagnosed in 14 episodes as
follow: pneumonia (6/14), sinusitis (4/14), endocarditis (1/14),
pleuropericarditis (1/14), skin abscesses (1/14), and colitis (1/14).
Group 3 – 11 episodes were classified as FUO.
Table 1
summarizes patients’ characteristics for each group. There were no
differences between groups when considering gender and presence of
mucositis, while patients with gram-negative bacteraemia were
significantly older. Days of hospitalization were similar between FN
groups. There were no differences in hematologic parameters (Hb
concentration, WBC and neutrophil count, as well as in platelets
count). The only parameter that was significantly lower in the
bacteraemia group was the monocytes count (Table 1).
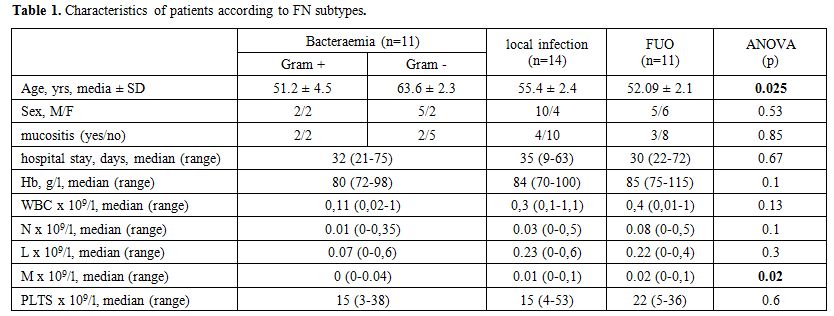 |
Table 1.
Characteristics of patients according to FN subtypes. |
Median concentrations and
p values for each biomarker are presented in Figure 2 and Table 2.
PCT concentrations did not differ significantly from those measured at
neutropenia onset in the local infection and FUO groups (not shown).
Similarly, there were no statistically significant differences in PS
and IL-8 values between FUO and local infection groups (Figure 2).
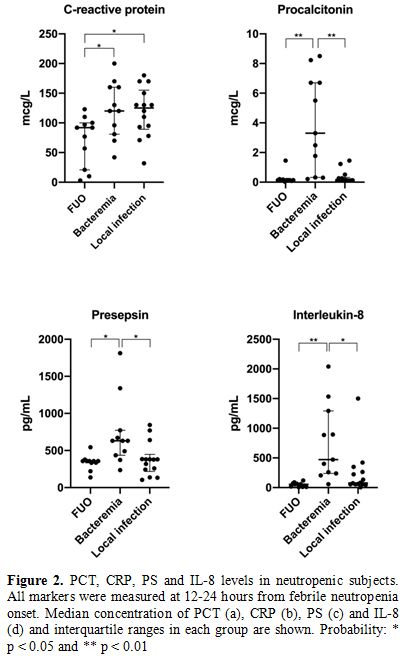 |
Figure 2. PCT, CRP, PS and IL-8
levels in
neutropenic subjects. All markers were measured at 12-24 hours
from febrile neutropenia onset. Median concentration of PCT (a), CRP
(b), PS (c) and IL-8 (d) and interquartile ranges in each group are
shown. Probability: * p < 0.05 and ** p < 0.01 |
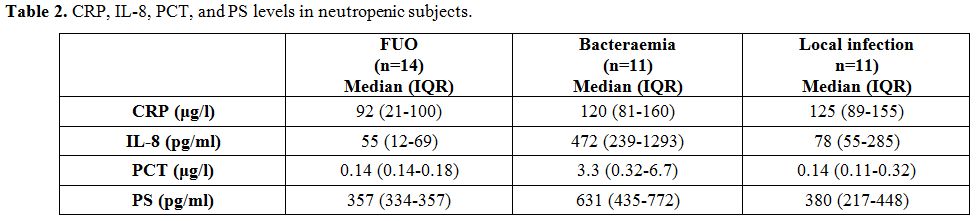 |
Table
2. CRP, IL-8, PCT, and PS levels in neutropenic subjects. |
Subjects
with bacteraemia showed significantly higher concentrations of all
biomarkers compared to patients with FUO and those with local
infections.
Only in the case of CRP, levels did not significantly differ in
subjects with bacteraemia compared to local infection (Figure 2). Monocyte count lower than
0.01 x 109/l
inversely correlated with the concentrations of PCT, IL-8 and PS
(r=-0.52, p=0.001; r=-0.45, p=0.005; r=-0.36, p=0.03
respectively).
To test whether CRP, IL-8, PCT and PS could
predict bacteraemia, we determined optimal cut-off values by ROC
analysis. The sensitivity, specificity, NPV, PPV, and diagnostic
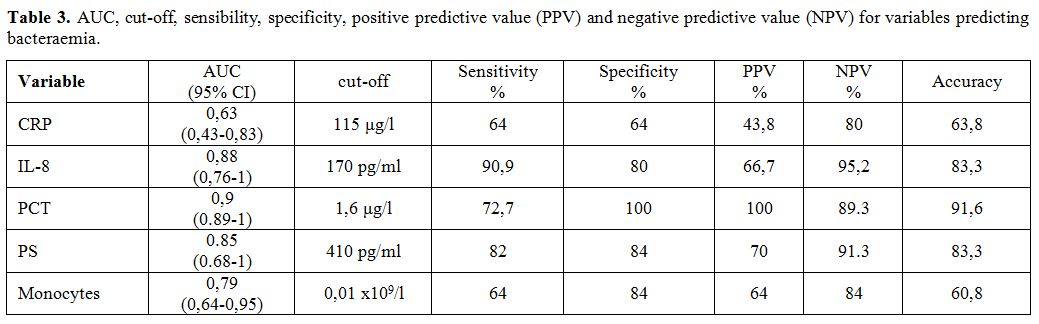
accuracy for distinct index test are presented in Table 3. ROC analysis showed that
PCT yielded higher AUC values than other biomarkers, with CRP showing
the lowest value (Table 3).
In addition, the optimal cut-off value for PCT as a biomarker of
bacteraemia was 1.6 μg/l, with a specificity of 100% and a sensitivity
of 72.7% (Table 3). The optimal
cut-off value for IL-8 was 170 pg/ml, with a sensitivity of 90,9% and a
specificity of 80%. Finally, the optimal cut-off for PS was 410 pg/ml,
with a sensitivity of 82% and specificity of 84 % (Table 3).
CRP could not predict bacteraemia well. In fact, with a cut off value
of 115 μg/l, CRP had the lowest AUC (0.63) with sensitivity and
specificity of only 64% for both (Table
3).
All patients with PCT concentrations above 1.6 μg/l had bacteraemia.
Patients with IL-8 concentrations superior to 170 pg/ml had a 40 times
higher risk for bacteraemia than the ones with lower levels. Patients
with PS concentrations superior to 410 pg/ml had 24 times higher risk
for bacteremia than the patients with lower levels. PS showed the
highest sensitivity in predicting Gram-negative bacteraemia.
 |
Table
3. AUC, cut-off, sensibility, specificity, positive predictive value
(PPV) and negative predictive value (NPV) for variables predicting
bacteraemia. |
When
combining two parameters for improving bacteraemia prediction (using
scatter plot graph and chi-square test), like PCT (cut-off > 1,6
μg/l) and PS (cut-off > 410 pg/ml) or PCT and IL-8 (cut-off > 170
pg/ml), no significant changes in the sensitivity or negative
predictive value compared to PCT alone were observed.
Discussion
In
this study, we found that PCT has higher specificity than PS, IL-8 and
CRP in predicting bacteraemia during FN in subjects with haematological
malignancies. PCT performed better even in term of the probability of
bacteraemia above a definite cut-off. In fact, a 64 times higher
probability of bacteraemia was found at a PCT cut-off value of 1,6 µg/ml,
compared to 40 times and 24 times higher probability for IL-8 and PS,
respectively for the best cut-off value of 170 ug/l and 410 pg/ml.
Interestingly, patients with monocyte count under 0,01 x 109/l,
had 9 times higher probability for bacteraemia than patients with
higher monocyte count. We found that CRP concentrations could not
predict bacteraemia well. This is not unexpected as CRP levels could be
influenced by the underlying malignant disease and tissue damage, all
factors affecting its specificity.[9]
Presepsin,
a molecule secreted from monocytes following phagocytosis, has gained
interest over the past few years in virtue of its rapid increase in
patients with sepsis.[6] A recent meta-analysis (18
studies, 3470 patients) has addressed the diagnostic accuracy of
presepsin in sepsis.[10]
Overall, data suggest that presepsin is a promising marker for the
diagnosis of sepsis, but no better performance of presepsin over PCT
could be demonstrated.
The role of PS in hematologic FN patients is even less clear as only
limited, and inconsistent results are available.[11-15]
Only one study found evidence in favour of presepsin as a predictor of
bacterial infection in FN. These authors concluded that PS could be
used as a discriminator of infectious versus non-infectious origin of
fever in children with oncohaematological disorders.[14]
Unfortunately, in this study, Baraka et al. did not provide details on
the methodology of sample collection. Therefore the comparison between
studies is difficult as the temporal profile of markers likely differs.
Of interest, in our study, PCT was informative even if blood samples
were collected by 2 hours from the onset of fever. One would have
expected a different dynamics of inflammatory markers, particularly of
PS and PCT, the latter rising later after the onset of inflammation.[16]
The fact that there is evidence of a pre-activation of monocytes before
the development of overt sepsis may explain our findings.[17]
In
agreement with our results, a retrospective study by Ebihara et al.
found that only PCT discriminates between neutropenic patients with
infection and uninfected subjects. However, the major limitation of
this retrospective study is that no baseline values were collected and
therefore, these authors concluded that these biomarkers could not be
used as diagnostic tools by themselves.[15] In a
population of adult haematological patients with FN, Koh et al.
demonstrated that PS levels increased significantly earlier than PCT,
but they concluded that the ability of PS to discriminate septic shock
from other conditions was inferior to that of PCT.[11]
We
found that IL-8 was the most sensitive biomarker. At a cut-off value of
170 pg/ml, IL-8 correlated with all cases of gram-positive bacteraemia
and 6/7 of gram-negative bacteraemia. Our result is consistent with
recent evidence correlating IL-8 with bacteraemia in paediatric
haematological patients, especially in Gram-negative bacteraemia.[18-21]
Interestingly, monocyte count lower than 0,01 x 109/l
inversely correlated with the concentrations of PCT, IL-8 and PS, in
agreement with a recent study by Koh et al.[11]
As already suggested, monocyte count in the bloodstream is not
representative of tissue monocytes during bacteraemia, On the
other hand, a low monocyte count in FN may also explain the lower
reliability of PS in compared to data from non-neutropenic subjects
with infection and sepsis.
Our findings are apparently in
contrast with the results of a recent study showing no added value of
PCT over CRP in haematological patients with prolonged and profound
neutropenia.[22] In particular, these authors
found that only 39% of bacteraemia episodes had PCT above the average
threshold at day two after fever onset. Also, they noted that CRP
values at the same time were significantly higher in microbiologically
documented infection compared to clinically documented infection. A
possible explanation for the discrepancy may relate to a different
subgroup composition of febrile neutropenic patients between ours and
Verlinden’s study as we did not include allogeneic transplants whereas
no patients with relapsed NHL were considered in Verlinden’s study.
Also, the timing of blood collection on the day of febrile neutropenia
onset slightly differed in our study as sampling was performed by 90
and 120 minutes from fever onset, always before patients were started
on antibiotic treatment, which may potentially affect procalcitonin
levels.[23]
One may argue that, in real life,
even a highly accurate and rapidly available marker does not change the
therapeutic approach as the decision to start a patient on antibiotic
therapy is always based on the clinical picture and standard
protocols. A biomarker would be most useful as a screening test
(i.e. a negative value confirms the absence of infection); hence it
should be characterized by a high sensitivity, not supported by our and
other studies.
Although there is general agreement over
combination therapy in septic shock, international guidelines recommend
against combination therapy in bacteremia and sepsis without shock.
However, in clinical scenarios of severe clinical illness, this
position does not preclude the use of multidrug therapy to broaden the
spectrum of antimicrobial treatment, and in this specific setting, the
identification of highly specific markers of bacteraemia like PCT may
be of help.
In agreement with previous studies, the neutrophil
count did not predict bacteraemia compared to other febrile neutropenia
subgroups.[14,24] Although sample
size limits substantial conclusions, this result is not unexpected as
all subjects with ANC <0.1 x 109/L
are considered at high risk, and other factors like duration of
neutropenia may contribute to the development of bacteraemia.[2]
Significant
limitations of this study are the fact that it is a single–centre study
with a relatively low number of febrile episodes (albeit comparable
with the majority of previously published monocentric studies on this
topic) and that comparison of our results with those from other studies
is limited because of the heterogeneity of populations and methodologic
approaches.
In conclusion, PCT has higher specificity than
PS, IL-8 and CRP in predicting bacteraemia during FN in subjects with
haematological malignancies.
References
- Klastersky J. Management of fever in neutropenic
patients with different risks of complications. Clin Infect Dis.
2004;39 Suppl 1: S32-37. https://doi.org/10.1086/383050
PMid:15250018
Freifeld
AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the
use of antimicrobial agents in neutropenic patients with cancer: 2010
update by the infectious diseases society of america. Clin Infect Dis.
2011;52: e56-93. https://doi.org/10.1093/cid/cir073
PMid:21258094
Póvoa P.
C-reactive protein: a valuable marker of sepsis. Intensive Care Med.
2002;28: 235-243. https://doi.org/10.1007/s00134-002-1209-6
PMid:11904651
Pierrakos
C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14: R15. https://doi.org/10.1186/cc8872
PMid:20144219 PMCid:PMC2875530
- Chenevier-Gobeaux
C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin
(sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta.
2015;450: 97-103. https://doi.org/10.1016/j.cca.2015.06.026
PMid:26164388
- Arai
Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K.
Phagocytosis by human monocytes is required for the secretion of
presepsin. J Infect Chemother. 2015;21: 564-569. https://doi.org/10.1016/j.jiac.2015.04.011
PMid:26026662
- Zhang
J, Hu ZD, Song J, Shao J. Diagnostic Value of Presepsin for Sepsis: A
Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:
e2158. https://doi.org/10.1097/MD.0000000000002158
PMid:26632748 PMCid:PMC5059017
- Pizzo
PA, Armstrong D, Bodey G, de Pauw B, Feld
R, Glauser M, Gaya H, Karp J, Klastersky
J, Todeschini G, Verhoef J, Wade J, Young
LS, Remington J. From the Immunocompromised Host Society. The
design, analysis, and reporting of clinical trials on the empirical
antibiotic management of the neutropenic patient. Report of a consensus
panel. J Infect Dis. 1990;161: 397-401. https://doi.org/10.1093/infdis/161.3.397
PMid:2179421
- Santolaya
ME, Cofre J, Beresi V. C-reactive protein: a valuable aid for the
management of febrile children with cancer and neutropenia. Clin Infect
Dis. 1994;18: 589-595. https://doi.org/10.1093/clinids/18.4.589
PMid:8038314
- Wu
CC, Lan HM, Han ST, et al. Comparison of diagnostic accuracy in sepsis
between presepsin, procalcitonin, and C-reactive protein: a systematic
review and meta-analysis. Ann Intensive Care. 2017;7: 91. https://doi.org/10.1186/s13613-017-0316-z
PMid:28875483 PMCid:PMC5585118
- Koh
H, Aimoto M, Katayama T, et al. Diagnostic value of levels of presepsin
(soluble CD14-subtype) in febrile neutropenia in patients with
hematological disorders. J Infect Chemother. 2016;22: 466-471. https://doi.org/10.1016/j.jiac.2016.04.002
PMid:27184936
- Urbonas
V, Eidukaitė A, Tamulienė I. The predictive value of soluble biomarkers
(CD14 subtype, interleukin-2 receptor, human leucocyte antigen-G) and
procalcitonin in the detection of bacteremia and sepsis in pediatric
oncology patients with chemotherapy-induced febrile neutropenia.
Cytokine. 2013;62: 34-37. https://doi.org/10.1016/j.cyto.2013.02.030
PMid:23510625
- Stoma
I, Karpov I, Uss A, Rummo O, Milanovich N, Iskrov I. Diagnostic value
of sepsis biomarkers in hematopoietic stem cell transplant recipients
in a condition of high prevalence of gram-negative pathogens. Hematol
Oncol Stem Cell Ther. 2017;10: 15-21. https://doi.org/10.1016/j.hemonc.2016.09.002
PMid:27793578
- Baraka
A, Zakaria M. Presepsin as a diagnostic marker of bacterial infections
in febrile neutropenic pediatric patients with hematological
malignancies. Int J Hematol. 2018;108: 184-191. https://doi.org/10.1007/s12185-018-2447-x
PMid:29616457
- Ebihara
Y, Kobayashi K, Ishida A, et al. Diagnostic performance of
procalcitonin, presepsin, and C-reactive protein in patients with
hematological malignancies. J Clin Lab Anal. 2017;31. https://doi.org/10.1002/jcla.22147
PMid:28133789
- Koizumi
Y, Shimizu K, Shigeta M, et al. Plasma presepsin level is an early
diagnostic marker of severe febrile neutropenia in hematologic
malignancy patients. BMC Infect Dis. 2017;17: 27. https://doi.org/10.1186/s12879-016-2116-8
PMid:28056845 PMCid:PMC5217328
- Lissauer
ME, Johnson SB, Bochicchio GV, et al. Differential expression of
toll-like receptor genes: sepsis compared with sterile inflammation 1
day before sepsis diagnosis. Shock. 2009;31: 238-244. https://doi.org/10.1097/SHK.0b013e3181834991
PMid:18665047
- Şahbudak
Bal Z, Karadaş Özdemir N, Şen S, et al. Diagnostic Accuracy of
Interleukin-6, Interleukin-8, and Interleukin-10 for Predicting
Bacteremia in Children with Febrile Neutropenia. Turk J Haematol.
2017;34: 254-257. https://doi.org/10.4274/tjh.2016.0434
PMid:28148470 PMCid:PMC5544046
- Santolaya
ME, Alvarez AM, Aviles CL, et al. Predictors of severe sepsis not
clinically apparent during the first twenty-four hours of
hospitalization in children with cancer, neutropenia, and fever: a
prospective, multicenter trial. Pediatr Infect Dis J. 2008;27: 538-543.
https://doi.org/10.1097/INF.0b013e3181673c3c
PMid:18458649
- Miedema
KG, Vermont CL, Ball LM, et al. The diagnostic value of interleukin-8
for the detection of bacteremia in pediatric hematopoietic stem cell
recipients with febrile neutropenia. Transplantation. 2014;98: e80-81. https://doi.org/10.1097/TP.0000000000000434
PMid:25318571
- Michel
CS, Teschner D, Wagner EM, Theobald M, Radsak MP. Diagnostic value of
sTREM-1, IL-8, PCT, and CRP in febrile neutropenia after autologous
stem cell transplantation. Ann Hematol. 2017;96: 2095-2101. https://doi.org/10.1007/s00277-017-3128-1
PMid:28920169
- Verlinden
A, De Vroey V, Goossens H, et al. Comparison of the Power of
Procalcitonin and C-Reactive Protein to Discriminate between Different
Aetiologies of Fever in Prolonged Profound Neutropenia: A Single-Centre
Prospective Observational Study. Mediterr J Hematol Infect Dis.
2019;11: e2019023. https://doi.org/10.4084/mjhid.2019.023
PMid:30858961 PMCid:PMC6402549
- Meisner M.
Update on procalcitonin measurements. Ann Lab Med. 2014;34: 263-273. https://doi.org/10.3343/alm.2014.34.4.263
PMid:24982830 PMCid:PMC4071182
- Masson
S, Caironi P, Spanuth E, et al. Presepsin (soluble CD14 subtype) and
procalcitonin levels for mortality prediction in sepsis: data from the
Albumin Italian Outcome Sepsis trial. Crit Care. 2014;18: R6. https://doi.org/10.1186/cc13183
PMid:24393424 PMCid:PMC4056046




