Barring the comorbidities typical of hemophilia, have the main diseases prevalent in older people without hemophilia have the same prevalence in PWH? This question applies not only to such frequent cardiovascular diseases as coronary artery and cerebrovascular atherothrombotic disease, atrial fibrillation and heart failure but also to cancer, chronic kidney disease, degenerative arthritis and diabetes. Because aging with hemophilia is a relatively recent picture, data are relatively scanty and published reports mainly stem from cross-sectional observational studies or case series that obviously have inherent limitations in design and case selection.[9-12] In most of them, the age limit to define older PWH is as early as 40-50 years. Even though these patients would not be defined old according to the commonly used criteria, this early threshold is meaningful in PWH, because those who have currently reached at least this or older ages were born at a time when regular replacement therapy with coagulation factors, and particularly their prophylactic use, was far from being largely available. In addition, the majority of them became infected with HCV and HIV in the 1970s and 1980. Thus, owing to the inevitable effects of associated chronic comorbidities (liver and kidney disease) these patients are aging earlier and are more frail and vulnerable at relatively younger ages than their age peers without hemophilia.
The great majority of the published reports are quite concordant to indicate that older PWH have a lower prevalence of morbidity and mortality from atherothrombotic cardiovascular diseases than age-matched peers from the general population.[9-12] Lower morbidity and mortality are usually attributed to the protective effect on thrombus formation by the lifelong deficiency of coagulation factors, particularly cogent in older PWH poorly treated until recently, considering that in them there is no lower prevalence of atherosclerosis[14,15] nor of the general cardiovascular risk factors,[9,12,16,17] except for lower serum cholesterol.[10,17] An important CVD risk factor as hypertension has a higher prevalence than in the populations taken for comparison,[9,10,17,18] even though the underlying reasons are partially elusive.[19] Pertaining to such other ailments frequently associated with aging as diabetes, cancer [20,21] and osteoporosis, PWH are not spared from them but evidence of substantial differences in morbidity rates from the general population is scanty. Arthritis is a very common and precocious consequence of the hemophilic arthropathy and the related chronic pain is looming large in PWH. Atrial fibrillation, a disease typical of aging, is less frequent, perhaps because the investigated cohorts included very few patients above the age of 80, when this cardiac arrhythmia reaches the very high rates of 10% or more. On the whole, there is a need for prospective cohorts studies or registries to accrue more data on the incident burden of cardiovascular disease and other ailments typical of aging in PWH. Some of these cohort studies are indeed ongoing in the frame of a joint UK-Dutch effort,[17] in the USA[18] and in Italy[22,23] (Table 1), and the clinical picture of older PWH at enrolment is published.[17,18,22,23] The prospective follow-up of these cases and the acquisition of the corresponding data on incident events should help to develop guidelines on management, that are currently lacking, particularly for coronary artery disease and atrial fibrillation,[24] or are based on the local experiences of a few large HTC.[25,26]
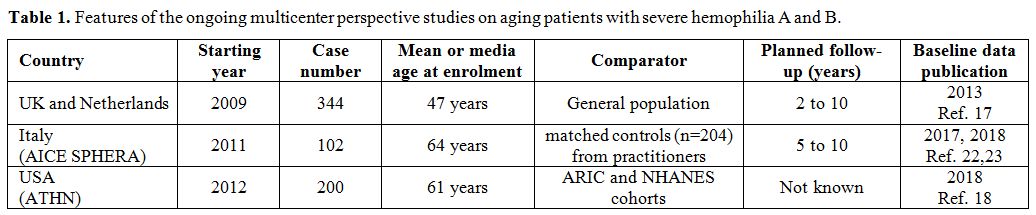 |
Table 1. Features of the ongoing multicenter perspective studies on aging patients with severe hemophilia A and B. |