Benedetto Maurizio Celesia1, Andrea Marino1, Rosa Fontana del Vecchio1, Roberto Bruno1, Filippo Palermo1, Maria Gussio1, Giuseppe Nunnari2 and Bruno Cacopardo1.
1 Division of
Infectious Diseases, Department of Clinical and Experimental Medicine,
ARNAS Garibaldi Hospital, University of Catania, Catania, Italy.
2 Department of Clinical and Experimental Medicine, Unit of Infectious Diseases, University of Messina, Messina, Italy.
Correspondence to: Benedetto Maurizio Celesia. Division of Infectious
Diseases, Department of Clinical and Experimental Medicine, ARNAS
Garibaldi Hospital, University of Catania, Catania, Italy.
E-mail:
bmcelesia@tin.it
Published: November 1, 2019
Received: July 17, 2019
Accepted: October 10, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019063 DOI
10.4084/MJHID.2019.063
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: CD4+
lymphocyte cell count represents the main immunological marker used to
monitor HIV infection. However, frequent monitoring may be unnecessary,
could cause anxiety to the patient as well as burdening healthcare with
extra expenses.
Objectives and methods:
A two-step retrospective (safety and cost-saving) analysis was
performed to evaluate the probability of maintaining a safe number of
more than 350 CD4+ cells/µl in HIV-positive subjects under treatment
during a three-year follow up and secondarily to estimate in real life
the cost of the CD4+ determinations in a 3 years period, speculating on
possible cost-saving strategies. The safety analyses was conducted with
Kaplan-Meyer method considering: 1) all patients independently from
their viral load (VL); 2) patients with 500 > CD4+ ≥ 350 cells/µl
versus (vs) CD4+ ≥ 500 cells/µl at baseline; 3) patients with VL <
20 copies/ml vs VL > 20 copies/ml. The cost-saving analysis
measuring the costs of CD4+ determinations was calculated from April 1,
2013, to March 31, 2016.
Results:
In the safety analysis, 253 subjects were enrolled. The median CD4+
count was 623 (489-805) cells/µl. Subjects maintaining ≥ 350 cells/µl
in the first, second, and third year were respectively 238 (94.1%), 229
(90.5%), and 226 (89.3%), independently from VL. Within subjects with ≥
350 CD4+/µl vs. ≥ 500 CD4+/µl at baseline, those who maintained ≥ 350
cells/µl until the third year were respectively 241 (95.3%) and 158
(98.1%). The probability of maintaining these values in the third year
was 89.3% for those who had CD4+ ≥ 350/µl at baseline and 98.1% for
those who had CD4+ ≥ 500/µl. This probability was around 90% vs. 99%
for subjects with HIV-RNA above or below 20 copies/ml. In the real-life
cost saving analysis, we evaluated subjects with a stable value or more
than 500 CD4+ (respectively 343, 364 and 383 in the first, second and
third period). We observed mean value of about two determinations
patient/year (2.41 in 2013/2014; 2.32 in 2014/2015; 2.18 in 2015/2016),
with a significant decrease between the first and the last period
(p<0.001). The mean cost patient/year was €101.51 in the first year,
€97.61 in the second, €92.00 in the third (p<0,001). Assuming to
extend these procedures to all our patients with stable CD4+ cells/µl
and monitoring CD4+ cell count once in a year, we were able to obtain
an overall saving of €19,152/year.
Conclusions:
A very high percentage of subjects maintained a high and safe number of
CD4+ cells (>350 cells/µl) during a three-year follow-up. It could
be possible to save up to 66% of the costs by reducing the number of
CD4+ count determinations in a year, to have other favorable
consequences as well, releasing new resources for patient management.
|
Introduction
It is estimated that HIV infection affects about 37 million people worldwide, with 2 million new infections in 2017 only.[1]
To date, CD4+ T-lymphocyte cell count represents the only immunological
diagnostic and prognostic marker validated in randomized clinical
trials and currently used in clinical practice.[2,3]
This parameter gives us information about the level of
immune-depression at baseline and it has led for a long time the
beginning of antiretroviral therapy (cART) administration; at the same
time, CD4+ cell count highlights the timing of opportunistic infections
prophylaxis.[4-6]
A key finding is that
untreated patients have a median decrease of the CD4+ cells count of
about 4% per year,[6] while subjects on ART have an increase of 50-100
cells μl/year;[7] this increase is strictly associated with the CD4+ nadir.[2,8]
Nevertheless, opportunistic infections were reported even with an absolute CD4+ count over the risk threshold (≥ 350 cells/µl),[2,5] although less frequently.[2]
Due to the high variability of absolute numbers, CD4+ percentage should
always be evaluated before clinical or therapeutic strategy
modification: a decrease below 14% is associated with an increased risk
of opportunistic infection.[2]
At this moment,
the key questions are: how to behave if the patient is virologically
suppressed? Is it safe to defer the CD4+ cell count monitoring in
stable patients on ART with ≥350 or 500 cells/µl? The role and
frequency of CD4+ cell count monitoring in stable HIV-infected subjects
remain controversial.[9,10] In 2012 the Department of Health and Human Services guidelines[11,12]
sentenced that “in patients with suppressed viral loads (VL) and a
consistent ART-related immune reconstitution, frequent CD4+ count
monitoring may be un-useful, given the continuous clinically irrelevant
fluctuations, in addition to causing stress and anxiety both in the
patient and in the physician. Therefore, in stable patients whose CD4+
cell count has increased well above the threshold for opportunistic
infection risk, CD4+ count can be monitored less frequently than the VL
(every 6 or 12 months)”.
For this reason, Howard Gale et al.[13]
supported a less frequent CD4+ monitoring in virologically suppressed
patients: they demonstrated that stable patients with VL <200
copies/mL and CD4+ counts ≥300 cells/µL had a 97.1% probability of
maintaining CD4+ ≥200 cells/µL for 4 years; when non-HIV causes of
lymphopenia were excluded, this probability rose to 99.2%.[7]
Caniglia and colleagues[14]
showed that decreasing monitoring frequency when CD4+ cell count>200
cells/μl (compared with when CD4+ cell count>500 cells/μl) results
in an increased risk of virologic failure at 24 months of follow up.
They also have shown that the mean CD4+ cell count at 18 months was
higher than 400 cells/µl in the patients virologically suppressed.
Therefore,
they concluded that less frequent monitoring for subjects on cART, with
confirmed virological suppression, does not change substantially
clinical outcomes in 18 months follow up.
Cost-saving is another considerable aspect: Hyle et al.,[15]
based on the need for redirecting financing in order to improve
healthcare, estimated a potential annual saving of 18 million dollars
by reducing CD4+ cell count monitoring.
The problem is how this
change could affect some clinical decisions, such as when to start ART
or prophylaxis for opportunistic infections (OI).
As far as we
know, rarely does a virologically suppressed patient with ≥300 CD4+
develop an OI or a rapid CD4+ decline (below 200 cells/µl).
Moreover, the most sensitive method to describe a treatment failure is by determining HIV RNA.[11]
For this reason, the CD4+ count is probably required only for those
subjects who are not virologically suppressed and for those with
advanced disease.[16] Thus, why do we keep requesting it? Katz MH said that “it is our habit and patients expect it”.[17]
Unfortunately, nowadays health funds are not unlimited, and we need to
save as much money as possible; this would be a right way to start as
well as a challenge for European and US physicians who deal with
guidelines.
Although different guidelines proposed CD4+ evaluation
every six or twelve months with convenience for patients and public
health system,[2,5] many doctors are not so confident with this strategy.
Objectives
A safety analysis
was conducted in our outpatient unit to measure the probability of
maintaining a safe (above the threshold for OI risk) number of CD4+
(≥350 cells/µl) when more than 350 cells/µl were obtained.
Secondarily, we performed a second cost-saving analysis to
evaluate, in a real-life context, if it could be possible to reduce the
number and the cost of CD4+ count determinations in a three-year
follow-up.
Materials and Methods
A
retrospective monocentric study, including patients on cART with CD4+
cell count steadily over 350 cells/µl in all determinations during
2011, was performed. Subjects with cirrhosis, OIs, active intravenous
drug users (IVDU), pregnant women, patients treated with chemotherapy
or peg-IFN plus ribavirin for HCV infection were excluded. All the
enrolled patients were monitored for 36 months. The whole CD4+ counts
and HIV-RNA determinations recorded were considered. Demographics and
clinical data were also evaluated.
Three analyses (Kaplan Meyer method) were performed considering:
1. all patients independently of HIV RNA levels;
2. patients with CD4+ cell count ranging from 350 to 499 cell/µl vs. patients with more than 500 cell/µl;
3. patients with HIV RNA levels below or above 20 copies/ml.
Statistical comparison of survival curves was performed using the Log-rank (Mantel-Cox) test.
Secondarily,
we retrospectively analysed the number of CD4+ cell count
determinations patients/year and calculated total- and single- patient
costs in all subjects on cART harboring a CD4+ cell count over 350
cells/µl in all determinations performed from April 1, 2013 to March
31, 2016. Three annual interval periods were analyzed. Statistical
analysis was performed using the Kruskal-Wallis test.
Results
In
the first safety analysis, we enrolled 253 patients. At the enrolment
(last visit during the year 2011) median age was 47 (IQR 41-54); 175
(69%) were males. The median CD4+ T-cells count was 623 cells/µl (IQR
489-805); 161 (63.6%) had CD4+ >500 cells/µl. 199 (78.7%) subjects
had HIV-RNA <50 copies/ml, 161 (63.3%) HIV-RNA <20 copies/ml.
Overall, 2613 samples were analyzed, with a median of 10.3 samples per
patient (Table 1).
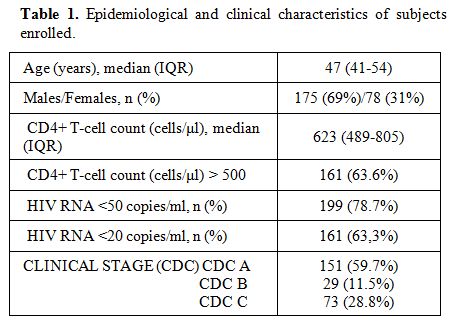 |
Table 1. Epidemiological and clinical characteristics of subjects enrolled. |
Among
subjects with more than 350 CD4+ lymphocyte/µl at baseline,
independently of HIV RNA levels, those with more than 350 CD4+ cell/µl
in all determinations in the first, second and third year were
respectively 238 (94.1%), 229 (90.5%), 226 (89.3%). (Figure 1).
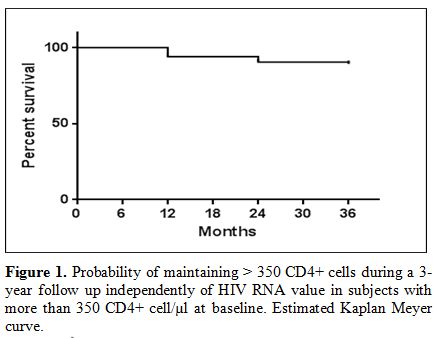 |
Figure
1. Probability of maintaining > 350 CD4+ cells during a 3-year
follow up independently of HIV RNA value in subjects with more than 350
CD4+ cell/µl at baseline. Estimated Kaplan Meyer curve. |
Within
the 161 subjects with CD4+ ≥500 cells/µl at baseline, 159 (98.7%), 159
(98.7%), and 158 (98.1%) respectively maintained CD4+ ≥350 cells/µl
during the first, second and third year.
Among 161 subjects with
more than 500 CD4+ cell count/µl at baseline, those who maintained more
than 500 cells/µl in all determinations in the first, second, and third
year were respectively 144 (89.4%), 139 (86.3%) and 138 (85.7%). The
probability of maintaining more than 350 cells/µl during a three-year
follow-up, regardless of HIV-RNA levels, was 89.3% for those who had
more than 350 CD4+ T-cells/µl at baseline and 98.1% for those who had
more than 500 cells/µl at baseline (Figure 2).
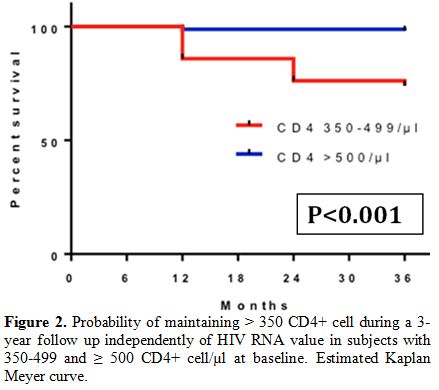 |
Figure 2. Probability of
maintaining > 350 CD4+ cell during a 3-year follow up independently
of HIV RNA value in subjects with 350-499 and ≥ 500 CD4+ cell/µl at
baseline. Estimated Kaplan Meyer curve. |
Within
the subjects with more than 350 or 500 CD4+ cells/µl and VL below 20
copies/ml at baseline (respectively 161 and 108), those who maintained
more than 350 CD4+ in all determinations in the first, second and third
year were respectively 152 (94.4%), 145 (90.0%), 144 (89.4%) and 107
(99.1%), 107 (99.1%), 107 (99.1%).
Out of 108 subjects with more
than 500 CD4+ cells at baseline and HIV-RNA below 20 copies/ml, those
who maintained more than 500 CD4+ at in the first, second and third
year were respectively 97 (89.8%), 97 (89.8%), 97 (89.8%).
The
probability of maintaining more than 350 cells/µl during a three-year
follow up for patients with HIV-RNA below 20 copies/ml at baseline was
around 90% for subjects with more than 350 CD4+ cells at baseline, and
around 99 % for subjects with more than 500 CD4+ cells at baseline.
This probability was lower for those who had a VL above 20 copies/ml (Figure 3).
 |
Figure 3. Probability of
maintaining > 350 CD4+ cells during a 3-year follow up in patients
with HIV RNA value more or below 20 copies/ml and with > 350 CD4+
cell/µl at baseline. Estimated Kaplan Meyer curve. |
Then,
we performed a cost-saving analysis, evaluating data from all subjects
with a stable value of 500 or more CD4+/µl throughout three years. They
were 343 in 2013/2014, 364 in 2014/2015, and 383 in 2015/2016; the
total number of determinations was respectively 829, 846, 839.
We
calculated a mean of 2.41 determinations per patient in the first
period, 2.32 in the second period, and 2.18 in the third period, with a
significant decrease between the first and the third period
(p<0,001).
The cost of a CD4+ determination laboratory kit is
about €42 times the whole number of determinations done each year; in
this way, we calculated the total spending for each period that is
€34,818 in 2013/2014, €35,532 in 2014/2015 and €35,238 in 2015/2016.
The
cost rose in the last period compared to the first, but there was an
increase in the outpatient number at the same time. We compared data
about the cost patient/year and we found a mean value of €101.51 in the
first period, €97.61 in the second and €92.00 in the third, with a
saving of €9.51 per patient. The median value was slightly less than
€100.00 per patient per year, with a significant decrease between the
first and the third year (p<0.0001).
Discussion
Tolerability
and potency of the new antiretroviral drugs, associated with the
increasing number of simple regimens based on a single pill or single
dose per day, represent an incredible opportunity for all patients in
cART to obtain and maintain good adherence and optimal
viro-immunological control over a long period.
The acceptance of
HIV infection and the reduction of HIV-related stigma could be improved
by reducing patients’ access to hospitals for blood testing or
pharmacological refill. Reducing CD4+ count determinations could help
with this purpose.
Absolute CD4+ cell number fluctuations are frequent, but their clinical significance is irrelevant.[18]
In
most cases, CD4+ cell percentage remains unmodified and any changes of
absolute CD4+ cell count are due to total leucocytes and/or lymphocytes
count oscillations, while the anxiety of patients frequently increases,
thus defining the request of a new CD4+ count determination. However,
the predictive value of these fluctuations remains inconsistent.[18]
It
was surprising to find that such a high number of subjects maintained a
stable and safe number of CD4+ cells during a three-year follow up
after they had already obtained the threshold of 350 CD4+ cells/µl.
Our follow up, conducted for a time double in length in comparison to the study of Mocroft et al,[7]
confirms that also in a longer follow up, the decrease of CD4+ is an
infrequent event, which becomes rare if more than 500 cells/µl are
obtained.
Even if HIV replication is not entirely suppressed,
our data showed that the probability of maintaining stable CD4+ cell
counts is high in these subjects.
Although our approach was very
conservative and prudential, the results confirm that a single
determination of CD4+ count in a year could not reduce the quality of
assistance and that the probability of having a value below the cut-off
of 350 cells/µl during the follow up is very low. Furthermore, we
cannot exclude that some of these low values are only insignificant
fluctuations.
More recently, researchers[19,20]
showed a predictive role for CD4+/CD8 ratio in disease progression and
immune activation, also in patients with undetectable HIV RNA.[21]
This issue remains controversial as debatable is, at the same time, the
selection of patients to address to this evaluation. However, the
reduction of CD4+ determinations could limit the recording of this data
for future analyses.
In the last years, because of the approval of new therapeutic guidelines,[22] more and more patients have begun the treatment earlier, regardless of baseline CD4+ count or HIV RNA viremia.
In
2014, in our setting, more than 72% of patients in treatment had
>500 CD4+/µl and therefore monitoring CD4+ cell count once a year
could be a safe strategy.
Although the safety was clearly
demonstrated, the cost-saving appears controversial. In fact, in the
cost-saving sub-study, during the three-year follow up we observed a
progressive light decrease of CD4+ determinations per patient for year.
These results were linked to the progressive change in the follow-up
strategies applied in the last years for stable patients, which
established the delay of blood testing every six months or more.
Although
this trend was statistically significant, more than 50% of patients
enrolled still do more than two CD4+ determinations in a year, so the
magnitude of the financial result was not as significant as we
expected. By extending these procedures to all patients with stable
CD4+ cells/µl and by monitoring CD4+ cell count once a year, we could
obtain, in this cohort, an overall saving of €19,152/year.
In
Italy more than 100,000 patients are in cART, more than 90% of them
show an HIV-RNA viremia below the cut off of detectability and more
than 75% present a CD4+ count above 350 cells/µl;[23]
taking these numbers into account, the strategy of reducing CD4+ cell
determinations could represent a considerable opportunity for saving
resources.
A scenario in which just more than 50% of patients
could be tested once a year could determine a considerable reduction of
costs and the opportunity of employing these funds to monitor other
markers.
Giving a precise evaluation of total cost saving is
complicated; different centers applied different follow-up strategies
(only CD4+; CD4+ and CD8+; CD3+, CD4+, and CD8+; or more complex
immunological panels). Moreover, the cost of the same single
determination could vary in different laboratories or geographic areas.
Also, the strategies to redirect saved money to alternative uses (from
management to treatment or assistance) could be controversial,
depending on each hospital's economic policy.
Conclusions
In
patients who have already achieved virological suppression with a
stable cART regimen, the role of CD4+ testing is questionable.[24]
Our
findings highlighted that a very high percentage of subjects maintained
a high and safe number of CD4+ cells (>350 cells/µl) during a
three-year follow-up. This probability is slightly less than 100% for
subjects with more than 500 cells/µl at baseline.
Many studies
suggest that, although most patients reach a CD4+ count greater than
500 cells/μL after several years of cART, CD4+ count restoration is
variable, and a few patients might fail to recovery despite virological
suppression.[25,26] Another critical fact is that the
variability only in CD4 recovery, with suppressed HIV-RNA, would not
change treatment decisions because there is no evidence for changing
cART in those with divergence between CD4+ and HIV-RNA.[27]
Furthermore,
the costs of CD4+ tests vary depending on the different laboratories,
but it is possible to save from 33% to 66% of the cost by reducing the
number of determinations in a year. This saving could have other
favorable consequences as well, providing new resources for patient’s
management. Although a progressive and significant reduction of CD4+
count determinations costs could be achieved in some laboratories, the
patient’s anxiety related to CD4+ fluctuations remained insolvable.
Nevertheless, it is time to rethink our strategies for reducing the
amount of CD4+ determinations conducted in a year.[24]
Acknowledgments
We thank Dr. Pietro Leanza for his kind English revision.
References
- UNAIDS. 2017 Global HIV Statistics. Fact sheet (2018).
- Antinori,
A. et al. Italian guidelines for the use of antiretroviral agents and
the diagnostic-clinical management of HIV-1 infected persons. Update
2016. New Microbiol. (2017).
- Venanzi
Rullo, E. et al. "Genetic evidence that Naïve T cells can contribute
significantly to the HIV intact reservoir: time to re-evaluate their
role". Clin. Infect. Dis. (2019). https://doi.org/10.1093/cid/ciz378 PMid:31063189
- Marino,
A. et al. Rapid emergence of cryptococcal fungemia, Mycobacterium
chelonae vertebral osteomyelitis and gastro intestinal stromal tumor in
a young HIV late presenter: A case report. BMC Infect. Dis. (2018). https://doi.org/10.1186/s12879-018-3573-z PMid:30587143 PMCid:PMC6307234
- EACS. European AIDS Clinical Society Guidelines Version 9.0 October 2017. European AIDS Clinical Society (EACS) (2017).
- Kaufmann,
G. R. et al. CD4 T-lymphocyte recovery in individuals with advanced
HIV-1 infection receiving potent antiretroviral therapy for 4 years:
The Swiss HIV cohort study. Arch. Intern. Med. (2003). https://doi.org/10.1001/archinte.163.18.2187 PMid:14557216
- Mocroft,
A. et al. Estimated average annual rate of change of CD4 + T-cell
counts in patients on combination antiretroviral therapy. Antivir.
Ther. (2010). https://doi.org/10.3851/IMP1559 PMid:20587849
- Nozza, S. et al. Antiretroviral therapy in geriatric HIV patients: The GEPPO cohort study. J. Antimicrob. Chemother. (2017). https://doi.org/10.1093/jac/dkx282 PMid:29091220
- Ford,
N., Meintjes, G., Vitoria, M., Greene, G. & Chiller, T. The
evolving role of CD4 cell counts in HIV care. Current Opinion in HIV
and AIDS (2017). https://doi.org/10.1097/COH.0000000000000348 PMid:28059957
- Hamers,
R. L. et al. Cost-effectiveness of laboratory monitoring for management
of HIV treatment in sub-Saharan Africa: A model-based analysis. AIDS
(2012). https://doi.org/10.1097/QAD.0b013e3283560678 PMid:22695297
- Panel
on Clinical Practices for Treatment of HIV infection. Panel on
Antiretroviral Guidelines for Adults and Adolescents. Guidelines for
the use of antiretroviral agents in HIV-1-infected adults and
adolescents. Rev. Panam. salud pública = Pan Am. J. public Heal.
(2011).
- Ford,
N. et al. CD4 changes among virologically suppressed patients on
antiretroviral therapy: A systematic review and meta-analysis. Journal
of the International AIDS Society (2015). https://doi.org/10.7448/IAS.18.1.20061 PMid:26257204 PMCid:PMC4530137
- Gale,
H. B. et al. Is frequent CD4+ T-lymphocyte count monitoring necessary
for persons with counts ≥300 cells/μL and HIV-1 suppression? Clin.
Infect. Dis. (2013). https://doi.org/10.1093/cid/cit004 PMid:23315315 PMCid:PMC3693489
- Caniglia,
E. C. et al. When to monitor CD4 cell count and HIV RNA to reduce
mortality and aids-defining illness in virologically suppressed
hiv-positive persons on antiretroviral therapy in high-income
countries: A prospective observational study. J. Acquir. Immune Defic.
Syndr. (2016).
- Hyle,
E. P., Sax, P. E. & Walensky, R. P. Potential Savings by Reduced
CD4 Monitoring in Stable Patients With HIV Receiving Antiretroviral
Therapy. JAMA Intern. Med. (2013). https://doi.org/10.1001/jamainternmed.2013.9329 PMid:23978894 PMCid:PMC3980729
- Johnson,
L. F. et al. Life Expectancies of South African Adults Starting
Antiretroviral Treatment: Collaborative Analysis of Cohort Studies.
PLoS Med. (2013). https://doi.org/10.1371/journal.pmed.1001418 PMid:23585736 PMCid:PMC3621664
- Katz, M. H. Directing Resources to Where They Are the Most Needed. JAMA Intern. Med. (2013). https://doi.org/10.1001/jamainternmed.2013.8590
- Young,
B., Ng, O. T., Lye, D. C. & Leo, Y. S. Derivation and validation of
an accurate estimation of CD4 counts from the absolute lymphocyte count
in virologically suppressed and immunologically reconstituted HIV
infected adults. BMC Infect. Dis. (2015). https://doi.org/10.1186/s12879-015-1079-5 PMid:26268903 PMCid:PMC4535254
- Mussini,
C. et al. CD4/CD8 ratio normalisation and non-AIDS-related events in
individuals with HIV who achieve viral load suppression with
antiretroviral therapy: An observational cohort study. Lancet HIV
(2015). https://doi.org/10.1016/S2352-3018(15)00006-5
- Chereau,
F. et al. Impact of CD4 and CD8 dynamics and viral rebounds on loss of
virological control in HIV controllers. PLoS One (2017). https://doi.org/10.1371/journal.pone.0173893 PMid:28380038 PMCid:PMC5381858
- Bellissimo,
F., Rita Pinzone, M., Maurizio Celesia, B., Cacopardo, B. &
Nunnari, G. Baseline CD4/CD8 T-Cell Ratio Predicts Prompt Immune
Restoration Upon cART Initiation. Curr. HIV Res. (2016). https://doi.org/10.2174/1570162X14666160414111554 PMid:27074946
- Ryom,
L. et al. Highlights of the 2017 European AIDS Clinical Society (EACS)
Guidelines for the treatment of adult HIV-positive persons version 9.0.
HIV Med. (2018).
- Camoni,
L. et al. Estimating minimum adult HIV prevalence: a cross sectional
study to assess the characteristics of people living with HIV in Italy.
AIDS Res. Hum. Retroviruses (2014). https://doi.org/10.1089/aid.2014.0154 PMid:25432098 PMCid:PMC4348082
- Ford, N. et al. The future role of CD4 cell count for monitoring antiretroviral therapy. The Lancet Infectious Diseases (2015). https://doi.org/10.1016/S1473-3099(14)70896-5
- Tuboi,
S. H. et al. Mortality associated with discordant responses to
antiretroviral therapy in resource-constrained settings. J. Acquir.
Immune Defic. Syndr. (2010). https://doi.org/10.1097/QAI.0b013e3181c22d19 PMid:20035163 PMCid:PMC2802453
- Ford,
N. et al. The future role of CD4 cell count for monitoring
antiretroviral therapy. Lancet Infect. Dis. 15, 241-247 (2015). https://doi.org/10.1016/S1473-3099(14)70896-5
- Gaardbo,
J. C., Hartling, H. J., Gerstoft, J. & Nielsen, S. D. Incomplete
immune recovery in HIV infection: Mechanisms, relevance for clinical
care, and possible solutions. Clinical and Developmental Immunology
(2012). https://doi.org/10.1155/2012/670957 PMid:22474480 PMCid:PMC3312328



