Giacomo Andreani1, Gianluca Fadda2, Dario Gned3, Matteo Dragani1, Giovanni Cavallo2, Valentina Monticone2, Alessandro Morotti1, Marco De Gobbi1, Angelo Guerrasio1, Anna Maria Barbui4, Antonio D’Avolio5 and Daniela Cilloni1.
1 Department of Clinical and Biological Sciences, University of Turin, San Luigi Gonzaga Hospital, Orbassano, Turin, Italy.
2 Department of Otolaringology, San Luigi Gonzaga Hospital, Orbassano, Turin, Italy.
3 Department of Diagnostic Imaging, San Luigi Gonzaga Hospital, Orbassano, Turin, Italy.
4 Microbiology and Virology Unit, Azienda Ospedaliera Universitaria Città della Salute e della Scienza, Turin, Italy.
5
Department of Medical Sciences, Laboratory of Clinical Pharmacology and
Pharmacogenetics, University of Turin, Amedeo di Savoia Hospital,
Turin, Italy.
Correspondence to: Giacomo Andreani, Department of Clinical and
Biological Sciences of the University of Turin, San Luigi Gonzaga
Hospital, Regione Gonzole 10, 10043, Orbassano, Turin, Italy. Tel.: +39
011 9026305; fax: +39 011 9026963. E-mail:
giacomo.andreani@unito.it ORCID ID:
https://orcid.org/0000-0003-4877-3929
Published: November 1, 2019
Received: July 12, 2019
Accepted: September 19, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019061 DOI
10.4084/MJHID.2019.061
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
A
diagnosis of rhino-orbital-cerebral mucormycosis was made in a
59-year-old man with a secondary acute myeloid leukemia a few days
after hematopoietic stem cell transplantation. Prompt treatment with
combined antifungal therapy (liposomal amphotericin B and
isavuconazole) followed by a procedure of endoscopic sinus surgery
resulted in the resolution of the infection. Therapeutic drug
monitoring of isavuconazole was performed during the year of treatment
showing an increment of plasma concentrations in correspondence with
the improvement of intestinal GvHD, thus suggesting that in this or
similar conditions TDM for isavuconazole can be of value.
A
literature review of cases of rhino-orbital-cerebral and rhino-cerebral
mucormycosis in allogeneic hematopoietic stem cell transplant
recipients was carried out.
|
Introduction
Mucormycosis
is an aggressive and potentially fatal invasive fungal infection which
can manifest by a variety of different syndromes. The genera in the
order of Mucorales most commonly found in humans are Rhizopus, Mucor, Rhizomucor.[1]
Fungal spores are ubiquitous in the atmosphere, but infection is a rare
event. Predisposing risk factors include diabetes mellitus, hematologic
malignancies, solid organ transplantation and hematopoietic stem cell
transplantation (HSCT), trauma, burns, iron overload and
immunosuppressive therapies. After Aspergillus, Mucorales are the most
common fungal pathogens affecting patients undergoing HSCT;
rhino-orbital-cerebral mucormycosis (ROCM) is the most common type of
mucormycosis defined as a fulminant infection involving nose, paranasal
sinuses, orbits, and brain. Management of ROCM requires a combination
of antifungal therapy, debridement of involved tissues and if possible
elimination of predisposing conditions. Despite early diagnosis and
aggressive treatment the prognosis is poor.[2]
Case Presentation
Here,
we report the case of a 59-year-old man with acute myeloid leukemia
(AML) arising in August 2017 from a 5q- myelodysplastic syndrome
treated with lenalidomide. A Mito-FLAG induction scheme (G-CSF from day
-1, fludarabine 60 mg/die days 1-5, cytarabine 2 g over 3 h every 12 h
days 1-5, mitoxantrone 12 mg/die day 1, 3 and 5) followed by two cycles
of consolidation chemotherapy were administrated (Mito-FLAG again and
one cycle with high-doses-cytarabine); meanwhile a donor search was
started. In March 2018, after a TBF reduced-intensity conditioning
regimen (thiotepa 390 mg day -6, busulfan 240 mg/die days -5 and -4;
fludarabine 80 mg day -3) the patient underwent allogeneic HSCT from a
matched unrelated donor. Micafungin was used for fungal prophylaxis.
Rabbit-derived anti-thymocyte globulins infusion (2.5 mg/Kg days -3,
-2, -1), cyclosporine A (3 mg/Kg, continuous infusion from day -1) and
methotrexate (30 mg day +1 then 20 mg days +3 and +6) were administered
for GvHD prophylaxis. Since the diagnosis of AML to HSCT a total of 47
units of red blood cells were transfused, deferasirox (an
iron-chelating agent) was started two months before HSCT producing a
ferritinemia reduction from 2713 to 1489 ng/ml few days before the
beginning of conditioning regimen; deferasirox administration was
stopped during the transplant procedure.
Day +1 from HSCT, the
patient complained, on the right side of the face, a sharp pain
mimicking trigeminal neuralgia and responsive to high doses of
morphine. CT scan of brain and sinuses showed a picture of
pansinusitis. At day +2, after a sudden onset of fever, empirical
antibacterial therapy (piperacillin/tazobactam plus amikacin) and
antifungal therapy with liposomal amphotericin B (L-Amb) were started.
Diplopia occurred in day +6, a palsy of VI right cranial nerve was
detected plus a sensitive deficit of V cranial nerve of the same side.
Magnetic resonance imaging (MRI) of brain and sinuses plus MR
angiography with contrast dye showed the presence of a mycetoma in the
right sphenoidal sinus associated with thrombophlebitis of the
cavernous sinus, thrombosis of the superior ophthalmic vein and
possible involvement of temporal meninges in contiguity with the bone
cavity.
Blood indirect biomarkers for the detection of a fungal
infection (1,3-beta-D-glucan and galactomannan) were negatives over the
entire period. Antifungal therapy was reinforced, a combination of
L-AmB at the dosage of 7.5 mg/Kg/die plus iv isavuconazole (ISC) 200 mg
(375 mg of isavuconazonium sulfate equivalent to 200 mg of ISC) every 8
hours for six doses followed by 200 mg/die was started. Day +11, the
patient, still in aplasia (WBC 10/mm3, Hb 7.0 g/dl, platelets 10000/mm3),
underwent a procedure of endoscopic sinus surgery (ESS) after
transfusions of blood components with no complications. The cultural
and histological examination does not reveal presence of hyphae;
Rhizomucor was identified by amplification and sequencing of two
Internal Transcribed Spacer regions (ITS1 and ITS2) in rRNA gene. The Sanger sequencing was performed after amplification using ITS1-Forward and ITS4-reverse primers.[3]
The
patient achieved myeloid engraftment at day +16 and became afebrile.
The trigeminal neuralgia regressed although the VI right cranial nerve
palsy persistence, antibiotics were stopped and the combined antifungal
therapy was continued. A new MRI, at day +41, revealed the presence of
a brain mycotic abscess with associated vasogenic cerebral edema of the
right temporal lobe (Figure 1).
Although the involvement of the cavernous sinus was detected, an
excisional neurosurgery intervention of the cerebral abscess was not
undertaken considering the procedural high-risk balanced to the
benefits of an incomplete drainage. The patient was discharged, ISC was
switched to oral formulation, and L-AmB was administered in an
outpatient setting for 74 days in total. Cyclosporine administration
was stopped one month after transplantation for fear to promote Rhizomucor
growth and further dissemination. The general condition of the patient
worsened; he lost 30 Kg of weight in total, parenteral nutrition was
started. We were forced to introduce steroid therapy (prednisone 1
mg/Kg/die) for severe gastrointestinal, cutaneous and ocular GvHD.
Because the response of cutaneous GvHD to steroids was not adequate,
extracorporeal photoapheresis was started with benefit. Megestrol
acetate, an orexigenic, was dispensed to treat cachexia. ISC dosage was
increased to 300 mg/die in consideration of ISC plasma concentrations (Figure 2); deferasirox was reintroduced in therapy.
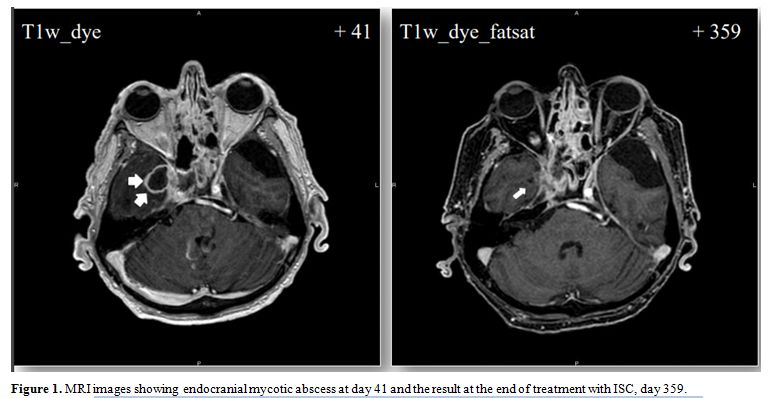 |
Figure 1. MRI images showing endocranial mycotic abscess at day 41 and the result at the end of treatment with ISC, day 359. |
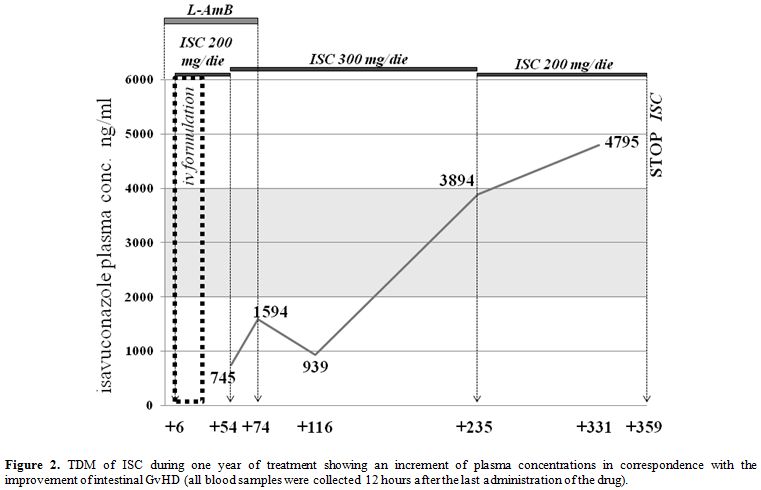 |
Figure 2. TDM of ISC
during one year of treatment showing an increment of plasma
concentrations in correspondence with the improvement of intestinal
GvHD (all blood samples were collected 12 hours after the last
administration of the drug). |
At
six months from HSCT, a new MRI of the brain showed the reduction of
the para-cavernous abscess with partial reabsorption of the purulent
quote and temporal lobe edema (Figure 1).
Complete thrombosis of the right internal carotid artery was detected
but patient remained completely asymptomatic for it. We decided to
continue antifungal therapy with ISC at 300 mg/die then we reduced the
dosage to 200 mg/die based on therapeutic drug monitoring (TDM).
Patient’s
mood improved and body weight with it, he started to have a complete
meal and to walk again with crutches. Palsy of VI right cranial nerve
regressed almost completely; prednisone was reduced and maintain to a
dose of 10 mg/die for hepatic and intestinal GvHD. Revaluation of bone
marrow at one year from HSCT confirmed full donor chimerism. Brain MRI
revealed further reduction of the endocranial mycotic abscess (8 x 3
mm), no purulent quote was present. In accordance with clinical
improvement we considered it a full response, and we decided to stop
isavuconazole after 354 days of treatment.
Literature Review of Cases of Rhino-Orbital-Cerebral Mucormycosis in Allogeneic Hematopoietic Stem Cell Transplant Recipients
We
searched all published journal articles in MEDLINE starting from 2005
with the search terms “Rhino-orbital-cerebral mucormycosis”,
“Rhino-cerebral mucormycosis”, “Hematopoietic stem cell
transplantation”. We included only case reports and case series of
ROCM, or rhino-cerebral mucormycosis (RCM) in which infection was
diagnosed during or after allogeneic HSCT, no other extensive reviews
were included due to lack of information. We identified 5 cases in
literature (3 ROCM and 2 RCM) (Table 1). The type of donors were: matched related donor (MRD) in 3 cases,[4-6] a HLA (human leukocyte antigen) haploidentical donor in one case,[7] and in another case a HLA-matched donor not otherwise specified;[8]
the underlying disease was AML in two cases, one case was a Ph+ B-ALL
(B-cell acute lymphoblastic leukemia), one was a pre-B cell ALL (a
5-year-old boy) and another case was a T-cell prolymphocytic leukemia.
The species in the order of Mucorales were different: two patients were
infected by Rhizopus oryzae, one patient by Cunninghamella bertholletiae, another patient by Lichthemia corymbifera, in one case the species of Mucor
was not mentioned. Fluconazole was used as fungal prophylaxis in three
cases and voriconazole in one case; in another case the type of
prophylaxis was not mentioned. MRI has been a fundamental tool in the
suspicious of ROCM/RCM; the diagnosis was performed by microscopy and
culture after biopsy or surgery debridement in 4 cases, one of which
also reported performance of the sequence analysis of ITS region; in
one case data was not reported. In four cases single antifungal therapy
with L-Amb was administered (two of them switched from caspofungin and
posaconazole respectively), in one case double antifungal therapy with
L-Amb plus posaconazole was administered. In three cases surgical
debridement was performed. All patients deceased few days or weeks
after the onset of the infection..
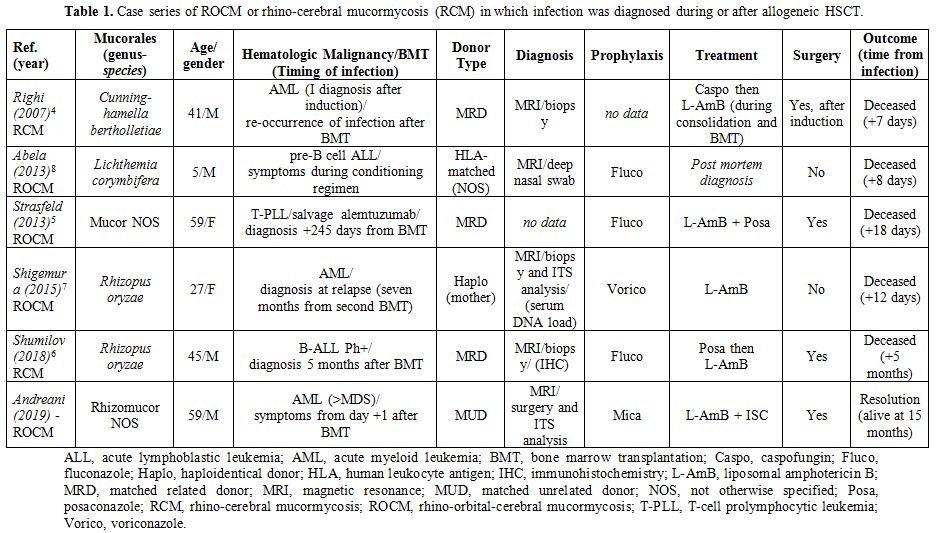 |
Table 1. Case series of
ROCM or rhino-cerebral mucormycosis (RCM) in which infection was
diagnosed during or after allogeneic HSCT. |
Discussion
The
mortality rate of ROCM is reported being between 15% and 85% with worst
prognosis for patients with brain, cavernous sinus, and carotid
involvement.[9] It is fundamental to rapidly start the
management, any delay on the treatment results in an increase in
mortality rate. The most common finding by CT scan of patients with
ROCM is simple sinusitis, and a negative CT scan does not rule out
mucormycosis. MRI is recommended because it is more sensitive for
detecting orbital and central nervous system (CNS) involvement.[10]
In the clinical case reported above, the mycetoma appeared on MRI as an
intrasinusal mass hypointense on T2w and non-homogeneously hyperintense
on T1w with a peripheral contrast enhancement due to inflammatory
thickening of the surrounding mucosa; artifacts consistent with the
presence of iron and manganese were detected in T2w*. Differential
diagnosis with granulomatous sinusitis is difficult on MRI; conversely
a contrast enhancement of the mass is more suspicious for the presence
of a squamous cell carcinoma or lymphoma of the sinus. Finally,
mucocele can be quite easily distinguished, among other things, for its
hyperintensity on T2w on MRI.
Serum test, 1,3-beta-D-glucan assay,
and galactomannan assay are not useful in patients suspected of having
mucormycosis because Mucorales do not have these components on cell
wall. Identification of the pathogen most often comes from microscopy,
culture, histopathological examination and/or sequencing of specific
DNA regions on biopsy samples but it is essential to know that no more
than 50% of cases are diagnosed by combined histopathology and culture;
molecular techniques are intriguing and increasingly used methods that
can be rapidly performed, on different samples (for instance on blood),
in the suspicious and/or in the monitoring of the infection with less
or no procedural risks for patients.[3]
Optimal
therapy requires a multidisciplinary approach consisting on a prompt
antifungal therapy, reversal of underlying predisposing conditions
(whenever possible, e.g. severe neutropenia) and surgical debridement
is recommended, when feasible.[11] The use of
deferasirox in mucormycosis is debated: it has been demonstrated that
deferasirox, contrary to deferoxamine, do not act as siderophore for
Mucorales indeed it has a fungicidal effect;[12] the
result of higher mortality rate at 90 days in the group of deferasirox
plus L-AmB compared to L-AmB alone in a very small (n= 20) prospective,
double-blind, placebo-controlled trial of hematologic patients appears
very difficult to interpret because of important imbalanced
characteristics between two treatment groups.[13] To
sum up we suggest to introduce deferasirox in mucormycosis treatment as
soon as possible; this is what we did with our patient. Moreover, we
administered iron chelator few weeks before transplantation to reduce
high levels of ferritinemia due to several blood transfusions, despite
this we consider iron overload in our patient as one of the major
culprits of the early onset of the infection after HSCT, together with
the condition of aplasia and immunosuppression.
Concerning
antifungal therapy tout-court, we initially adopted a combined
treatment with L-AmB and ISC, followed by a prompt surgical
debridement. Next we stepped down therapy to ISC only, because of
patient inability to attend daily the outpatient clinic (Italian
National Health Service does not allow L-Amb administration in a home
setting therapy). ISC is a broad-spectrum azole drug with activity
against yeasts, mould and dimorphic fungi, and it has been approved by
FDA as first-line treatment for mucormycosis and by EMA in cases in
which L-AmB is inappropriate.[14] Considering the
condition of profound immunosuppression of our patient and in light of
CNS involvement we opted for a combined antifungal therapy ab initio,
as already reported from other groups.[15] Although
many works declare no apparent relationship between exposure and
efficacy to suggest routine TDM for ISC, the same raise the issue for
patients with intestinal GvHD in which drug absorption through the oral
route can be decreased and in cases of CNS involvement.[16]
In our patient ISC plasma concentrations after switch to oral
formulation were below expected range of values, 2000-4000 ng/ml
considering ISC Minimum Inhibitory Concentrations for Rhizomucor from
previous studies[17] (ISC plasma concentrations were assessed by an HLPC/GC-mass spectrometry assay),[18]
coinciding with a severe intestinal GvHD so we opted for an increment
in dosage to 300 mg/die. However, ISC plasma concentrations grew up
dramatically only after improvement of intestinal GvHD although the
reduction in ISC dosage. A recently published work assessing ISC plasma
concentration in 19 patients with hematologic malignancies (six of them
previously undergone allogeneic HSCT) retrospectively observed an
increment in ISC concentration overtime during treatment, authors
speculate that the increasing trend could be due to expected drug
accumulation in tissues and consequently in plasma.[19]
Regardless of different possible explications we can formulate for the
trend of TDM, it is crucial to identify ISC efficacy concentration
thresholds. The aim is to pursue a correct dose adjustment in those
patients, like HSCT recipients, in whom intestinal absorption is
reduced and in whom concomitant medications can modify ISC
concentrations.[20] In lack of defined thresholds we
believe that in this hematologic setting of large interpatient
pharmacokinetic variability, TDM of ISC can be a tool of value to
sustain clinician in the decision-making giving the possibility to
compare drug exposure of the patient to results that came out from
clinical trials and to those they are coming out on real-world practice.
References
- Vaughan C, Bartolo A, Vallabh N, Leong SC. A
meta-analysis of survival factors in rhino-orbital-cerebral
mucormycosis - has anything changed in the past 20 years? Clinical
Otolaryngology. 2018;43:1454-1464. https://doi.org/10.1111/coa.13175 PMID:29947167
- Jeong
W, Keighley C, Wolfe R, et al. The epidemiology and clinical
manifestations of mucormycosis: a systematic review and meta-analysis
of case reports. Clin Microbiol Infect. 2019;25(1):26-34. https://doi.org/10.1016/j.cmi.2018.07.011 PMID:30036666
- Korabecna
M. The Variability in the Fungal Ribosomal DNA (ITS1, ITS2, and 5.8
rRNA Gene): Its Biological Meaning and Application in Medical Mycology.
In Communicating Current Research and Educational Topics and Trends in
Applied Microbiology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain,
2007; pp. 783-787.
- Righi
E, Giacomazzi CG, Lindstrom V, et al. A Case of Cunninghamella
bertholettiae Rhino-cerebral Infection in a Leukemic Patient and Review
of Recent Published Studies. Mycopathologia. 2018;165:407-410. https://doi.org/10.1007/s11046-008-9098-z PMID:18340546
- Strasfeld
L, Espinosa-Aguilar L, Gajewsi JL et al. Emergence of Cunninghamella As
a Pathogenic Invasive Mold Infection in Allogeneic Transplant
Recipients. Clin Lymphoma Myeloma Leuk. 2013;13:622-8. https://doi.org/10.1016/j.clml.2013.05.002 PMID:23850285
- Shumilov
E, Bacher U, Perske C, et al. In Situ Validation of the Endothelial
Cell Receptor GRP78 in a Case of Rhinocerebral Mucormycosis. Antimicrob
Agents Chemother. 2018;62(5). https://doi.org/10.1128/AAC.00172-18 PMID:29483124
- Shigemura
T, Nishina S, Nakazawa H, et al. Early detection of Rhizopus DNA in the
serum of a patient with rhino-orbital-cerebral mucormycosis following
allogeneic hematopoietic stem cell transplantation. Int J Hematol.
2016;103:354-355. https://doi.org/10.1007/s12185-016-1938-x PMID:26781616
- Abela
L, Toelle SP, Hackenberg A, et al. Fatal outcome of
rhino-orbital-cerebral mucormycosis due to bilateral internal carotid
occlusion in a child after hematopoietic stem cell transplantation.
Pediatr Infect Dis J. 2013;32(10):1149-50. https://doi.org/10.1097/INF.0b013e31829e69e7 PMID:24067555
- Roden
MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of
zigomycosis: a review of 929 reported cases. Clin Infect Dis.
2005;41:634-53. https://doi.org/10.1086/432579 PMID:16080086
- Spellberg B, Ibrahim AS. Recent Advances in the Treatment of Mucormycosis. Curr Infect Dis Rep. 2010; 12:423-429. https://doi.org/10.1007/s11908-010-0129-9 PMID:21308550
- Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-Orbital-Cerebral Mucormycosis. Curr Infect Dis Rep. 2012;14:423-434. https://doi.org/10.1007/s11908-012-0272-6 PMID:22684277
- Spellberg
B, Andes D, Perez M, et al. Safety and outcomes of open-label
deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents
Chemother. 2009;53:3122-3125. https://doi.org/10.1128/AAC.00361-09 PMID:19433555
- Spellberg
B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy
for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded,
placebo-controlled trial. J Amtimicrob Chemother. 2012; 67: 715-722. https://doi.org/10.1093/jac/dkr375 PMID:21937481
- Maertens
JA, Raad II, Marr KA et al. Isavuconazole versus voriconazole for
primary treatment of invasive mould disease caused by Aspergillus and
other filamentous fungi (SECURE): a phase 3, randomised-controlled,
non-inferiority trial. Lancet. 2016;387:760-69. https://doi.org/10.1016/S0140-6736(15)01159-9 PMID:26684607
- Candoni
A, Klimko N, Busca A, et al.; on behalf of SEIFEM Group
(Epidemiological Surveillance of Infections in Haematological
Diseases). Fungal infections of the central nervous system and
paranasal sinuses in onco-haematologic patients. Epidemiological study
reporting the diagnostic-therapeutic approach and outcome in 89 cases.
Mycoses. 2019;62:252-260. https://doi.org/10.1111/myc.12884 PMID:30565742
- Stott
KE, Hope WW. Therapeutic drug monitoring for invasive mould infections
and disease: pharmacokinetic and pharmacodynamic considerations. J
Antimicrob Chemoter. 2017;72 Suppl 1: i12-i18. https://doi.org/10.1093/jac/dkx029 PMID:28355463
- Rybak
JM, Marx KR, Nishimoto AT, Rogers PD. Isavuconazole: Pharmacology,
Pharmacodynamics, and Current Clinical Experience with a New Triazole
Antifungal Agent. Pharmacotherapy. 2015;35(11):1037–1051. https://doi.org/10.1002/phar.1652 PMID:26598096
- Fatiguso
G, Favata F, Zedda I, et al. A simple high performance liquid
chromatography-mass spectrometry method for Therapeutic Drug Monitoring
of isavuconazole and four other antifungal drugs in human plasma
samples. J Pharm Biomed Anal. 2017;145:718.724. http://doi.org/10.1016/j.jpba.2017.07.040 PMID:28806568
- Furfaro
E, Signori A, Di Grazia C, et al. Serial monitoring of isavuconazole
blood levels during prolonged antifungal therapy. J Antimicrob
Chemother. 2019;74(8):2341-2346. http://doi.org/10.1093/jac/dkz188 PMID:31119272
- Andes
D, Kovanda L, Desai A, et al. Isavuconazole Concentration in Real-World
Practice: Consistenly with Results from Clinical Trials. Antimicrob
Agents Chemother. 2018;62(7). http://doi.org/10.1128/AAC.00585-18 PMID:29735569


