Francesca Maria Quaglia1,2, Gian Matteo Rigolin2, Elena Saccenti2, Massimo Negrini3, Eleonora Volta2, Melissa Dabusti2, Maria Ciccone2, Antonio Urso2, Michele Laudisi2 and Antonio Cuneo2.
1 Stem Cell Research Laboratory, Section of Hematology, Department of Medicine, University of Verona, Verona, Italy.
2
Hematology Section, Department of Medical Sciences, Azienda
Ospedaliero-Universitaria, Arcispedale S.Anna, University of Ferrara,
Ferrara, Italy.
3 Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy.
Correspondence to: Gian Matteo Rigolin. Hematology Section, Department
of Medical Sciences, Azienda Ospedaliero-Universitaria, Arcispedale
S.Anna, University of Ferrara, Via Aldo Moro, 8, 44124, Cona, Ferrara,
Italy. Tel. +39 0532 239674, Fax +39 0532 236049. E-mail:
rglgmt@unife.it
Published: September 1, 2019
Received: June 19, 2019
Accepted: August 16, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019057 DOI
10.4084/MJHID.2019.057
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
In
2014 a 66-year-old woman presented with anemia and an IgAk monoclonal
spike. Bone marrow (BM) biopsy showed 80% lymphocytes and
lymphoplasmacytoid cells. Computed Tomography (CT) scan documented
neither adenopathy nor splenomegaly. Diagnosis of IgA lymphoplasmacytic
lymphoma was made. After three lines of treatment, progressive disease
with adenopathies, splenomegaly, and ascites were documented on a CT
scan. Our patient developed thrombocytopenia, transfusion-dependent
anemia, and clinical deterioration. We performed genetic studies of
peripheral blood lymphocytes with the NGS approach. Given the
identification of MYD88 L265P mutation, in February 2018 our patient
started ibrutinib off-label. Hb and PLT improved from day +35. In July
2018 no ascites and 50% reduction of adenopathies and spleen were shown
on a CT scan. In April 2019 the patient was still on ibrutinib with
transfusion independence and good performance status.
|
Introduction
Lymphoplasmacytic
lymphoma (LPL) is a rare chronic lymphoproliferative neoplasm
characterized by the proliferation of B lymphocytes with varying
degrees of plasmacytic differentiation involving bone marrow (BM),
lymph nodes, or spleen.[1] Waldenstrom
macroglobulinemia (WM) is a subset of LPL that has a detectable level
of monoclonal IgM gammopathy, with BM involvement by LPL.[2,3] Indeed in over 95% of LPL cases, the malignant clone produces an IgM paraprotein consistent with WM.[4]
However, the remaining LPL cases do not fulfill the diagnostic criteria
of WM. These conditions are mainly represented by rare cases of primary
nonsecretory lymph node-based presentations of LPL or by
lymphoplasmacytic B-cell proliferation in the BM associated with IgA or
IgG gammopathies.[1,5] Accurate
diagnosis of LPL can be difficult because of the absence of
morphologic, immunophenotypic, or chromosomal markers, especially in
non-WM cases where there is no IgM gammopathy to support the diagnosis.[1] The identification of the MYD88 L265P gene mutation represented a major advance in the diagnosis of LPL[3,5] although the real incidence of this mutation in LPL patients is unknown and a small number of WM patients with unmutated MYD88 exist. Indeed in a study by Treon et al.[6] about 90% of WM or LPL have MYD88 L265P mutation and a small subgroup of patients with marginal zone lymphoma (MZL) were shown to carry this genetic lesion.[6] In contrast, MYD88
L265P mutation was absent in tissue samples from patients with myeloma,
including samples from patients with IgM secreting myeloma.[6] MYD88
L265P mutation may, therefore, be useful in distinguishing LPL from
B-cell disorders showing partially overlapping clinicopathological
features.[6] Few cases of non-IgM LPL have been reported demonstrating the presence of MYD88 L265P.[5,7-11]
MYD88 L265P triggers survival signaling through BTK and HCK, and MYD88 L265P expressing cell lines undergo apoptosis in response to ibrutinib, which targets both of these kinases.[4] Moreover, Ibrutinib has demonstrated significant activity in patients with relapsed/refractory B-Cell malignancies.[12,13]
In 2015, the FDA and the EMA approved ibrutinib for the treatment of
symptomatic WM but not for LPL, based on a clinical trial in previously
treated patients. The patients with LPL not fulfilling the diagnostic
criteria of WM were excluded from WM trials and should be treated as
the other indolent lymphoproliferative neoplasms, while recent
guidelines[14-17] included recommendations on the
usage of ibrutinib specifically for WM. For these reasons, the use of
ibrutinib in non-IgM LPL has not yet been reported. We present here the
first report of a patient with MYD88-mutated IgA LPL who underwent salvage therapy with ibrutinib.
Case Report
In
September 2014 a 66-year-old woman presented with symptomatic anemia
(Hemoglobin, Hb: 9 g/dl), with IgA/k monoclonal spike (1.6 g/dl) (Figure 1)
and an otherwise unremarkable serum chemistry profile. A Computed
Tomography (CT) scan documented neither adenopathy nor splenomegaly. BM
biopsy showed an 80% infiltrate by lymphocytes and lymphoplasmacytoid
cells with admixed atypical plasma cells (25%). Flow cytometry showed a
kappa-restricted B-cell population (strong sIg kappa positivity) that
expressed CD20, CD19, CD22, CD38, CD138, FMC7, and was negative for
CD5, CD3, CD10, CD56, CD79a, CD23. The malignant plasma cells showed
IgA+ kappa-restriction. The karyotype on BM cells was normal and so, no
t(4,14), t(14,16), t(11,14), or del(17p) aberrations were detected by
fluorescence in situ hybridization (FISH) using a probe-panel for
multiple myeloma (MM); a deletion of 13q14 DLEU was detected by FISH
using a 5 probe-panel for CLL (13q14, chromosome-12 centromere, 11q22,
17p13, 6q21). A diagnosis of IgA-secreting LPL was made.
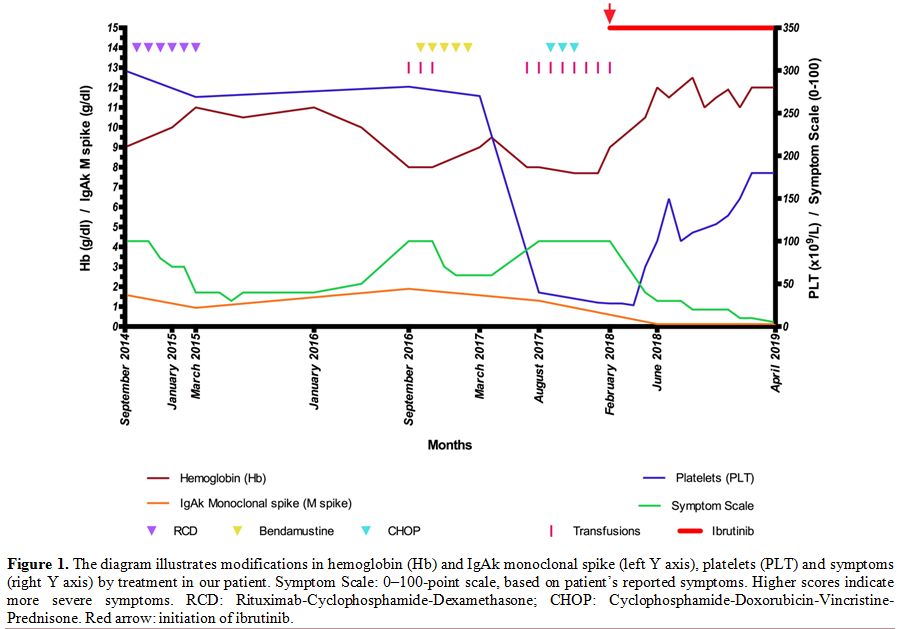 |
Figure
1. The diagram illustrates modifications in hemoglobin (Hb) and IgAk
monoclonal spike (left Y axis), platelets (PLT) and symptoms (right Y
axis) by treatment in our patient. Symptom Scale: 0–100-point scale,
based on patient’s reported symptoms. Higher scores indicate more
severe symptoms.
RCD:
Rituximab-Cyclophosphamide-Dexamethasone; CHOP:
Cyclophosphamide-Doxorubicin-Vincristine-Prednisone. Red arrow:
initiation of ibrutinib. |
The patient was treated with rituximab-cyclophosphamide-dexamethasone (RCD regimen) with Partial Response (PR)[18] (Figure 1). Eighteen months later the patient, who presented with progressive disease (PD) (Hb 8 g/dl, lymphocytes 3.56 x 109/L
with 80% lymphoplamacytic cells, IgAk monoclonal spike 1.9 g/dl), was
treated with bendamustine. In January 2017, after five cycles, the BM
aspirate showed 90% lymphoid cells and adenopathies, splenomegaly and
ascites were noted on a CT scan. Thus,
cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) was started
and, after three cycles, the patient developed thrombocytopenia
(Platelets, PLT: 30 x 10^9/L), transfusion-dependent anemia (Hb 7.7
g/dl), persistent lymphocytosis (Lymphocytes: 7.15 x 109/L) in the peripheral blood (PB) and clinical deterioration (Figure 1).
We performed genetic studies of PB lymphocytes (after separation over a
Ficoll gradient, yielding >80% clonal B lymphocytes) with a targeted
NGS approach detecting mutations in 20 genes frequently mutated in CLL (ATM,
BIRC3, BRAF, CDKN2A, PTEN, CDH2, DDX3X, FBXW7, KIT, KLHL6, KRAS, MYD88,
NOTCH1, NRAS, PIK3CA, POT1, SF3B1, TP53, XPO1, ZMYM3). The MYD88 L265P mutation was identified in 65.7% of the reads. Given the identification of MYD88
L265P in the peripheral blood, ibrutinib appeared a reasonable option.
In February 2018, our patient started ibrutinib off-label, 420 mg once
daily (Figure 1). Hb and PLT
improved from day +35 (Hb 10-12 g/dl, PLT > 100 x 10^9/L). In July
2018 no ascites and 50% reduction of adenopathies and spleen were shown
on a CT scan. In April 2019, the patient was still on full dose
ibrutinib with transfusion independence and good performance status.
This
patient is unique in that it represents - to the best of our knowledge
- the first reported case of response to ibrutinib in symptomatic
aggressive IgA secreting LPL with MYD88 mutation refractory to multiple
lines of treatment. Guidelines for treatment of WM pose indication for
ibrutinib in relapsed or untreated patients who are not candidates for
chemoimmunotherapy.[14-17] Our case clearly indicates
that ibrutinib may represent a valuable therapeutic option for
chemorefractory LPL not fulfilling the diagnostic criteria of WM. Our
patient had previously been exposed to alkylators, immunomodulators,
anti-CD20 monoclonal antibodies and steroids. Her therapeutic options
at the time of her most recent relapse were limited and given the
identification of the MYD88
L265P mutation in the peripheral blood, ibrutinib appeared as a
reasonable option. In our patient, ibrutinib produced a response within
4–6 weeks, that is a typical time-frame during a response is usually
observed. The partial response has been sustained for approximately 15
months at the time of this report. The kynetics of response in
different disease compartments (blood, nodal, extranodal, spleen) were
similar to those observed in WM patients[15,19] and CLL patients on single-agent ibrutinib[20]
with few treatment emergent adverse events consisting in grade 1
bruising, arthralgias and diarrhea, which improved and resolved with
continued treatment.
In conclusion, we present the case of a heavily pretreated patient with MYD88-mutated
IgA LPL, who has obtained a partial response to ibrutinib that is
ongoing after more than one year of therapy. This observation suggests
that ibrutinib appears to be potentially effective in this
difficult-to-treat-condition.
References
- Naderi N, Yang DT. Lymphoplasmacytic Lymphoma and Waldenstrom Macroglobulinemia. Arch Pathol Lab Med. 2013;137:580-585; https://doi.org/10.5858/arpa.2012-0034-RS PMid:23544948
- Mazzucchelli
M, Frustaci AM, Deodato M, Cairoli R, Tedeschi A. Waldenstrom's
macroglobulinemia: an update. Mediterr J Hematol Infect Dis . 2018; 10
(1): e2018004. https://doi.org/10.4084/mjhid.2018.004 PMid:29326801 PMCid:PMC5760071
- Swerdlow
SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R,
Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the
World Health Organization classification of lymphoid neoplasms. Blood.
2016; 9;127(20):2375-2390. doi: 10.1182/blood-2016-01-643569. Epub 2016
Mar 15. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727 PMCid:PMC4874220
- Castillo
JJ, Ghobrial IM, Treon SP. Response to ibrutinib in a patient with IgG
lymphoplasmacytic lymphoma carrying the MYD88 L265P gene mutation. Leuk
Lymphoma. 2016;57(11):2699-2701. DOI: 10.3109/10428194.2016.1157875. https://doi.org/10.3109/10428194.2016.1157875 PMid:26980069
- Martino
G, Marra A, Ascani S, Sportoletti P. Uncommon lymphoplasmacytic
lymphoma with IgA paraproteinemia: a challenging clinical diagnosis
solved by MYD88 mutation analysis. Ann Hematol. 2018. https://doi.org/10.1007/s00277-018-3545-9 PMid:30426157
- Treon
SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson
CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL,
Laramie JM, Skifter DA, Lincoln SE, Hunter ZR. MYD88 L265P Somatic
Mutation in Waldenström's Macroglobulinemia. N engl j med.
2012;367(9):826-833. https://doi.org/10.1056/NEJMoa1200710 PMid:22931316
- Mori
N, Ohwashi M, Yoshinaga K, Mitsuhashi K, Tanaka N, Teramura M, Okada M,
Shiseki M, Tanaka J, Motoji T. L265P mutation of the MYD88 gene is
frequent in Waldenstrom's macroglobulinemia and its absence in myeloma.
PloS One. 2013;8(11):e80088. https://doi.org/10.1371/journal.pone.0080088 PMid:24224040 PMCid:PMC3818242
- Swerdlow
SH, Kuzu I, Dogan A, Dirnhofer S, Chan JKC, Sander B, Ott G, Xerri L,
Quintanilla-Martinez L, Campo E. The many faces of small B cell
lymphomas with plasmacytic differentiation and the contribution of
MYD88 testing. VirchowsArch. 2016;468(3):259-275. https://doi.org/10.1007/s00428-015-1858-9 PMid:26454445 PMCid:PMC5002945
- Cao
X, Medeiros LJ, Xia Y, Wang X, Thomas SK, Loghavi S, Li X, Shah JJ,
Gustafson SA, Weber DM, Miranda RN, Xu-Monette ZY, Orlowski RZ, Young
KH. Clinicopathologic features and outcomes of lymphoplasmacytic
lymphoma patients with monoclonal IgG or IgA paraprotein expression.
Leuk Lymphoma. 2016;57(5):1104-13. https://doi.org/10.3109/10428194.2015.1096357 PMid:26421453
- Jiménez
C, Sebastián E, Chillón MC, Giraldo P, Mariano Hernández J, Escalante
F, González-López TJ, Aguilera C, de Coca AG, Murillo I, Alcoceba M,
Balanzategui A, Sarasquete ME, Corral R, Marín LA, Paiva B, Ocio EM,
Gutiérrez NC, González M, San Miguel JF, García-Sanz R. MYD88 L265P is
a marker highly characteristic of, but not restricted to, Waldenstrom's
macroglobulinemia. Leukemia. 2013;27:1722-1728. https://doi.og/10.1038/leu.2013.62 PMid:23446312
- King
RL, Gonsalves WI, Ansell SM, Greipp PT, Frederick LA, Viswanatha DS, He
R, Kyle RA, Gertz MA, Kapoor P, Morice WG, Howard MT. Lymphoplasmacytic
Lymphoma With a Non-IgM Paraprotein Shows Clinical and Pathologic
Heterogeneity and May Harbor MYD88 L265P Mutations. Am J Clin Pathol.
2016;145(6):843-851. https://doi.org/10.1093/ajcp/aqw072 PMid:27329639
- Advani
RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS,
Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A,
Hedrick E, Fowler NH. Bruton Tyrosine Kinase Inhibitor Ibrutinib
(PCI-32765) Has Significant Activity in Patients With
Relapsed/Refractory B-Cell Malignancies. J Clin Oncol. 2013;
31(1):88-94. https://doi.org/10.1200/JCO.2012.42.7906 PMid:23045577 PMCid:PMC5505166
- Noy
A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F,
Collins GP, Ma S, Coleman M, Peles S, Smith S, Barrientos JC, Smith A,
Munneke B, Dimery I, Beaupre DM14, Chen R15.Targeting Bruton tyrosine
kinase with ibrutinib in relapsed/refractory marginal zone lymphoma.
Blood. 2017; 129(16):2224-2232. https://doi.org/10.1182/blood-2016-10-747345 PMid:28167659 PMCid:PMC5399483
- Leblond
V, Kastritis E, Advani R, Ansell SM, Buske C, Castillo JJ, García-Sanz
R, Gertz M, Kimby E, Kyriakou C, Merlini G, Minnema MC, Morel P, Morra
E, Rummel M, Wechalekar A, Patterson CJ, Treon SP, Dimopoulos MA.
Treatment recommendations from the Eighth International Workshop on
Waldenström's Macroglobulinemia. Blood 2016;128:1321-8. https://doi.org/10.1182/blood-2016-04-711234 PMid:27432877
- Treon
SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV,
Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC,
Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR,
Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML, Advani RH.
Ibrutinib in Previously Treated Waldenström's Macroglobulinemia. N engl
J Med. 2015; 372(15): 1430-1440. https://doi.org/10.1056/NEJMoa1501548 PMid:25853747
- Castillo
JJ, Palomba ML, Advani R, Treon SP. Ibrutinib in Waldenström
macroglobulinemia: latest evidence and clinical experience. Ther Adv
Hematol. 2016; 7(4):179-186. https://doi.org/10.1177/2040620716654102 PMid:27493708 PMCid:PMC4959643
- Kastritis
E, Leblond V, Dimopoulos MA, Kimby E, Staber P, Kersten MJ, Tedeschi A,
Buske C. ESMO Guidelines Committee. Waldenstrom's macroglobulinaemia:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 2018;29(Supplement 4):iv41-iv50. https://doi.org/10.1093/annonc/mdy146 PMid:29982402
- Cheson
BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister
TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern
Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium;
Italian Lymphoma Foundation; European Organisation for Research;
Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de
Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's
Study Group; Japanese Lymphorra Study Group; Lymphoma Study
Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group;
Southwest Oncology Group; United Kingdom National Cancer Research
Institute. Recommendations for initial evaluation, staging, and
response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano
classification. J Clin Oncol. 2014 Sep 20;32(27):3059-68. https://doi.org/10.1200/JCO.2013.54.8800 PMid:25113753 PMCid:PMC4979083
- Dimopoulos
MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, Tournilhac O,
Ma S, Oriol A, Heffner LT, Shustik C, García-Sanz R, Cornell RF, de
Larrea CF, Castillo JJ, Granell M, Kyrtsonis MC, Leblond V, Symeonidis
A, Kastritis E, Singh P, Li J, Graef T, Bilotti E, Treon S, Buske C;
iNNOVATE Study Group and the European Consortium for Waldenström's
Macroglobulinemia. Ibrutinib for patients with rituximab-refractory
Waldenström's macroglobulinaemia (iNNOVATE): an open-label substudy of
an international, multicentre, phase 3 trial. Lancet Oncol. 2017
Feb;18(2):241-250. https://doi.org/10.1016/S1470-2045(16)30632-5
- Ahn
IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S, Lotter J, Housel S,
Stetler-Stevenson M, Yuan CM, Maric I, Calvo KR, Nierman P, Hughes TE,
Saba NS, Marti GE, Pittaluga S, Herman SEM, Niemann CU, Pedersen LB,
Geisler CH, Childs R, Aue G, Wiestner A. Depth and durability of
response to ibrutinib in CLL: 5-year follow-up of a phase 2 study.
Blood. 2018;131(21):2357-2366. https://doi.org/10.1182/blood-2017-12-820910 PMid:29483101 PMCid:PMC5969380