Hye Jo Shin1, Eui Soo Lee1, Seung Beom Han1,2, Jae Wook Lee1,3, Nack-Gyun Chung1,3, Bin Cho1,3 and Jin Han Kang1,2..
1 Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
2 The Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
3 Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Correspondence to: Jin Han Kang, MD, PhD, Professor. Department of
Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, The
Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591,
Republic of Korea. Tel.: 82 2 2258 6183; Fax.: 82 2 537 4544. E-mail:
kjhan@catholic.ac.kr
Published: September 1, 2019
Received: May 22, 2019
Accepted: August 9, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019052 DOI
10.4084/MJHID.2019.052
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Vaccination
for hepatitis B virus (HBV) after chemotherapy among pediatric patients
with acute Leukemia is still a debated issue. We investigated HBV
immunity before and after chemotherapy and assessed immune response to
re-vaccination after chemotherapy.
Methods:
We retrospectively analyzed data of children and adolescents aged
<19 years requested for vaccination after chemotherapy for acute
leukemia to evaluate hepatitis B surface antibody (HBsAb) status before
and after chemotherapy and to identify factors related to HBsAb
positivity after chemotherapy.
Results:
Of 89 enrolled patients, 61 (68.5%) with acute leukemia were HBsAb
positive before chemotherapy.Of these 61 patients, 48 (78.7%)
seroconverted to HBsAb negative status after chemotherapy; there were
76 (85.4%) HBsAb negative patients after chemotherapy. HBsAb positive
patients when compared to HBsAb negative patients after chemotherapy
had a significantly higher HBsAb positive rate (100.0% vs. 63.2%, p=0.008)
before chemotherapy. Following HBsAb testing after one dose of the HBV
vaccination, 33 (43.4%) of the 76 HBsAb negative patients seroconverted
to an HBsAb positive status. HBsAb positive patients after a single
dose of HBV vaccination had a significantly higher HBsAb positive rate
at the time of diagnosis compared to HBsAb negative patients (84.8% vs.
48.8%, p=0.001).
Conclusions:
Based on these results, HBV re-vaccination after chemotherapy is
recommended for all children and adolescents with acute leukemia. In
addition, further investigation is required to improve the
immunogenicity of HBV re-vaccination.
|
Introduction
Given
higher survival rates in patients with acute leukemia due to
advancements in chemotherapy technologies and conservative treatment
for cancer patients,[1] it has become more important to prevent
long-term complications after treatment. In particular, pediatric
cancer patients have a longer survival period after treatment than
adult patients, and they are more likely to be affected by
vaccine-preventable diseases (VPDs) during this period. Therefore, it
is necessary to establish a vaccination policy for pediatric cancer
patient survivors. Hepatitis B virus (HBV) infection is a well-known
VPD. The worldwide prevalence of HBV infection significantly decreased
after HBV vaccination during infancy was introduced.[2]
Even in
low HBV infection endemic areas, there is a need to focus on prevention
of HBV infection after anti-cancer treatment as well as prevention and
treatment of reactivation of a previous HBV infection caused by
immune-compromised conditions or de novo HBV infection during
chemotherapy for acute leukemia.[3] The 2013 Guideline for Vaccination
of the Immunocompromised Host by the Infectious Diseases Society of
America states that all patients, who received hematopoietic cell
transplantations (HCTs), are recommended to have HBV vaccination after
transplantation.[3] However, not all patients who underwent
chemotherapy for acute leukemia are advised to undergo HBV vaccination
after chemotherapy, except in certain cases based on age and risk
factors.[3] One previous study reported that the positive rate of
hepatitis B surface antibody (HBsAb) remained high after chemotherapy
for pediatric acute leukemia; thus, it was recommended that such
patients should continue the standard vaccination schedule.[4] In
contrast, other studies have reported that universal HBV vaccination
was required due to a significantly reduced HBsAb positive rate after
chemotherapy for acute leukemia.[5-7] Additionally, a recent
vaccination guideline for patients with hematological malignancies of
the 2017 European Conference on Infections in Leukaemia (ECIL7)
supports this universal vaccination strategy.[8]
The subjects in
the present study were pediatric patients with acute leukemia from
Korea, a low intermediate HBV infection endemic area where almost all
children and adolescents have acquired immunity from vaccination during
infancy. To facilitate the development of an HBV vaccination policy
after chemotherapy for children and adolescents treated with
chemotherapy only, we evaluated the HBsAb positive rate before and
after treatment for acute leukemia. We also evaluated the
immunogenicity of HBV vaccine after chemotherapy.
Patients and Methods
Subjects.
Patients included in this study were aged <19 years at the time of
leukemia diagnosis, received chemotherapy for acute leukemia in the
Department of Pediatrics at Seoul St. Mary’s Hospital, The Catholic
University of Korea, and were referred to the division of Pediatric
Infection Diseases between January 2015 and June 2018 for vaccination
after chemotherapy. Of the 187 referred patients, 64 who had received
HCTs were excluded. From the remaining 123 patients, treated with
chemotherapy only, we excluded 33 who did not have an HBsAb test at the
time of diagnosis with acute leukemia, and one who received a
qualitative radioimmunoassay (RIA) test for HBsAb instead of a
quantitative enzyme-linked immunosorbent assay (ELISA). Retrospective
analysis of the medical records for the remaining 89 patients was
performed. The present study was performed after obtaining approval
from the Institutional Review Board of Seoul St. Mary’s Hospital
(Approval number: KC18RESI0503).
Data collection and definitions.
Demographic data, including sex and age, were gathered at the diagnosis
of leukemia. Clinical data, including type of underlying acute leukemia
and its treatment results, the risk group of acute lymphoblastic
leukemia (ALL), time intervals between completion of chemotherapy and
HBsAb testing, and between completion of chemotherapy and HBV
vaccination, HBsAb titers at the time of acute leukemia diagnosis and
after completing chemotherapy, and the complete blood cell count at the
time of the HBsAb testing, were investigated. Furthermore, HBsAb titers
after HBV vaccination were also investigated in patients who received
HBV vaccination after chemotherapy. HBsAb tests were performed using a
commercial quantitative ELISA kit (Elecsys® Anti-HBs, Roche Diagnostics
GmBH, Mannheim, Germany); titers were summarized as follows: titers
<2 IU/L, corresponding to the threshold of the test, were
categorized as 1 IU/L and those >1,000 IU/L were categorized as
1,000 IU/L. Positive and negative antibodies were defined as HBsAb
levels of ≥10 IU/L and <10 IU/L, respectively. Patients testing
negative for HBsAb after chemotherapy received one dose of HBV vaccine
(Hepavax-Gene® TF, Janssen Korea Ltd., Seoul, Korea) at a dose of 0.5
mL (10 μg of hepatitis B surface antigen [HBsAg]) for patients aged
<11 years, and 1.0 mL (20 μg of HBsAg) for those aged ≥11 years.
After at least 4 weeks post-vaccination, the HBsAb test was again
performed. HBsAb negative patients whose HBsAb level increased to ≥10
IU/L after HBV vaccination were considered to have an anamnestic
response. For patients still negative for HBsAb after one dose of HBV
vaccine, a second and third dose were administered at least 1 month and
6 months after the first HBV vaccination, respectively.
Statistical analysis.
The subjects were divided into HBsAb negative and positive patients
based on their HBsAb titer after chemotherapy. Demographic and clinical
data were compared between these two patient groups. Based on the HBsAb
retest results determined after one dose of HBV vaccine in patients who
received an HBV vaccination after chemotherapy, patients were divided
into those with and without an anamnestic response, and then the two
groups were compared. For comparison of continuous data and categorical
data between the patient groups, Mann-Whitney U and Fisher’s exact
tests were applied, respectively. The SPSS 21 program (IBM Corporation,
Armonk, NY, USA) was used for statistical analyses, and statistical
significance was defined as a p-value <0.05.
Results
The
89 study subjects included 58 (65.2%) males and 31 (34.8%) females with
a median age of 8 years (range, 1-18 years). At baseline, there were 79
(88.8%) patients with ALL and 10 (11.2%) with acute myeloid leukemia
(AML); all were at their first complete remission state. At the time of
their diagnosis with acute leukemia, 61 (68.5%) patients were HBsAb
positive (Figure 1); their
median HBsAb titer was 67.15 IU/L (range, 11.02-1,000.00 IU/L). HBsAb
positivity at the time of leukemia diagnosis was not associated with
sex or type of underlying leukemia. However, significantly more
patients aged <7 years were HBsAb positive compared to those and ≥7
years old (86.4% vs 62.7%, p=0.038) at the time of diagnosis.
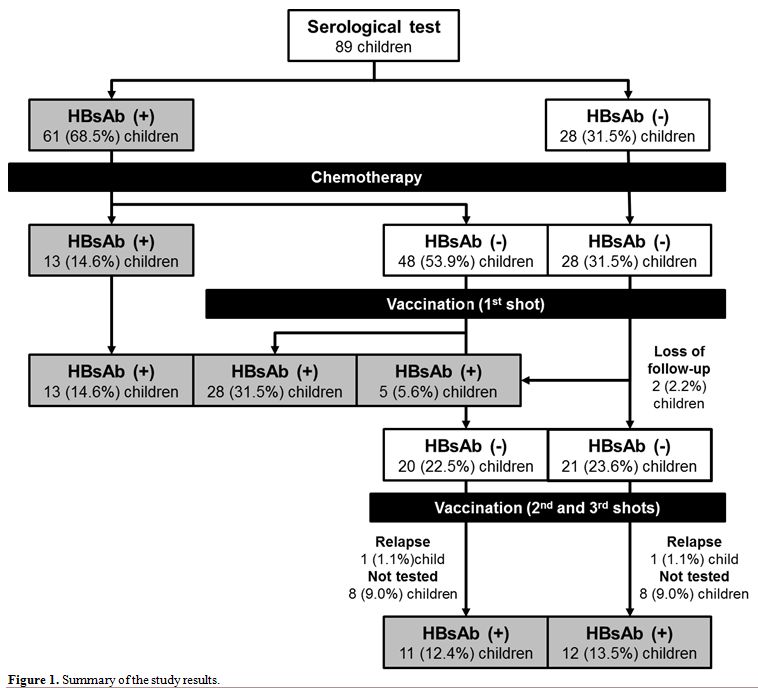 |
Figure 1. Summary of the study results. |
Comparison between HBsAb positive and negative patients after chemotherapy.
HBsAb testing was performed at a median of 3 months (range, 0-14
months) after completing chemotherapy. Two (2.2%) patients had received
1 g/kg of intravenous immunoglobulin 7 months before an HBsAb test.
Regarding the 61 patients who were HBsAb positive at the time of
leukemia diagnosis, 48 (78.7%) seroconverted to HBsAb negative after
chemotherapy (Figure 1). For
the remaining 13 (21.3%) patients who remained HBsAb positive after
chemotherapy, the median HBsAb titer of 40.91 IU/L (range, 11.48-256.70
IU/L) was significantly lower than the 169.20 IU/L (range, 16.30-1,000
IU/L) reported at the time of their leukemia diagnosis (p=0.003). This
median titer for these 13 patients at the time of leukemia diagnosis
was significantly higher than the HBsAb titer determined at the time of
leukemia diagnosis for the other 48 patients who seroconverted to
negative after chemotherapy; the median was 53.57 IU/L (range,
11.02-819.00 IU/L, p=0.021). Only 30.6% (11/36) and 32.0% (8/25) of
patients with an HBsAb titer of ≥50.00 IU/L and ≥100.00 IU/L before
chemotherapy, respectively, remained HBsAb positive after chemotherapy.
Compared to patients who were HBsAb negative after chemotherapy, more
HBsAb positive patients had AML as an underlying disease (p<0.001)
and were HBsAb positive at the time of leukemia diagnosis (p=0.008, Table 1).
The risk group of ALL was not significantly associated with HBsAb
positivity after chemotherapy. Younger age significantly correlated
with HBsAb positivity at the time of diagnosis, but this correlation
was not observed following chemotherapy. White blood cell, neutrophil
and lymphocyte counts determined on the day of HBsAb testing were not
significantly different between the two patient groups (data not shown).
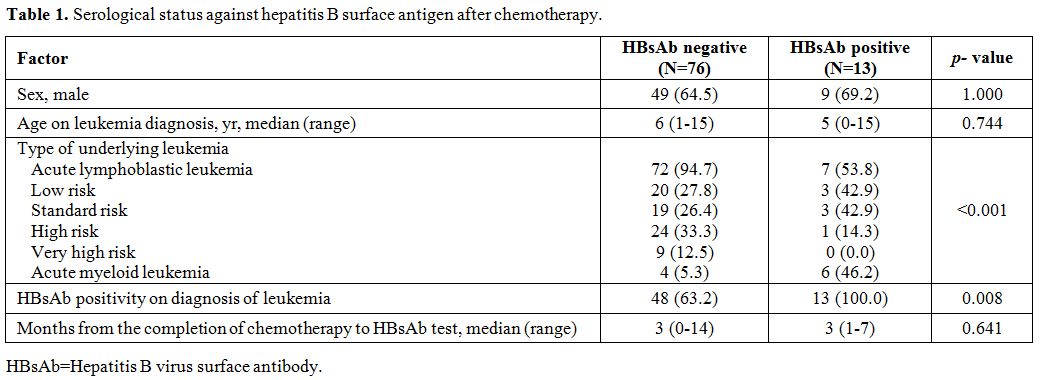 |
Table 1. Serological status against hepatitis B surface antigen after chemotherapy. |
Anamnestic response to the additional HBV vaccination after chemotherapy.
All 76 patients who were HBsAb negative after chemotherapy received one
dose of HBV vaccine at a median of 7 months (range, 3-18 months) after
completing chemotherapy; of these, 74 were subjected to an antibody
test at least 4 weeks after vaccination. Of these 74 patients, 33
(44.6%) seroconverted to HBsAb positive (Figure 1, Table 2),
with a median titer of 45.72 IU/L (range, 10.27-1,000 IU/L). In
comparison to the 41 patients who remained HBsAb negative after
vaccination, the 33 seropositive patients were significantly more
likely to be HBsAb positive (p=0.001, Table 2)
and to have a higher HBsAb titer (median 8.22 IU/L vs 54.05 IU/L,
p<0.001) at the time of leukemia diagnosis. White blood cell,
neutrophil and lymphocyte counts determined on the day of first HBsAb
test after chemotherapy were not significantly associated with
anamnestic response to HBV vaccination after chemotherapy (data not
shown). Of the 41 HBsAb negative patients, after one dose of HBV
vaccination, 40 (excluding one patient with ALL relapse) received a
second HBV vaccination, and 36 received a third vaccination (excluding
one patient with ALL relapse after the second vaccination, one patient
who was lost to follow-up after the second vaccination, and two who
dropped out for unknown reasons). Regarding the 36 patients who
received the third vaccination, 23 who had an antibody test performed
after vaccination seroconverted to HBsAb positive, with a median HBsAb
titer of 1,000 IU/L (range, 40.69-1,000 IU/L).
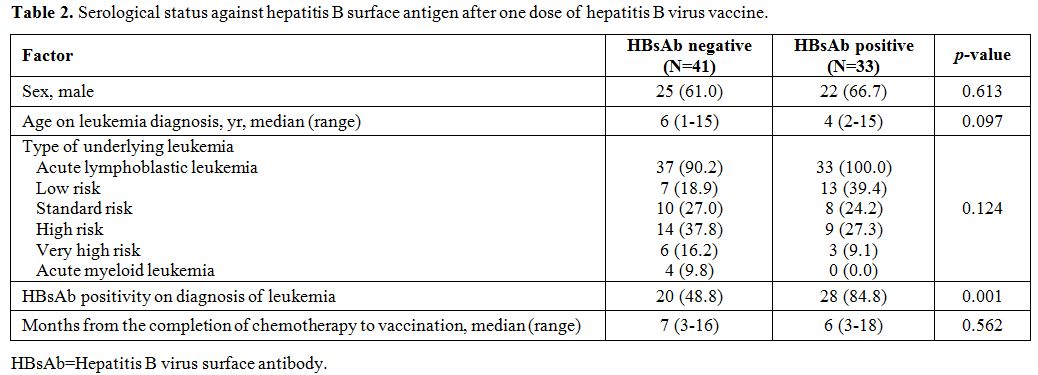 |
Table 2. Serological status against hepatitis B surface antigen after one dose of hepatitis B virus vaccine. |
Discussion
This
study investigated HBV immunity before and after chemotherapy for acute
leukemia and immune response to re-vaccination after chemotherapy. We
found that 68.5% of children and adolescents who were diagnosed with
acute leukemia were HBsAb positive at leukemia diagnosis. Of these
HBsAb positive patients, 78.7% seroconverted to HBsAb negative after
chemotherapy. As a result, 85.4% of all patients were HBsAb negative
after chemotherapy. Of these patients, 44.6% seroconverted to HBsAb
positive after a single HBV vaccination. Hence, 51.7% of all patients
were HBsAb positive after one dose (Figure 1). All patients, who were subjected to testing after a third dose, exhibited seroconversion to an HBsAb positive status.
Although
the HBV vaccination history could not be determined for all patients in
the present study, 80.9% of the enrolled patients have received their
three doses of vaccination during infancy; a rate that was determined
based on their vaccination records. Furthermore, since mandatory HBV
vaccination (thrice at birth and then at 1 and 6 months after birth)
was incorporated into the National Immunization Program of Korea in
1995 with a maintenance of an HBV vaccination rate >93% after the
2000s,[9] almost all study subjects should have acquired HBsAb from HBV
vaccination during infancy. Although the majority (95%) of healthy
infants were HBsAb positive after three doses of primary HBV
vaccination,[2] antibody titer and seroprevalence decreased over time,
resulting in a 50-60% HBsAb positivity rate among children aged 6-8
years.[10-12] The HBsAb positive rate of the children enrolled in the
present study (with a median age of 6 years) was similar to that of
healthy children at 68.5%. HBsAb seroprevalence in healthy Korean
children has been found to decrease by 17.0% from the age of six to
eight,[10] whereas, the rate of seroconversion to HBsAb negative status
of the patients enrolled in the present study was 78.7% during
approximately 3 years of chemotherapy. This seroconversion rate was
higher than the previously reported rates of 27.4-53.8% in pediatric
patients receiving chemotherapy for hematological
malignancies.[7,13,14] We speculate that the seroconversion rate could
have been affected by differences in either intensity of chemotherapy
among patients, antibody test methods, or the sensitivity and
specificity of the ELISA kit used. Overall HBsAb positive rate after
chemotherapy for hematological malignancy including acute leukemia
ranges from 14.0-80.6% in studies, including this one.[4-7,13-16]
However, all studies, except the one that showed the 80.6% positive
rate, were below a 50% positive rate. Therefore, there is a need for
universal HBV vaccination policy for patients with acute leukemia after
chemotherapy rather than vaccination based on HBsAb test results,
reconfirming the recent ECIL7 guideline for vaccination in patients
with hematological malignancies.[8] HBsAb positivity in patients with
acute leukemia after chemotherapy exhibited no significant correlation
with age, sex, underlying leukemia, or the intensity of
chemotherapy.[5-7,14,16] In the present study, patients who were HBsAb
positive after chemotherapy had a significantly higher HBsAb positive
rate and a significantly higher HBsAb titer before chemotherapy
compared to those who were HBsAb negative after chemotherapy. Although
a similar result was reported in another study,[14] the other study
reported no significant correlation in HBsAb positivity before and
after chemotherapy.[16] In healthy children, those with a higher HBsAb
titer after their primary HBV vaccination during infancy exhibited a
higher anamnestic response rate to re-vaccination after several
years.[17-19] As such a higher HBsAb titer before chemotherapy may be
considered to be a contributing factor to HBsAb positivity after
chemotherapy. However, with low rates of patients with either HBsAb ≥10
IU/L (21.5%) or ≥100 IU/L (33.3%) before chemotherapy remaining HBsAb
positive after chemotherapy, the prediction of HBsAb positivity after
chemotherapy based on HBsAb positivity before chemotherapy would have
limited usefulness in real-world clinical settings.
In the present
study, about half of the patients had an anamnestic response after
their initial HBV vaccination after chemotherapy. This implied that the
remaining 48.3% of patients were HBsAb negative despite a single HBV
vaccination after chemotherapy. In previous studies, higher percentages
(63.2-95.7%) of patients who were HBsAb negative after chemotherapy
were found with an anamnestic response after HBV vaccination.[5,7,15]
Almost all (92.9-100%) healthy adolescents who receive their three
doses of primary HBV vaccine during infancy have an anamnestic response
to HBV re-vaccination, even after 10-20 years.[20-22] However, as low
as 47.9% of healthy adolescents show an anamnestic response to
re-vaccination after 15 years of HBV vaccination in infancy.[23] Such
low anamnestic responses occurred when all three doses of the primary
vaccination were administered within 6 months of birth, or when a
low-dose vaccine (2.5 or 5.0 μg of HBsAg) was used.12 In Korea, a 10 μg
HBsAg vaccine has been used for infants; however, the vaccination is
performed thrice within their first 6 months of age. It is, in fact,
impossible to alter the national primary vaccination schedule for
patients with acute leukemia only; thus, it is necessary to identify
adjustable factors after chemotherapy that can improve HBV vaccine
immunogenicity. Accordingly, it makes sense to consider the appropriate
time interval between completion of chemotherapy and HBV vaccination.
Although restoration of B-cells can be achieved 3-6 months following
completion of chemotherapy, it may take several years for the
restoration of memory B-cell counts.[24] In the present study, HBsAb
testing and HBV vaccination were performed at medians of 3 and 7 months
after completion of chemotherapy, respectively. Considering that
previous studies described a >90% HBsAb seroconversion rate with HBV
vaccination at least 15 months after chemotherapy,[5,7] an improved
anamnestic response through delayed vaccination after the full
restoration of memory cells should be possible. Therefore, vaccination
may need to be postponed in countries with a low prevalence of HBV
infection until there is a full restoration of lymphocytes in number
and functionality, and one dose of vaccine rather than three doses may
be adequate after chemotherapy with this strategy. Follow-up studies
are needed to determine the appropriate time of immunity restoration
after chemotherapy, in order to allow for the acquisition of full
vaccine immunity without morbidity from VPDs. In addition, the
immunogenicity of HBV vaccines after chemotherapy may be enhanced by
the use of new adjuvant vaccines, vaccines containing new antigens, or
vaccines with increasing dose of antigens.[25]
The present study
has some limitations. Since there was no screening for antibodies
against the HBV core antigen, it was not possible to establish which
patients were previously HBV-infected. Patient history of HBV
vaccination before chemotherapy was also not determined for all of the
enrolled patients. However, with the recent high HBV vaccination rate
during infancy and decreasing HBV infection prevalence in Korea, most
patients should receive HBV vaccination before chemotherapy; thus, only
few children should be HBV-infected. The present study also excluded
patients not screened for HBV serology at the time of diagnosis, or who
had RIA test instead of ELISA. Therefore, unified test items, times,
and methods are necessary from the time of diagnosis with acute
leukemia for future studies to establish an accurate vaccination
schedule after chemotherapy.
Conclusions
The
present study was unable to identify the significant factors of HBsAb
positivity after chemotherapy and seroconversion by HBV vaccination
after chemotherapy. However, it is useful to know that all patients
should receive HBV vaccination after chemotherapy for acute leukemia in
regions where almost all children have acquired HBV immunity from
vaccination during infancy, and there is a low prevalence of HBV
infection, in accordance with the recent ECIL7 guideline. In addition,
there should be on-going studies on the appropriate vaccination time
after chemotherapy and improving the immunogenicity of vaccines in
immunocompromised patients.
References
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541-1552. https://doi.org/10.1056/NEJMra1400972 PMid:26465987
- World Health Organization. Hepatitis B vaccines: WHO position paper - July 2017. Wkly Epidemiol Rec. 2017;92:369-392.
- Rubin
LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A,
Dhanireddy S, Sung L, Keyserling H, Kang I; Infectious Diseases Society
of America. 2013 IDSA clinical practice guideline for vaccination of
the immunocompromised host. Clin Infect Dis. 2014;58:e44-100. https://doi.org/10.1093/cid/cit684 PMid:24311479
- Fioredda
F, Plebani A, Hanau G, Haupt R, Giacchino M, Barisone E, Balbo L,
Castagnola E. Re-immunisation schedule in leukaemic children after
intensive chemotherapy: a possible strategy. Eur J Haematol.
2005;74:20-23. https://doi.org/10.1111/j.1600-0609.2004.00340.x PMid:15613102
- Fayea
NY, Kandil SM, Boujettif K, Fouda AE. Assessment of hepatitis B virus
antibody titers in childhood cancer survivors. Eur J Pediatr.
2017;176:1269-1273. https://doi.org/10.1007/s00431-017-2970-4 PMid:28730317
- Viana
SS, Araujo GS, Faro GB, da Cruz-Silva LL, Araujo-Melo CA, Cipolotti R.
Antibody responses to Hepatitis B and measles-mumps-rubella vaccines in
children who received chemotherapy for acute lymphoblastic leukemia.
Rev Bras Hematol Hemoter. 2012;34:275-279. https://doi.org/10.5581/1516-8484.20120071 PMid:23049440 PMCid:PMC3460395
- Zignol
M, Peracchi M, Tridello G, Pillon M, Fregonese F, D'Elia R, Zanesco L,
Cesaro S. Assessment of humoral immunity to poliomyelitis, tetanus,
hepatitis B, measles, rubella, and mumps in children after
chemotherapy. Cancer. 2004;101:635-641. https://doi.org/10.1002/cncr.20384 PMid:15274078
- Mikulska
M, Cesaro S, de Lavallade H, Di Blasi R, Einarsdottir S, Gallo G,
Rieger C, Engelhard D, Lehrnbecher T, Ljungman P, Cordonnier C;
European Conference on Infections in Leukaemia group. Vaccination of
patients with haematological malignancies who did not have
transplantations: guidelines from the 2017 European Conference on
Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e188-199. https://doi.org/10.1016/S1473-3099(18)30601-7
- Choung
JM, Kim JC, Eun SH, Hwang PH, Nyhambat B, Kilgore P, Kim JS. Study on
vaccination state in children : Jeonbuk province, 2000. Korean J
Pediatr. 2002;45:1234-1240.
- Kim
YJ, Li P, Hong JM, Ryu KH, Nam E, Chang MS. A Single Center Analysis of
the Positivity of Hepatitis B Antibody after Neonatal Vaccination
Program in Korea. J Korean Med Sci. 2017;32:810-816. https://doi.org/10.3346/jkms.2017.32.5.810 PMid:28378555 PMCid:PMC5383614
- Qawasmi
M, Samuh M, Glebe D, Gerlich WH, Azzeh M. Age-dependent decrease of
anti-HBs titers and effect of booster doses using 2 different vaccines
in Palestinian children vaccinated in early childhood. Hum Vaccin
Immunother. 2015;11:1717-1724. https://doi.org/10.1080/21645515.2015.1041687 PMid:25996579 PMCid:PMC4514285
- Schonberger
K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of
long-term protection after hepatitis B vaccination in infancy: a
meta-analysis. Pediatr Infect Dis J. 2013;32:307-313. https://doi.org/10.1097/INF.0b013e31827bd1b0 PMid:23249904
- Alavi
S, Rashidi A, Arzanian MT, Shamsian B, Nourbakhsh K. Humoral immunity
against hepatitis B, tetanus, and diphtheria following chemotherapy for
hematologic malignancies: a report and review of literature. Pediatr
Hematol Oncol. 2010;27:188-194. https://doi.org/10.3109/08880011003602141 PMid:20367262
- Keskin
Yildirim Z, Buyukavci M. Assessment of humoral immunity to hepatitis B,
measles, rubella, and mumps in children after chemotherapy. J Pediatr
Hematol Oncol. 2018;40:e99-102. https://doi.org/10.1097/MPH.0000000000001072 PMid:29309372
- Baytan
B, Gunes AM, Gunay U. Efficacy of primary hepatitis B immunization in
children with acute lymphoblastic leukemia. Indian Pediatr.
2008;45:265-270.
- Karaman
S, Vural S, Yildirmak Y, Urganci N, Usta M. Assessment of hepatitis B
immunization status after antineoplastic therapy in children with
cancer. Ann Saudi Med. 2011;31:573-576. https://doi.org/10.4103/0256-4947.87091 PMid:22048500 PMCid:PMC3221126
- Chaves
SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, Stevenson
K, Yano VM, Armstrong GL, Samandari T, Kamili S, Drobeniuc J, Hu DJ.
Persistence of long-term immunity to hepatitis B among adolescents
immunized at birth. Vaccine. 2012;30:1644-1649. https://doi.org/10.1016/j.vaccine.2011.12.106 PMid:22245310
- Wu
JS, Hwang LY, Goodman KJ, Beasley RP. Hepatitis B vaccination in
high-risk infants: 10-year follow-up. J Infect Dis. 1999;179:1319-1325.
https://doi.org/10.1086/314768 PMid:10228050
- Dentinger
CM, McMahon BJ, Butler JC, Dunaway CE, Zanis CL, Bulkow LR, Bruden DL,
Nainan OV, Khristova ML, Hennessy TW, Parkinson AJ. Persistence of
antibody to hepatitis B and protection from disease among Alaska
natives immunized at birth. Pediatr Infect Dis J. 2005;24:786-792. https://doi.org/10.1097/01.inf.0000176617.63457.9f PMid:16148845
- Bagheri-Jamebozorgi
M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT,
Nejad-Ghaderi M, Jamalizadeh A, Shokri F, Jafarzadeh A. The persistence
of anti-HBs antibody and anamnestic response 20 years after primary
vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin
Immunother. 2014;10:3731-3736. https://doi.org/10.4161/hv.34393 PMid:25483689 PMCid:PMC4514033
- Van
Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in
adolescents 15-16 years of age vaccinated in infancy with three doses
of hepatitis B vaccine. Vaccine. 2016;34:2745-2749. https://doi.org/10.1016/j.vaccine.2016.04.013 PMid:27095043
- Zanetti
AR, Mariano A, Romano L, D'Amelio R, Chironna M, Coppola RC, Cuccia M,
Mangione R, Marrone F, Negrone FS, Parlato A, Zamparo E, Zotti C,
Stroffolini T, Mele A; Study Group. Long-term immunogenicity of
hepatitis B vaccination and policy for booster: an Italian multicentre
study. Lancet. 2005;366:1379-1384. https://doi.org/10.1016/S0140-6736(05)67568-X
- Bialek
SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, Bell
BP. Persistence of protection against hepatitis B virus infection among
adolescents vaccinated with recombinant hepatitis B vaccine beginning
at birth: a 15-year follow-up study. Pediatr Infect Dis J.
2008;27:881-885. https://doi.org/10.1097/INF.0b013e31817702ba PMid:18756185
- van
Tilburg CM, van Gent R, Bierings MB, Otto SA, Sanders EA, Nibbelke EE,
Gaiser JF, Janssens-Korpela PL, Wolfs TF, Bloem AC, Borghans JA,
Tesselaar K. Immune reconstitution in children following chemotherapy
for haematological malignancies: a long-term follow-up. Br J Haematol.
2011;152:201-210. https://doi.org/10.1111/j.1365-2141.2010.08478.x PMid:21114483
- Saco
TV, Strauss AT, Ledford DK. Hepatitis B vaccine nonresponders: Possible
mechanisms and solutions. Ann Allergy Asthma Immunol. 2018;121:320-327.
https://doi.org/10.1016/j.anai.2018.03.017 PMid:29567355


