Chiara Visintini1, Margherita Venturini1, Stefano Botti2, Gianpaolo Gargiulo3 and Alvisa Palese1..
1 School of Nursing, Department of Medical Sciences, University of Udine, Italy.
2 Hematology Unit, Azienda USL-IRCCS Reggio Emilia, Italy.
3 Hematology and Haematopoietic Stem Cell Transplantation centre, “Federico II” University Hospital of Naples, Italy.
Correspondence to: PhDc Alvisa Palese, MNS. School of Nursing, Udine
University, Viale Ungheria, 20, 33100 Udine, Italy; Tel.: +39 0432
590926; E-mail:
alvisa.palese@uniud.it
Published: September 1, 2019
Received: May 14, 2019
Accepted: August 8, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019051 DOI
10.4084/MJHID.2019.051
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Haemorrhagic
cystitis (HC) is a severe complication occurring after haematopoietic
stem cell transplantation (HSCT) in 13-40% of patients, caused by
infectious and/or non-infectious factors that increase the in-hospital
length of stay and the risk of mortality of transplanted recipients.
Although different management interventions have been suggested in the
literature, available knowledge on interventions performed by Italian
nurses in their daily practices has not been documented to date.
Aim of the study:
The aim of this study is to describe HC preventive and treatment
interventions in patients undergoing HSCT as performed by Italian
nurses in their daily practice.
Material and methods:
A multicentre survey was conducted in 2018 by inviting all 110 Italian
HSCT centres belonging to the Italian Group for Bone Marrow
Transplantation (GITMO). Data collection was performed with an online
questionnaire submitted to GITMO reference nurses working in each HSCT
centre. Descriptive statistics were performed.
Results:
A total of 38 Italian centres participated. The preventive intervention
most applied in daily care was the mesna administration (n=37; 97.4%),
followed by intravenous hyperhydration (n=33; 86.8%) and forced
diuresis with furosemide (n=24; 63.1%). Preventive continuous bladder
irrigation (CBI) was performed in 13 centres (34.2%). Transfusions of
blood products (n=32; 84.2%), CBI (n=31; 81.6%) and intravenous
hydration (n=28; 73.7%) were the most applied treatments, beyond the
administration of analgesics (n=38; 100.0%) and antispasmodics (n=26;
68.4%).
Conclusion: A
great variability both in the HC prevention and treatment interventions
applied in daily practice across centres have emerged suggesting that
no strong recommendations in the field are available to date.
Therefore, there is a need to increase the evidence available in the
field by providing methodological studies of higher quality,
multicentre and prospective.
|
Introduction
In
patients undergoing haematopoietic stem cell transplantation (HSCT),
haemorrhagic cystitis (HC) is a severe complication with an estimated
incidence of 13% to 40%.[1–4] Infectious and/or
non-infectious factors contribute to HC occurrences, such as adenovirus
(ADV) or BK polyomavirus (BKPyV) reactivation,[5,6] conditioning regimens,[4,7] graft-versus-host disease (GVHD),[2,8] and the stem cell sources or donor-recipient incompatibility.[1,9]
HC is responsible for the bleeding from the bladder mucosa and a
widespread symptomatology including burning, bladder pain, and severe
haematuria with clots retention with possible renal failure.[10]
HC has been classified as early-onset (EOHC) when it occurs within 48
hours after the conditioning regimens, or late-onset (LOHC) when it
occurs after 48 hours.[11] Moreover, HC has been documented to increase the in-hospital length of stay and the risk of mortality.[3,6]
As emerged from a recent scoping review,[12] urine alkalinisation, hyperhydration and forced diuresis have been the most recommended preventive HC measures;[1,2]
however, conflicting data have been reported regarding the
effectiveness of the preventive application of the continuous bladder
irrigation (CBI).[1,2,13] The agent
2-mercaptoethanol sodium sulphonate (mesna) has been documented to
reduce the urothelial exposure to chemotherapy, particularly
cyclophosphamide.[1,2,13] Even
ciprofloxacin as a prophylactic measure has been reported to be
effective in reducing the incidence of severe BKPyV-associated
haemorrhagic cystitis (BKPyV-HC).[14]
Regarding
the HC treatment, no gold standard has been established to date;
however, cidofovir (CDV) seems to be the most effective against
BKPyV-HC.[15-18] Mackey (2012)[19]
has demonstrated the use of intravesical CDV as capable of limiting the
risk of renal damage, compared to its intravenous administration. Other
promising antivirals against ADV or cytomegalovirus (CMV)-associated HC
have also been documented.[18,20] Moreover, in cases of refractory HC, the administration of intravesical prostaglandins has been suggested[21,22] in addition to local therapies, e.g., formalin and alum,[23] hyaluronic acid,[24] and fibrin glue.[25]
Furthermore, recent studies have suggested the administration of
specific T-BKPyV cells as a new therapeutic option to treat HC and to
minimise the risks of GVHD,[26,27] while
cystoscopies, cauterisations and surgical interventions have been found
as useful in severe grades of HC or life-threatening conditions.[21,28] Supportive measures such as CBI, analgesics and blood products have also been suggested.[15,19,21,22,28]
However, which preventive or treatments are daily performed at the
bedside have been rarely documented. Cesaro, on behalf of the ECIL-6
working group (2018),[29] has recently updated the
Guidelines for the Management of BKPyV-associated HC in HSCT
recipients; nevertheless, no recommendation above grade C (=marginal
support for use) has been established in the field of HC treatment.
To date, only three surveys[30,31,32] have been published on this subject. Gargiulo et al. (2014)[32]
in their prospective study among 30 Italian HSCT centres reported an
overview of interventions applied by Italian nurses and physicians in
paediatric and adult transplanted patients. As reported by the involved
experienced professionals, quinolones (87.3%) followed by
hyperhydration (85.3%) and urine alkalinisation (62.2%) were the most
common preventive interventions, while the bladder catheter insertion
was reported by 11.8% of the centres. Among treatments, hyperhydration
(56.3%), bladder catheter placement (56.3%), CBI (27.2%) and CDV
(12.7%) were the most applied. The survey conducted in 2016 by
Schneidewind et al. (2017)[31] was addressed to
haematologists and urologists among the European Bone Marrow
Transplantation (EBMT) centres in Germany, focusing on the management
of BKPyV-associated HC in the adult population by using a
questionnaire. According to the findings, local bladder therapy was the
most effective treatment in the opinion of 63.3% of haematologists,
followed by CDV medication (26.7%) and other therapies (10.0%).
Urologists mainly reported the use of CBI (92.6%) and local therapies
(27.8%, as cidofovir, leflunomide, tranexamic acid and alum), while
systemic therapy was applied less often (14.8%). More recently, Cesaro
and colleagues (2018)[30] performed a survey on ADV
infections management in Europe, Russia and the Middle East among 89
EBMT centres. CDV was the medication most applied. The reduction of
immunosuppression (84%) and the administration of brincidofovir (27%)
were largely adopted, especially among the paediatric population to
treat ADV infection.
With the intent to advance the available
knowledge on prevention and treatment as performed in their daily
practices, the principal aim of this study was to describe
interventions of Italian clinical nurses to prevent and manage HC. A
secondary aim was to describe the professional experience of nurses in
managing HC as well as their perceived HC effects on patients.
Materials and Methods
A
national-wide online survey was performed in 2018 involving the Italian
Group for Bone Marrow Transplantation (GITMO) network. Eligible
participants were clinical nurses who at the time of the survey: (a)
were members of GITMO, (b) were active clinical nurses in one of the
Italian HSCT centres included in the GITMO network, and (c) were
willing to participate in the survey. A total of 110 clinical nurses
were found eligible.
Data collection.
A questionnaire was developed by researchers in cooperation with the
GITMO nurses’ board members and piloted by five clinical nurses not
involved in this final survey. After the piloting, no changes were
required. In the questionnaire were included all interventions
documented in the literature which were aimed at preventing and
managing HC, as emerged from a scoping review.[12] The final version of the anonymous online survey was based upon 22 items divided into the following four sections:
(1) Section
1: exploring the main demographic characteristics of responding nurses,
as well as the number of HC cases they recalled to have managed in
2017, by using closed (n = 3), multiple choice (n = 2) and short
open-answer questions (8 items),
(2) Sections 2 and 3:
assessing preventive and treatment interventions as performed in daily
practice using multiple-choice questions (n = 3); open questions (n =
2) were set regarding timing and specification of the interventions
applied (e.g., administration routes),
(3) Section 4:
exploring nurses’ experiences while managing patients with HC (n = 3)
regarding (i) the encountered difficulties, (ii) the perceived impact
of HC on patients and (iii) on the nurses, by using 5-degree Likert
scales (e.g., 1 = no impact, 5 = maximum impact). Area of investigation
and items for each area have been established based on the available
literature[12] and the available data from the GITMO network, where previous research in the field has been conducted.
Nurses
were left free to fill in the questionnaire with the support of
physicians; only 12 (31.6%) filled in the questionnaire autonomously,
while the remaining 26 (68.4%) had the cooperation of a physician
working in the same centre, as emerged from the last question of the
questionnaire.
A presentation letter reporting the aims of the
study, as well as access instructions and the questionnaire link, was
sent to all GITMO network centres. The completion of the questionnaire
was intended as the consent to participate in the survey. Two reminders
were performed by e-mail and by phone to promote responses. All
procedures were in accordance with the ethical standards of the GITMO
and with the 1964 Helsinki Declaration.
Data Analysis.
The answers were reported and analysed with Microsoft Excel 2013 and
then processed with the SPSS V 24.00. Descriptive statistics were
performed (frequencies, percentages, means, standard deviations [SD,
±], confidence intervals [CIs] at 95%). Open answers were read by two
researchers and then summarised according to their commonalities and
differences.
Results
A
total of 38 clinical nurses participated, mainly caring for adult
patients (24; 63.1%). Of the 110 centres, 34.5% were represented. Most
of the participants were female (29; 76.3%) with an average age of 46.3
± 8.4 years. The majority of them were educated at the university level
(20; 52.6%), and at the time of the study had been working an average
of 15.8 ± 8.3 years, as reported in table 1.
According
to that recalled by participants, an average of 62.1 ± 37.2 (range
14–185) transplants were performed in 2017. The median number of HC
cases in the same reference year was 2.4 ± 2.7 (CI 95% 1.5–3.2),
leading to a prevalence rate of 3.8% as reported in table 2.
The HC cause recalled by clinical nurses was mainly infectious (19;
65.5%), such as BK virus (15; 51.7%). The recalled onset after HSCT was
on average 25.0 ± 27.7 days (ranging from 3–180) and the HC duration
was 23.7 ± 23.35 days (ranging from 5–150). The grading of the common
terminology criteria (CTC) of the National Cancer Institute (NCI)[33]
was the most used assessment tool for HC (22; 57.9%). In 52.6% of
centres (n = 20), there were protocols or checklists guiding nursing
care interventions, as reported in table 2.
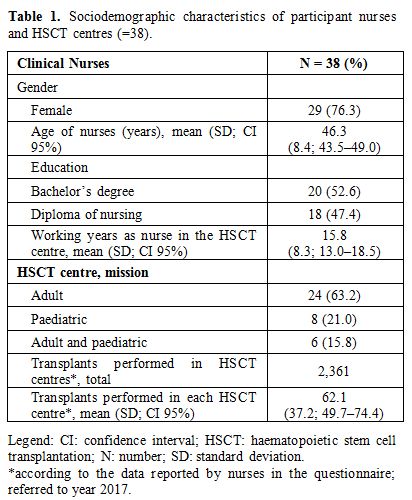 |
Table 1. Sociodemographic characteristics of participant nurses and HSCT centres (=38). |
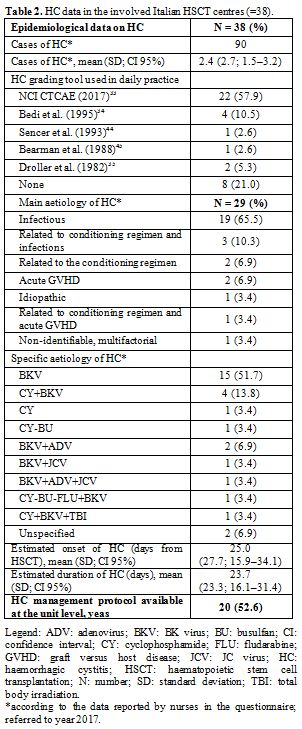 |
Table
2. HC data in the involved Italian HSCT centres (=38). |
.Preventive interventions.
In order to prevent HC, the most applied intervention reported by
nurses was the intravenous mesna (37 of 38 centres; 97.4%), followed by
hyperhydration with normal saline solution (33; 86.8%) and forced
diuresis with furosemide (24; 63.1%). The placement of a three-way
urinary catheter to supply CBI was carried out in 13 centres (34.2%).
The least applied preventive intervention was the oral hydration and
the placement of the bladder catheter, as reported in table 3.
Several centres (10; 26.3%) applied four preventive interventions per
patient, whereas 1 (2.6%), 4 (10.5%), 8 (21.0%), 6 (15.8%), 6 (15.8%)
and 3 (7.9%) centres have applied one, two, three, five, six and seven
interventions per patient, respectively. Intravenous mesna
administration was usually started at the beginning of cyclophosphamide
(CY) administration in more than half of the centres (25 of 37; 67.6%);
five centres specified “at the beginning of the conditioning regimen”.
The administration ended at the time of the CY administration (4 of 37;
10.8%) and the conditioning regimen in general (3 of 37; 8.1%), or from
6–48 hours after the last dose of CY (17 of 37; 45.9%); however, 11
(29.7%) centres did not respond.
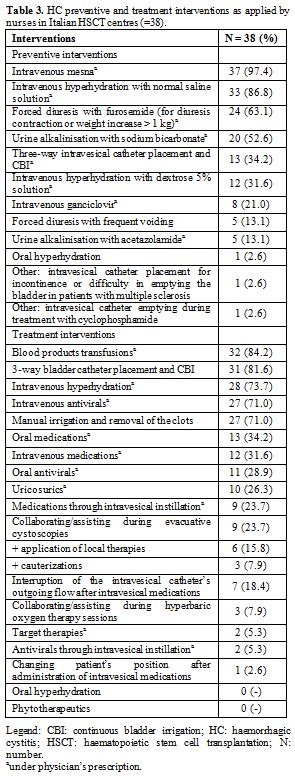 |
Table 3. HC preventive and treatment interventions as applied by nurses in Italian HSCT centres (=38). |
Hyperhydration
with normal saline was started from the patient admission in the centre
to 6 hours before the conditioning (10 of 33; 30.3%), or from the
beginning of the conditioning regimen (11 of 33; 33.3%); three
participants specified “at the beginning of CY administration”. It
continued until the end of chemotherapy administration in 8 of 33
centres (24.2%). However, 10 (30.3%) and 14 (42.4%) of 33 centres using
hyperhydration with normal saline did not respond regarding when this
preventive measure is used to be started and ended, respectively.
Forced
diuresis with furosemide was usually started at the beginning of the
conditioning regimen in 6 of 24 centres (25.0%) - three participants
specified “at the beginning of CY administration” - or at the end of
the conditioning (2 of 24; 8.3%). The administration of furosemide
usually ended when the conditioning regimen also ended (3 of 24;
12.5%), specifically from 24–48 hours later (4 of 24; 16.7%). However,
also, in this case, 14 (58.3%) and 15 (62.5%) centres did not respond
regarding when this preventive measure is used to be started and ended,
respectively.
Treatment interventions. As shown in table 3,
the most applied HC treatment is the transfusion of blood products (32;
84.2%), followed by CBI (31; 81.6%) and the intravenous hyperhydration
(28; 73.7%). Manual irrigation and administration of intravenous
antivirals were reported by 27 centres (71.0%). No centres reported the
use of oral hyperhydration and phytotherapeutics. Several centres
applied six or seven interventions on the same patient (12 of 38;
31.6%). Among transfused blood components, platelets (24 of 32; 75.0%),
packed red blood cells (13 of 32; 40.6%), fresh frozen plasma (5 of 32;
15.6%) and albumin (1 of 32; 3.1%) were reported as being used most
often. Most centres, to manually irrigate and remove clots, used a
50–60 mL syringe by injecting a normal saline solution (7 of 27;
25.9%), water for injectable preparations (3 of 27; 11.1%) or distilled
water (1 of 27; 3.7%). However, 16 centres (59.2%) did not specify the
solution used.
Concerning the CBI, most centres reported using as
infusing solution the normal saline (10 of 31; 32.2%) or water for
injectable solutions (3 of 31; 9.7%). One centre out of 31 (3.2%)
administered CBI using a volumetric pump, and 3 (9.7%) reported using
solutions prepared at a lower temperature than that of the environment.
Moreover, three centres of 31 (9.7%) started the administration at the
onset of microhematuria or large clots, while one centre (3.2%)
reported stopping it at haematuria’s resolution. Other centres did not
report data regarding the timing of CBI use.
The infused solutions
for hyperhydration were reported as containing normal saline (15 of 28;
53.6%), sodium bicarbonate (4 of 28; 14.3%) and dextrose 5% (2 of 28;
7.1%). Two centres specified “dextrose 5% with potassium chloride
corrections” and “electrolysing rehydrating solutions”, respectively.
As
antivirals, oral acyclovir (5 of 11; 45.4%), oral CDV (11 of 27;
40.7%), intravenous ganciclovir (6 of 27; 22.2%), intravenous foscavir
(9 of 27; 33.3%) and intravenous acyclovir (3 of 27; 11.1%) emerged as
highly used. CDV was given by intravesical instillation (2 of 38;
5.3%), interrupting the outflow from the bladder catheter for 30
minutes in 3 of 7 centres (42.8%) after the end of the instillation.
Among other medications, ciprofloxacin (8 of 13; 61.5%) and
levofloxacin, oxybutynin, leflunomide, meropenem, ceftazidime (5 of 13;
38.5%) were reported as used by oral administration. Among those nurses
who reported to have supported physicians in evacuative cystoscopies (9
of 38; 23.7%), 3 of them (33.3%) have applied local fibrin glue while
one has applied the hyaluronic acid (out of 9; 11.1%) and two the
platelet gel (out of 9; 22.2%), respectively. Moreover, three nurses
(7.9%) reported having supported clinicians in performing
cauterisations.
Figure 1
shows the medications usually given by nurses to relieve pain. The
administration of intravenous opioid analgesics (31; 81.6%) and of
antispasmodics by prescription (26; 68.4%) were the most applied
medications.
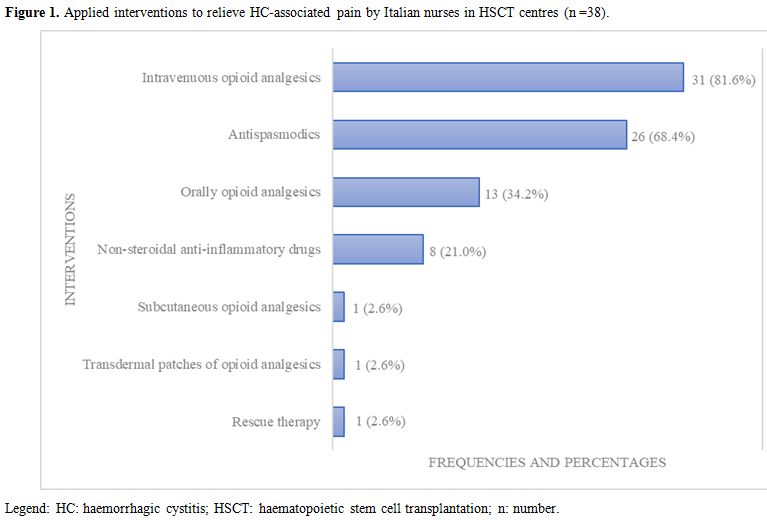 |
Figure 1. Applied interventions to relieve HC-associated pain by Italian nurses in HSCT centres (n =38). |
Nurses’ experiences. As seen in table 4,
the greatest difficulty that nurses encountered in the management of
patients with HC was the large degree of support that these patients
required to improve their adverse emotional state due to this
complication (average 3.31 out of 5; SD 1.16). The lowest difficulty
was the technical management of urinary devices (1.97 out of 5; SD
0.97). According to the experience of nurses, the highest impact of HC
is at the patient level, affecting the quality of life (QoL) (4.60 out
of 5; SD 0.59). The impact on healthcare professional workloads and
processes was the least (3.50 out of 5; SD 1.06). A sense of impotence
(2.74 out of 5; SD 1.20), frustration (2.58 out of 5; SD 1.48),
dissatisfaction (2.47 out of 5; SD 1.37) and anxiety (2.26 out of 5; SD
1.29) were the most-perceived impacts upon nurses while managing a
patient with HC..
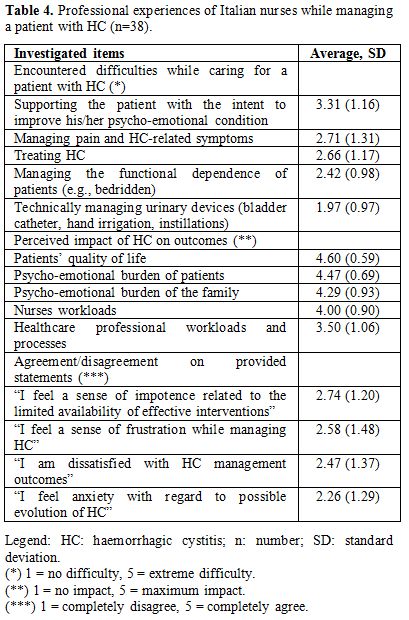 |
Table 4. Professional experiences of Italian nurses while managing a patient with HC (n=38). |
Discussion
To
the best of our knowledge, this is the first survey at the national
level that included nurses and explored the HC preventive and treatment
interventions as performed in daily practice. The majority of them
completed the questionnaire with physicians, suggesting that in these
settings, multidisciplinary care is fundamental. Although higher than
that reported in the EBMT Survey on ADV (20%),[30] the response rate was low (34.5%) as compared to that reported by a German survey (>70%).[31]
The
prevalence of HC was estimated on the basis of the recall of
participants – as well as for the HC onset, the duration and the
aetiology. Prevalence was around 3.8%, lower than that reported in the
literature (19.0%,[1] 12.2%,[32] 32.5%[2]).
However, the centres were performing both autologous and allogeneic
HSCTs. Autologous HSCTs have been less associated with the risk of
developing HC as compared to allogeneic ones.[1,13]
The large variability reported regarding the onset of HC after HSCT,
and its duration, have been documented as heterogeneous also in the
literature.[31,32,34] However,
although data collected were reported by nurses with long professional
experience (>15 years, thus experts in the field), this may not
reflect the actual data of the centres.
The most described HC
aetiology is related to infections (specifically to BKPyV). This could
be attributed to the advances in prophylaxis against the urotoxic
effects with the conditioning regimens and to the more known role of
BKPyV and other viruses as risk factors as documented in the recent
literature mainly focused upon the viral HCs.[6,15,19,31]
The role of busulfan-cyclophosphamide as a risk factor for HC was not
confirmed by the multivariate analysis of Tsuboi et al. (2003).[4]
The CTC of NCI[33] in its many versions has been the most applied assessment tool in the haematological centres involved, and several authors[21,26] used this classification. However, Gargiulo et al. (2014)[32] used the score developed by Droller and colleagues (1982),[35]
which was adopted by only two centres in our survey. Increasing
comparability of the data in future multicentre studies by applying a
common assessment tool is recommended.
Preventive interventions. The most applied preventive intervention is the administration of mesna as reported by several studies.[1,2,13] Its administration is more common compared to Gargiulo and colleagues’ data (2014),[32] where mesna was administered in 50.6% of patients. However, fluoroquinolones that Gargiulo et al. (2014)[32]
reported as the most applied intervention were not administered in our
survey. Rather, the use of quinolones such as ciprofloxacin (8; 21.0%)
and levofloxacin (1; 2.63%) has been considered as therapeutic
measures. After Leung et al. (2005),[36] Miller et al. (2011)[14]
administered ciprofloxacin 500 mg orally every 12 hours until 60 days
after HSCT in 44 patients (48 patients did not receive prophylaxis)
showing a cumulative incidence of HC of 2.6% in the ciprofloxacin group
as compared to 20.9% in the non-treated group (P=0.01). Thus, they
demonstrated that ciprofloxacin was effective in reducing the incidence
of severe BKPyV-HC.
From the 37 centres administering mesna, some
centres preferred intermittent intravenous boluses and some preferred
continuous infusion. From the scoping review by Visintini et al.
(2019),[12] mesna can be administered by intermittent boluses[2,34] or by continuous infusion.[13]
Large variability of practices exists concerning when the mesna should
be started and ended. Conflicting data has also emerged, thus causing
uncertainty and the absence of standard care plans. Hadjibabaie et al.
(2008)[2] administered mesna before CY infusion and every 6 hours for a total of three doses and Vose et al. (1993)[13]
tested mesna administration from 1 hour before CY, by continuous
infusion, until 24 hours after the last CY dose. Bedi et al. (1995)[34]
conducted a randomised controlled trial among 147 American HSCT
recipients with HC. Their regimen consisted of 5 intravenous doses at
30 min before CY and 3, 6, 9 and 12 hours after each dose of CY.
As
the second- and third-most applied preventive interventions,
hyperhydration with normal saline and forced diuresis with furosemide
have both been recommended for the prevention of HC.[34] An administration speed of 250 mL/h was used in the randomised controlled trial by Vose et al. (1993),[13] while 2500 mL/day emerged from the retrospective analysis of Gonella et al. (2015).[1] Bedi and colleagues (1995)[34]
preferred 2 mL/kg/h until 24 hours after the last CY dose. Dextrose
water and normal saline containing potassium chloride have also been
utilised.[2] According to our data, only one Italian
centre reported the use of oral hyperhydration, a natural way to intake
fluids but probably more uncomfortable for patients, considering the
quantity of daily water to drink (at least 2 L). In addition, forced
diuresis with furosemide was tested by Bedi et al. (1995)[34]
as compared to mesna and findings showed equally preventive
effectiveness of both interventions (P=0.41). Forced diuresis through
frequent voiding has not been documented by studies available as
effective in reducing HC as compared to CBI.[37,38]
Data about the effectiveness of preventive CBI are still conflicting.[1,2,13] However, 13 centres have reported using it in daily care. Gonella et al. (2015)[1]
suggested considering the benefits and harms of preventive
catheterisation and CBI, especially concerning patients’ discomfort and
an increased risk of urinary tract infections. Moreover, according to
the recent ECIL 6 Guidelines (2018)[29] the
prophylaxis of BKPyV-HC relying on hyperhydration and bladder
irrigation, in particular when using myeloablative conditioning based
on CY or other alkylating agents, have been suggested with low levels
of evidence and strength of recommendations (hyperhydration=BII, level
of evidence from at least one well-designed clinical trial, moderately
recommended for use; bladder irrigation; CII=level of evidence from at
least one well-designed clinical trial without randomization,
marginally recommended for use). Furthermore, the use of specific
antivirals and fluoroquinolones have not been recommended (DII).
Treatment interventions.
The administration of blood products has been the most applied
treatment for HC. Actually, it is considered a supportive treatment,
able to improve HC but not effective alone in its resolution.[15,21,34,38,39]
However, blood transfusions could be supportive not only for HC but
even for the myeloablative effects of chemotherapies, and their common
use can be considered a standard of care in patients undergoing HSCT.
Bladder catheter placement and CBI have emerged as being widely used.
Gargiulo et al. (2014)[32] divided the catheter
placement (56.3%) from the CBI (27.2%), and therefore our data could
not be compared. Only one centre reported using a 3-way bladder
catheter. However, the documented effectiveness of CBI[1,15,40] has been studied in association with other interventions such as the instillation of hyaluronic acid.[24]
Local
therapies have been reported as the most up-to-date therapeutic
measures by urologists (92.6%) and haematologists (63.3%).[31]
From our survey, the administration of intravesical antivirals was
reported by only 2 centres using CDV, while 7 reported instillations of
hyaluronic acid. Local treatments have been used rarely in Italy until
2014 (CDV instillation, 3.6%; hyaluronic acid instillation, 5.5%).[32]
However, intravesical CDV might be an option for symptomatic
improvement in patients with BKPyV-HC, without systemic adverse effects
such as renal failure.[19] Furthermore, 7 centres
(18.4%) reported interrupting the catheter’s outgoing flow at the end
of intravesical instillation while 3 interrupted it for 30 minutes
(less than the hour suggested in the literature).[21,39]
Moreover, among the nine centres implementing evacuative cystoscopies,
around one-third of them applied topical fibrin glue at the same time.
This measure has been studied mainly in Italy: In the pilot study
performed by Tirindelli and colleagues (2009),[25]
five patients with refractory HC were treated with fibrin glue through
an endoscopic applicator. The treatment showed a positive response in
three patients suggesting that fibrin glue may be an effective solution
for refractory HC.
Multiple treatments such as intravenous
tranexamic acid, immunoglobulins, fibrinogen and antithrombin III have
also been used. According to the literature available, CDV - the most
frequently reported intravenous antiviral - appeared to have the
highest level of activity against BKPyV-HC.[6,15,16,19]
However, the recent ECIL 6 Guidelines attributed the highest level of
evidence (II= evidence from uncontrolled trials) for CDV that is still
recommended as grade C (=marginal support for use), due to its
uncertainty ineffectiveness and best dose schedule.[29]
Furthermore, the access to CDV has been limited in recent years
according to the rules of importation of the medication from abroad and
this has certainly influenced its adoption across Italian centres.
In
addition to CDV, leflunomide and other therapies such as ciprofloxacin
and intravenous immunoglobulins have been studied by haematologists.[31] The use of the antivirals ganciclovir and foscavir, as described in our study, has also been documented in the literature.[18]
The role of tranexamic acid as a haemostatic agent was associated with
a high risk of thromboembolic events, and it is contraindicated in the
management of HC.[41] In our survey, 21.0% of centres
reported its administration suggesting the need to improve knowledge on
its negative effects among clinicians through educational initiatives.
Immunoglobulins, however, appeared safe and effective in association
with intravesical prostaglandin E2.[22] These findings reveal a wide range of therapeutic tools with no strong recommendations to guide the clinical practice.
Regarding
the manual irrigation and removal of the clots, a procedure which has
been largely reported to be used by our centres, only one study based
upon a randomised controlled trial[21] reported to have handily removed the clots before carboprost instillation. No data emerged from the German survey,[31] likely because it was addressed to physicians, and this measure is performed by nurses.
Among supportive measures, Ippoliti et al. (1995)[21] and Laszlo et al. (1995)[22]
administered analgesics and antispasmodics to reduce the discomfort and
pain associated with catheter and intravesical instillations. Patients
were premedicated with oral oxybutynin with or without belladonna (a
phytotherapeutic) and opium suppositories.[21] These
interventions confirm the trend of our survey: intravenous opioid
analgesics and antispasmodics were the most used in the daily practice
of Italian nurses. Moreover, supportive therapies as hyperhydration,
bladder irrigation, platelet transfusions and pain treatment are
recommended by the ECIL 6 Guidelines (AIII) according to the evidence
from opinions of respected authorities, thus strongly recommended for
the clinical use.[29]
Nurses experiences.
To date, no studies have been published regarding nurses’ perceptions
towards encountered difficulties while caring for patients with HC, nor
on perceived outcomes. Helping patient and family caregivers to
overcome the emotional burden emerged as the greatest difficulty
encountered by nurses, followed by pain and symptom management and by
the HC treatment itself. Frustration, dissatisfaction and anxiety
emerged because of the perception of impotence. Nurses who stay close
to these patients need additional support. Management of HC has been
reported by our participants to increase both nurses’ and other health
care professionals’ workloads. The impact of HC has been reported at
the patient level mainly as worsening of the QoL according to previous
literature.[42,43] Yasar and Akin (2016),[42]
in a descriptive study including 100 Turkish patients undergoing HSCT,
reported a moderate change in QoL; additionally, transplanted patients
reported an inability to carry out social activities (23%) or to fulfil
responsibilities (31%), and reported feeling alone (23%). Having
“confidence in my nurse” has been reported as increasing the overall
QoL. However, the study was not focused on patients with HC.
Our
national survey is affected by several limitations, such as the low
response rate that could have introduced a selection bias; moreover, we
have required nurses to report their experience in terms of HC
occurrence, patients, and both preventive and treatment intervention:
recall bias can have affected the findings. According to the findings,
no common standard of care is used in daily practice regarding HC
preventive and treatment measures. Also, similar agents can be
administered differently (for example, in timing and doses); moreover,
any differentiation have been reported both in preventive and
therapeutic measures for EOHC and LOHC. This uncertainty and the
variability in daily practices reflect the limitation in preventive and
especially treatment options in the field, confirmed even at the
international level by the recent ECIL 6 Guidelines.[29]
Therefore, to improve this clinical field, multicentre prospective
clinical studies are strongly necessary: interventions based upon the
best evidence could improve patients’ outcomes as well as alleviate the
burden on nurses who manage this clinical condition.
Acknowledgments
We
would like to thank the colleagues of the Italian HSCT centres
belonging to the GITMO that voluntarily participated in this survey and
for their dedication to patients’ healthcare.
References
- Gonella S, Di Pasquale T, Palese A. Preventive
measures for cyclophosphamide-related hemorrhagic cystitis in blood and
bone marrow transplantation: an Italian multicenter retrospective
study. Clin J Oncol Nurs. 2015;19:E8-14. https://doi.org/10.1188/15.CJON.E8-E14 PMid:25689665
- Hadjibabaie
M, Alimoghaddam K, Shamshiri AR, Iravani M, Bahar B, Mousavi A, Jahani
M, Khodabandeh A, Anvari Y, Gholami K, Ghavamzadeh A. Continuous
bladder irrigation prevents hemorrhagic cystitis after allogeneic
hematopoietic cell transplantation. Urol Onc. 2008;26:43-46. https://doi.org/10.1016/j.urolonc.2006.12.015 PMid:18190829
- Hassan
Z, Remberger M, Svenberg P, Elbander M, Omazic B, Mattsson J, Conrad R,
Svahn BM, Ahlgren A, Sairafi D, Aschan J, Le Blanc K, Barkholt L,
Ringde'n O. Hemorrhagic cystitis: a retrospective single‐center survey.
Clin Transplant. 2007;21:659-667. https://doi.org/10.1111/j.1399-0012.2007.00705.x PMid:17845642
- Tsuboi
K, Kishi K, Ohmachi K, Yasuda Y, Shimizu T, Inoue H, Matsumoto M,
Hattori K, Yoshiba F, Watanabe S, Ogawa Y, Kawada H, Yabe H, Yabe M,
Kato S, Hotta T. Multivariate analysis of risk factors for hemorrhagic
cystitis after hematopoietic stem cell transplantation. Bone Marrow
Transplant. 2003;32:903-907. https://doi.org/10.1038/sj.bmt.1704240 PMid:14561991
- Sakurada
M, Kondo T, Umeda M, Kawabata H, Yamashita K, Takaori-Kondo A.
Successful treatment with intravesical cidofovir for virus-associated
hemorrhagic cystitis after allogeneic hematopoietic stem cell
transplantation: a case report and a review of the literature. J Infect
Chemother. 2016;22:495-500. https://doi.org/10.1016/j.jiac.2016.01.013 PMid:26898668
- Cesaro
S, Pillon M, Tridello G, Aljurf M, Martino R, Schroyens W, Nozzoli C,
Barba P, Faraci M, Fagioli F, Cappelli B, Cordonnier C, Al-Mohareb F,
Floisand Y, Greil J, Panizzolo IS, Santarone S. Relationship between
clinical and BK virological response in patients with late hemorrhagic
cystitis treated with cidofovir: a retrospective study from the
European Group for Blood and Marrow Transplantation. Bone Marrow
Transplant. 2013;48:809-813. https://doi.org/10.1038/bmt.2012.247 PMid:23222380
- Lee
GW, Lee JH, Choi SJ, Kim S, Seol M, Kim WK, Lee JS, Lee KH. Hemorrhagic
cystitis following allogeneic hematopoietic cell transplantation. J
Korean Med Sci. 2003;18:191-195. https://doi.org/10.1038/bmt.2015.162 PMid:26168069 PMCid:PMC5343753
- Federoff A. BK virus in hematopoietic stem cell transplantation recipients. Clin J Oncol Nurs. 2008;12:895-900. https://doi.org/10.1188/08.CJON.895-900 PMid:19064383
- Xu
LP, Zhang HY, Huang XJ, Liu KY, Liu DH, Han W, Chen H, Chen YH, Gao ZY,
Zhang YC, Lu DP. Hemorrhagic cystitis following hematopoietic stem cell
transplantation: incidence, risk factors and association with CMV
reactivation and graft-versus-host disease. Chin Med J.
2007;120:1666-1671. https://doi.org/10.1097/00029330-200710010-00004 PMid:17935666
- Gaziev
J, Paba P, Miano R, Germani S, Sodani P, Bove P, Perno CF, Marziali M,
Gallucci C, Isgrò A, Paciaroni K, Roveda A, Simone MD, De Angelis G,
Alfieri C, Lucarelli G. Late-onset hemorrhagic cystitis in children
after hematopoietic stem cell transplantation for thalassemia and
sickle cell anemia: a prospective evaluation of polyoma (BK) virus
infection and treatment with cidofovir. Biol Blood Marrow Transplant.
2010;16:662-671. https://doi.org/10.1016/j.bbmt.2009.12.009 PMid:20026413
- Russell
SJ, Vowels MR, Vale T. Haemorragic cystitis in paediatric bone marrow
transplant patients: An association with infective agents, GVHD, and
prior cyclophosphamide. Bone Marrow Transplant. 1994;13:533-539.
PMid:8054906
- Visintini
C, Venturini M, Palese A. Haemorrhagic cystitis, preventive and
treatment interventions in patients undergoing hematopoietic stem cell
transplantation: a scoping review. Eur J Oncol Nurs., in press
- Vose
JM, Reed EC, Pippert GC, Anderson JR, Bierman PJ, Kessinger A, Spinolo
J, Armitage JO. Mesna compared with continuous bladder irrigation as
uroprotection during high-dose chemotherapy and transplantation: a
randomized trial. J Clin Oncol. 1993;11:1306-1310. https://doi.org/10.1200/JCO.1993.11.7.1306 PMid:8315426
- Miller
AN, Glode A, Hogan KR, Schaub C, Kramer C, Stuart RK, Costa LJ.
Efficacy and safety of ciprofloxacin for prophylaxis of polyomavirus BK
virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem
cell transplantation recipients. Biol Blood Marrow Transplant.
2011;17(8):1176-1181. https://doi.org/10.1016/j.bbmt.2010.12.700 PMid:21185389
- Philippe
M, Ranchon F, Gilis L, Schwiertz V, Vantard N, Ader F,
Labussiere-Wallet H, Thomas X, Nicolini FE, Wattel E,
Ducastelle-Leprêtre S, Barraco F, Lebras L, Salles G, Michallet M,
Rioufol C. Cidofovir in the treatment of BK virus-associated
hemorrhagic cystitis following allogeneic hematopoietic stem cell
transplantation: a retrospective study and a literature review. Biol
Blood Marrow Transplant. 2016;22:723-730. https://doi.org/10.1016/j.bbmt.2015.12.009 PMid:26718666
- Gilis
L, Morisset S, Billaud G, Ducastelle-Leprêtre S, Labussiere-Wallet H,
Nicolini FE, Barraco F, Detrat M, Thomas X, Tedone N, Sobh M, Chidiac
C, Ferry T, Salles G, Michallet M, Ader F, on behalf of the Lyon BK
virus Study group. High burden of BK virus-associated hemorrhagic
cystitis in patients undergoing allogeneic hematopoietic stem cell
transplantation. Bone Marrow Transplant. 2014;49:664-670. https://doi.org/10.1038/bmt.2013.235 PMid:24488049
- Lee
SS, Ahn JS, Jung SH, Ahn SY, Kim JY, Jang HC, Kang SJ, Jang MO, Yang
DH, Kim YK, Lee JJ, Kim HJ. Treatment of BK virus-associated
hemorrhagic cystitis with low-dose intravenous cidofovir in patients
undergoing allogeneic hematopoietic cell transplantation. Korean J
Intern Med. 2015;30:212-219. https://doi.org/10.3904/kjim.2015.30.2.212 PMid:25750563 PMCid:PMC4351328
- Paduch DA. Viral lower urinary tract infections. Curr Urol Rep. 2007;8:324-335. https://doi.org/10.1007/s11934-007-0080-y PMid:18519018
- Mackey
MC. Intravesicular cidofovir for the treatment of
polyomavirus-associated hemorrhagic cystitis. Ann Pharmacother.
2012;46:442-446. https://doi.org/10.1345/aph.1Q430 PMid:22395246
- Miyamura
K, Hamaguchi M, Taji H, Kanie T, Kohno A, Tanimoto M, Saito H, Kojima
S, Matsuyama T, Kitaori K, Nagafuji K, Sato T, Kodera Y. Successful
ribavirin therapy for severe adenovirus hemorrhagic cystitis after
allogeneic marrow transplant from close HLA donors rather than distant
donors. Bone Marrow Transplant. 2000;25:545-548. https://doi.org/10.1038/sj.bmt.1702195 PMid:10713633
- Ippoliti
C, Przepiorka D, Mehra R, Neumann J, Wood J, Claxton D, Gajewski J,
Khouri I, Van Besien K, Andersson B, Deisseroth AB, Dinney CP.
Intravesicular carboprost for the treatment of hemorrhagic cystitis
after marrow transplantation. Urology. 1995;46:811-815. https://doi.org/10.1016/S0090-4295(99)80349-5
- Laszlo
D, Bosi A, Guidi S, Saccardi R, Vannucchi AM, Lombardini L, Longo G,
Fanci R, Azzi A, De Santis R, Rossi Ferrini P. Prostaglandin E2 bladder
instillation for the treatment of hemorrhagic cystitis after allogeneic
bone marrow transplantation. Haematologica. 1995;80:421-425.
PMid:8566882
- Roskopf J, Fitzsimmons W, Ahsan N, Laskow D. The pharmacologic treatment of human polyomavirus infection. Graft. 2002;5:88-97. https://doi.org/10.1177/1522162802238461
- Miodosky
M, Abdul-Hai A, Tsirigotis P, Or R, Bitan M, Resnick IB, Gesundheit B,
Zilberman I, Ioffe L, Leubovic A, Slavin S, Shapira MY. Treatment of
post-hematopoietic stem cell transplantation hemorrhagic cystitis with
intravesicular sodium hyaluronate. Bone Marrow Transplant.
2006;38:507-511. https://doi.org/10.1038/sj.bmt.1705474 PMid:16921402
- Tirindelli
MC, Flammia G, Sergi F, Cerretti R, Cudillo L, Picardi A, Postorino M,
Annibali O, Greco R, Avvisati G, Arcese W, for the Rome Transplant
Network. Fibrin glue for refractory hemorrhagic cystitis after
unrelated marrow, cord blood, and haploidentical hematopoietic stem
cell transplantation. Transfusion. 2009;49:170-175. https://doi.org/10.1111/j.1537-2995.2008.01934.x PMid:18954405
- Tzannou
I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G,
Sasa G, Lulla P, Watanabe A, Kuvalekar M, Gee AP, Wu MF, Liu H, Grilley
BJ, Krance RA, Gottschalk S, Brenner MK, Rooney CM, Heslop HE, Leen AM,
Omer B. Off-the-shelf virus-specific t cells to treat bk virus, human
herpesvirus 6, cytomegalovirus, epstein-barr virus, and adenovirus
infections after allogeneic hematopoietic stem-cell transplantation. J
Clin Oncol. 2017;35:3547-3557. https://doi.org/10.1200/JCO.2017.73.0655 PMid:28783452 PMCid:PMC5662844
- Mani
J, Jin N, Schmitt M. Cellular immunotherapy for patients with
reactivation of JC and BK polyomaviruses after transplantation.
Cytotherapy. 2014;16:1325-1335. https://doi.org/10.1016/j.jcyt.2014.04.003 PMid:24934303
- Dropulic
LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant
recipients. Bone Marrow Transplant. 2008;41:11-18. https://doi.org/10.1038/sj.bmt.1705886 PMid:17952131 PMCid:PMC3066131
- Cesaro
S, Dalianis T, Rinaldo CH, Koskenvuo M, Pegoraro A, Einsele H,
Cordonnier C, Hirsch HH, Members of the ECIL-6. ECIL Guidelines for the
prevention, diagnosis and treatment of BK polyomavirus-associated
haemorrhagic cystitis in haematopoietic stem cell transplant
recipients. J Antimicrob Chemother. 2018;73(1):12-21. https://doi.org/10.1093/jac/dkx324
- Cesaro
S, Berger M, Tridello G, Mikulska M, Ward KN, Ljungman P, van der Werf
S, Averbuch D, Styczynski J; Infectious Disease Working Party of EBMT.
A survey on incidence and management of adenovirus infection after
allogeneic HSCT. Bone Marrow Transplant. 2018. https://doi.org/10.1038/s41409-018-0421-0 PMid:30546071
- Schneidewind
L, Neumann T, Kranz J,Knoll F, Pelzer AE, Schmidt C, Krüger W.
Nationwide survey of BK polyomavirus associated hemorrhagic cystitis in
adult allogeneic stem cell transplantation among haematologists and
urologists. Ann Hematol. 2017;96:797-803. https://doi.org/10.1007/s00277-017-2935-8 PMid:28160087
- Gargiulo
G, Olando L, Alberani F, Crabu G, Di Maio A, Duranti L, Errico A,
Liptrott S, Pitrone R, Santarone S, Soliman C, Trunfio A, Selleri C,
Bruno B, Mammoliti S, Pane F. Haemorrhagic cystitis in haematopoietic
stem cell transplantation (HSCT): a prospective observational study of
incidence and management in HSCT centres within the GITMO network
(Gruppo Italiano Trapianto Midollo Osseo). Ecancermedicalscience.
2014;8:420. https://doi.org/10.3332/ecancer.2014.420 PMid:24834115 PMCid:PMC3998658
- National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Quick Reference 5x7. 2017:119. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed 20 June 2019).
- Bedi
A, Miller CB, Hanson JL, Goodman S, Ambinder RF, Charache P, Arthur RR,
Jones RJ. Association of BK virus with failure of prophylaxis against
hemorrhagic cystitis following bone marrow transplantation. J Clin Onc.
1995;13:1103-1109. https://doi.org/10.1200/JCO.1995.13.5.1103 PMid:7738616
- Droller
MJ, Saral R, Santos G. Prevention of cyclophosphamide- induced
hemorrhagic cystitis. Urology. 1982;20:256-258. PMid:7123717 https://doi.org/10.1016/0090-4295(82)90633-1
- Leung
AYH, Chan MTL, Yuen KY, Cheng VCC, Chan KH, Wong CLP, Liang R, Lie AKW,
Kwong YL. Ciprofloxacin decreased polyoma BK virus load in patients who
underwent allogeneic hematopoietic stem cell transplantation. Clin
Infect Dis. 2005;40(4):528-537. https://doi.org/10.1086/427291 PMid:15712075
- Breitz
H, Wendt R, Stabin M, Bouchet L, Wessels B. Dosimetry of high dose
skeletal targeted radiotherapy (STR) with 166Ho-DOTMP. Cancer Biother
Radiopharm. 2003;18(2):225-230. https://doi.org/10.1089/108497803765036391 PMid:12804048
- Giralt
S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, Alexanian R,
Weber D, Maloney D, Holmberg L, Rajandran J, Breitz H, Ghalie R,
Champlin R. 166Ho-DOTMP plus melphalan followed by peripheral blood
stem cell transplantation in patients with multiple myeloma: results of
two phase 1/2 trials. Blood. 2003;102:2684-2691. https://doi.org/10.1182/blood-2002-10-3250 PMid:12730103
- Ganguly
N, Clough LA, DuBois LK, Mcguirk JP, Abhyankar S, Aljitawi OS, O'Neal
N, Divine CL, Ganguly S. Low-dose cidofovir in the treatment of
symptomatic BK virus infection in patients undergoing allogeneic
hematopoietic stem cell transplantation: a retrospective analysis of an
algorithmic approach. Transpl Infect Dis. 2010;12:406-411. https://doi.org/10.1111/j.1399-3062.2010.00513.x PMid:20487411
- Bridges
B, Donegan S, Badros A. Cidofovir bladder instillation for the
treatment of BK virus hemorrhagic cystitis after allogeneic stem cell
transplantation. Am J Hematol. 2006;81:535-537. https://doi.org/10.1002/ajh.20567 PMid:16755571
- Miller
LJ, Chandler SW, Ippoliti CM. Treatment of cyclophosphamide-induced
hemorrhagic cystitis with prostaglandins. Ann Pharmacother.
1994;28(5):590-594. https://doi.org/10.1177/106002809402800508 PMid:8068996
- Yasar
N, Akin S. Evaluation of quality of life and care needs of Turkish
patients undergoing hematopoietic stem cell transplantation. Nurs Res
Pract. 2016. ID 9604524. https://doi.org/10.1155/2016/9604524 PMid:28116155 PMCid:PMC5220483
- Grulke
N, Albani C, Bailer H. Quality of life in patients before and after
haematopoietic stem cell transplantation measured with the European
Organization for Research and Treatment of Cancer (EORTC) Quality of
Life Core Questionnaire QLQ-C30. Bone Marrow Transplant.
2012;47(4):473-482. https://doi.org/10.1038/bmt.2011.107 PMid:21602898
- Sencer
SF, Haake RJ, Weisdorf DJ. Hemorrhagic cystitis after bone marrow
transplantation. Risk factors and complications. Transplantation.
1993;56(4):875-879. https://doi.org/10.1097/00007890-199310000-00020 PMid:8212210
- Bearman
SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, Thomas
ED. Regimen-related toxicity in patients undergoing bone marrow
transplantation. J Clin Oncol. 1988;6(10):1562-1568. https://doi.org/10.1200/JCO.1988.6.10.1562 PMid:3049951




