Nikolaos Papadopoulos1, Dimitrios Kountouras2, Katerina Malagari3, Maria Tampaki2, Maria Theochari2 and John Koskinas2.
1 1st Department of Internal Medicine, 417 Army Share Fund Hospital of Athens.
2 2nd
Department of Medicine, National and Kapodistrian University of Athens,
Medical School, Hippokration General Hospital of Athens.
3 2nd and 1st Department of Radiology, National and Kapodistrian University of Athens, Medical School, Evgenidion Hospital of Athens.
Corresponding
author: Nikolaos Papadopoulos M.D., Ph.D. Tel: +302117100671. E-mail:
nipapmed@gmail.com
Published: March 1, 2020
Received: September 9, 2019
Accepted: February 4, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020013 DOI
10.4084/MJHID.2020.013
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background/Aim:
The incidence of hepatocellular carcinoma (HCC) in patients with
transfusion dependent thalassemia (TDT) has been increasing, where
viral hepatitis and iron overload are the two established HCC risk
factors. The aim of this study was to investigate the etiological
factors of HCC development and to evaluate the possible factors
associated with survival in our cohort of TDT patients with HCC.
Methods:
Records of patients with TDT diagnosed with HCC from 2008 to 2018 were
reviewed. Liver iron concentration (LIC) has been assessed by the
signal-intensity-ratio MRI. The diagnosis of HCC was made by a 3-phase
contrast magnetic resonance imaging (MRI) and patients were staged and
treated for HCC according to Barcelona Clinic Liver Cancer (BCLC)
grading system.
Results:
Forty-two TDT patients with HCC have been included. Most of them
(78.5%) were anti-HCV positive, 59.5% HCV-RNA positive, and 16.5% had
serological markers of resolved HBV infection. Patients with HCV
infection have been treated successfully with either Peg-IFNa±Ribavirin
or with the new direct antivirals (DAAs). At the time of HCC diagnosis,
all patients with chronic HCV infection were HCV-RNA negative, 78.5%
had underlying cirrhosis, and the vast majority (98%) had average or
mild elevated LIC values. According to the BCLC system, patients were
classified as 0-A: 28.5%, B: 57% and C-D: 14.5%. HCC has been
treated with loco-regional treatment in 78.5% of our patients, while
the rest have received sorafenib. Twenty-eight patients (66.5%) died
due to HCC with a median survival time of 6 months (range: 2-60). Using
the Cox proportional hazard model, the only factors associated with
poor survival were BCLC stages C and D.
Conclusions: In conclusion, BCLC staging is the main prognostic factor of survival in patients with TDT who develop HCC.
|
Introduction
The
incidence of hepatocellular carcinoma (HCC) in patients with
thalassemia has been increasing in recent years, where chronic viral
hepatitis and iron overload are the two established HCC risk factors in
this particular patient population.[1] Importantly all these factors are both preventable and treatable.
Both
alpha- and beta-thalassemia are more prevalent in tropical and
subtropical regions of the world. The southern regions of Europe, such
as Italy and Greece, are the most likely areas to be affected in
Europe. Greece, a country of approximately 11 million people, has a
mean frequency of thalassaemia carriers at 7%.[2]
The
role of viral hepatitis as a risk factor for hepatocarcinoma is
essential in thalassemia, mainly in older patients, since the risk of
viral transmission through blood transfusion was significantly reduced
after the 1990s with the identification of the HCV virus and the
universal screening of blood donors.[3] The estimation
of the current prevalence of Hepatitis C in Greece ranges from 0.5% to
2% according to the population studied, while the prevalence of HBsAg
was 0.84% in a 6-year blood donor based study in Athens.[4,5]
HBV-induced hepatocarcinogenesis is a multifactorial process that
involves the presence of chronic hepatitis and cirrhosis and also the
direct role of hepatitis B virus (HBV), development of HCC in the
absence of cirrhosis, mainly through HBV-DNA integration into the host
DNA which alters the function of endogenous genes or induces
chromosomal instability.[6] On the other hand,
HCV-induced hepatocarcinogenesis of HCC is a gradual process that
relates to chronic hepatitis and the duration of disease leading to
liver cirrhosis.[7-10] Furthermore, the risk of HCC is
greatly reduced in HCV cirrhotic patients who obtained a sustained
virological response (SVR) after HCV treatment but is not eliminated.[11,12]
Both
the incidence and the etiology of HCC changed over the last 25 years.
In a recently published study performed in Crete island, authors
indicate a rose from 6.0 new cases per 100,000 inhabitants in the first
five-year period (1990-1994) to 16.8 in the last five years
(2010-2014). The change was mostly attributable to a gradual increase
in the incidence of HB, alcohol and non-alcoholic fatty liver disease
(NAFLD) related cases, especially during the last decade.[13]
The
hepatocarcinogenicity of iron seems to be a very complex phenomenon. It
is well established that patients with hereditary hemochromatosis have
a risk of developing HCC even in the absence of cirrhosis.[14]
Even more, several reports have described the development of HCC in
non-cirrhotic patients with thalassemia syndromes who were negative for
HBV and HCV but had a hepatic iron overload. The primary hypothesis is
that free iron, even in the absence of cirrhosis, is hepatocarcinogenic
due to the generation of reactive oxygen species (ROS), which causes
peroxidation of membrane fatty acids that impair protein synthesis and
disrupt DNA synthesis.[15]
Data concerning
etiological factors and treatment outcomes of HCC appear to be lacking
in patients with transfusion dependent thalassemia (TDT). The aim of
this study was to investigate the etiological factors of HCC
development and to evaluate the possible factors associated with
survival among Greek patients with TDT and HCC.
Patients and Methods
We
review the records of all patients with TDT who developed HCC from a
referral tertiary liver center of Athens from 2008-2018. The database
included patient demographic and epidemiological characteristics,
medical history data, as well as clinical and laboratory data. All
patients were under systemic transfusions at 2-4-wk intervals (age of
initial treatment: 3 months to 5 years) and iron chelation therapy
either with deferoxamine (DFO) or deferiprone (DFP) or deferasirox
(DFX) (age of initial treatment: 2-15 years). Hematological
/biochemical parameters including ferritin levels, serological markers
of hepatitis B (hepatitis B surface antigen, antibodies to hepatitis B
surface and core antigens) and hepatitis C (antibodies to hepatitis C
virus) have been determined by commercially available assays. Serum
α-fetoprotein (αFP) levels have been determined by a commercially
available assay (R&D) with a lower cut-off value of 20 ng/mL. All
anti-HCV positive patients were further evaluated with HCV-RNA by
commercially available quantitative PCR assays (COBAS TaqMan HCV v1.0
or v2.0; Roche Diagnostics) with a lower limit of detection of 43 and
15 IU/ml respectively.
Τhe average of the last four measurements
before the diagnosis of HCC has been used to represent the levels of
ferritin and hemoglobin for the purpose of our analysis. All included
patients in the study had serum ferritin levels less than 1,000 ng/ml.
Hepatic magnetic resonance imaging (MRI) is now considered the gold
standard method for estimating and monitoring liver iron concentration
(LIC) in these patients. Thus, liver iron overload has been assessed by
the signal-intensity-ratio MRI (Rennes University algorithm) at the
time of HCC diagnosis.[16,17] Patients with LIC
values ≤40μmolFe/g were considered as normal, those with LIC values
between 40 and ≤100 μmolFe/g as having mild hemosiderosis, those with
LIC values between 100 and ≤200 μmolFe/g as having moderate
hemosiderosis and those with LIC values above 200 μmolFe/g as having
severe iron overload.[16] Liver cirrhosis has been
diagnosed using imaging methods (computed tomography scan or liver
ultrasound), while in four of them (9.5%) has been documented by liver
biopsy. The diagnosis of HCC was done by magnetic resonance imaging
(MRI) with contrast (3 phase) and was confirmed by guided liver biopsy
in uncertain by MRI cases. All patients were assessed using Barcelona
Clinic Liver Cancer (BCLC) grading system and were classified as very
early or early stage (0-A), intermediate stage (B), and as advanced or
terminal stage (C-D).[18] The very early stage (BCLC
0) is defined as the presence of a single nodule < 2 cm in diameter
in patients with well-preserved liver function (Child-Pugh A). The
early stage (BCLC A) corresponds to patients with one nodule < 5 cm
or up to three nodules each < 3 cm. Patients with BCLC stages 0 and
A are candidates for potentially curative treatment options such as
surgical resection, liver transplantation, or local radiofrequency
ablation (RFA). The intermediate stage (BCLC B) includes asymptomatic
patients with large or multifocal tumors. The advanced stage (BCLC C)
concerns patients with cancer-related symptoms, macrovascular invasion,
or extrahepatic spread. Patients with BCLC B and C are treated with
palliative approaches, such as transarterial chemoembolization (TACE)
or systemic therapy with sorafenib. Patients in the terminal stage
(BCLC D) receive best supportive care.[19] The study
has been performed according to the World Medical Association
Declaration of Helsinki and has been approved by the hospital ethics
committee. All patients were informed and consent to access their
confidential data from the hospital’s medical records.
Statistical analysis.
All data were analyzed using the statistical package MedCalc (version
18.11). Statistical analysis was performed using a t-test for
comparisons of continuous variables between groups and corrected
chi-squared test for comparisons of qualitative data. A two-tailed p
value <0.05 was considered to be statistically significant. The
survival time for each patient, in terms of months, was defined as the
interval between the dates of HCC diagnosis and the date of death or
the end of the study. Cumulative survival rates were estimated using
the Kaplan-Meier method, while cox proportional hazards model was also
used to identify risk factors relating to survival.
Results
In
total, 42 patients were included. Their mean age was 45.5±5.8 years,
whereas 27 of them (64.5%) were males. At the time of HCC diagnosis,
most of the patients (78.5%) were diagnosed with compensated cirrhosis
(Child-Pugh A).
Out of 42 patients, 33 (78.5%) were anti-HCV
positive. Most of them 25/42 (59.5%) were HCV-RNA positive within the
last 10 years. The rest of the anti-HCV positive patients (8/42) were
always HCV-RNA negative indicative of spontaneous viral clearance after
exposure to the virus. All viraemic patients have been treated either
with Pegylated - interferon alpha (Peg-IFN) ± ribavirin (RBV) [8/25,
(32%)] or with the new interferon-free, direct acting antivirals (DAAs)
regimens [17/25, (68%)]. All patients had achieved sustained
virological response (SVR) at least 12 months before HCC diagnosis.
Regarding
HBV infection, none of our patients was HBsAg positive. Thus, no one
had received anti-viral treatment. However, 7 patients (16.5%) had
serological evidence of resolved HBV infection
[HBsAg(-)/anti-HBc(+)/anti-HBs(+)] without prior anti-HBV treatment.
All of these patients had a history of acute HBV infection, and
finally, they have achieved HBsAg clearance.
The mean LIC values
were 48.6±29.5 μmolFe/g. More than half of our patients (55%) had
average LIC values, 18 patients (43%) were classified as having mild
hemosiderosis and only one patient (2%) has been classified as having
moderate hemosiderosis.
αFP levels have been assessed in all patients and were ≤100 ng/ml in 26 of them (62%).
The
tumor has been diagnosed as multifocal (≥3) in 25 patients (59.5%),
while those with maximum diameter ≥5cm have been diagnosed in 18
patients (43%). According to the BCLC grading system, patients were
classified as 0-A: 12/42 (28.5%), B: 24/42 (57%) and as C-D: 6 patients
(14.5%).
HCC has been treated with TACE or RFA in 33 patients
(78.5%) while the rest 9 patients were treated with sorafenib. Two
patients with very early stage and no other contraindication underwent
surgical excision but eventually, they have been treated with
loco-regional therapies due to HCC relapse in the first six months.
These two patients have been included in TACE/RFA group.
Overall,
28 patients (66.5) have eventually died due to HCC, with a median
survival time of 6 months (2-60), while a median survival time of 60
months (6-96) has been observed in still living patients at the end of
the study (Table 1).
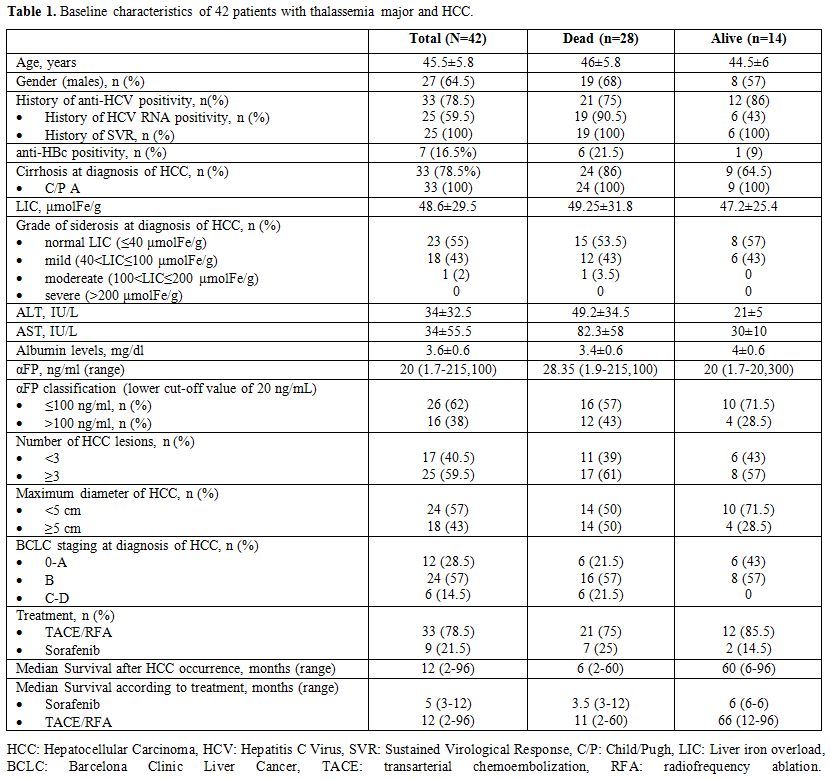 |
Table
1. Baseline characteristics of 42 patients with thalassemia major and HCC. |
The overall Kaplan-Meier survival curve of the patients is shown in figure 1. In order to reveal possible risk factors relating to survival, we used the cox proportional hazard model adjusted for age (Table 2). BCLC stages C and D were associated with poor survival (HR: 5.4179, 95% CI: 1.4936-19.6531, p=0.0102) (Figure 2).
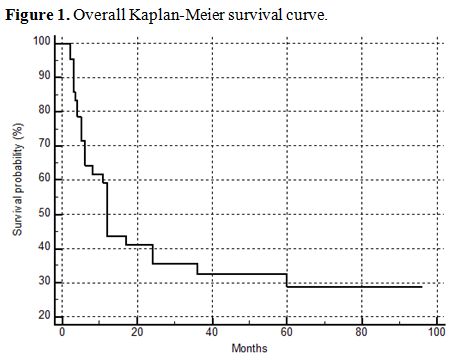 |
Figure 1.
Overall Kaplan-Meier survival curve. |
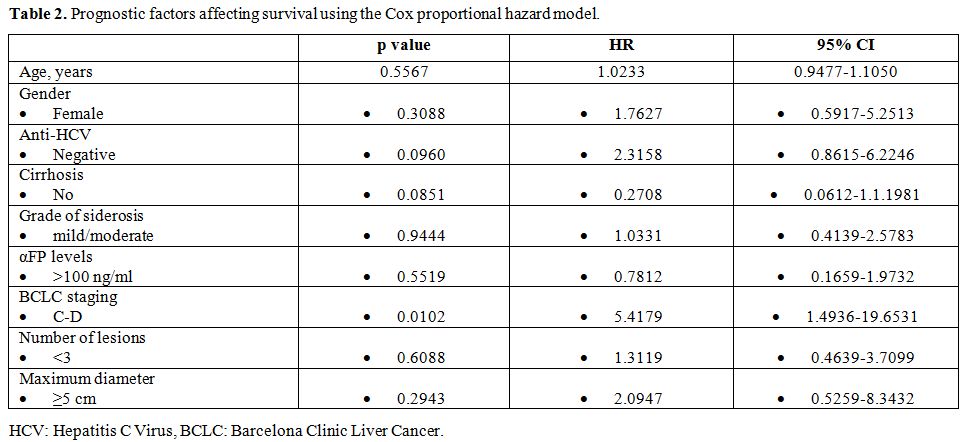 |
Table 2. Prognostic factors affecting survival using the Cox proportional hazard model. |
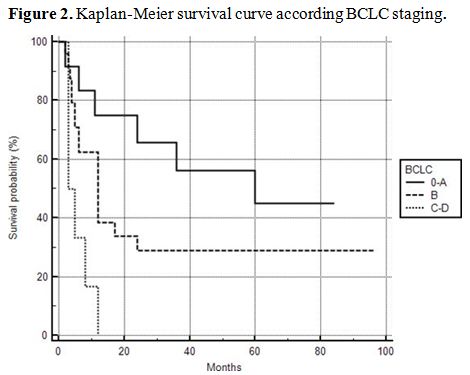 |
Figure 2. Kaplan-Meier survival curve according BCLC staging. |
Discussion
In
patients with TDT, the development of HCC represents evolving morbidity
and mortality in the last years. The main risk factors for HCC are
chronic iron accumulation, chronic HCV or HBV infection and cirrhosis.
It is well established that patients with hereditary hemochromatosis
have an increased risk of HCC irrespective of the presence of
cirrhosis.[14,15] Patients with TDT are exposed, at a
very young age, to iron through transfusions, and most studies,
including ours, denote that HCC develops below 50 years of age.[20,21]
Free iron induced oxidative stress and increased levels of ROS lead to
mitochondrial injury, DNA damage and consequently, to
hepatocarcinogenesis.[22] In agreement with these
reports, our patients had a mean age of 45.5±5.8 years old. However,
all of them were under iron chelation treatment, had serum ferritin
levels less than 1,000 ng/ml and the vast majority did not have (55%)
or had only mild hemosiderosis (43%) at the time of HCC diagnosis.
However, the amount and duration of exposure to excess iron are crucial
to the development of liver injury. Moreover, the association between
the iron overload and the development of HCC has been confirmed in
studies in rats fed a high-iron diet. After 15 months, the iron-loaded
liver developed HCC in the absence of cirrhosis.[23]
Furthermore, while TDT outcomes have been improving in recent years,
particularly those related to heart disease due to iron chelation
treatments, HCC has emerged as a new complication of liver disease.[3,24]
Both chronic viral hepatitis B and C and related cirrhosis remain important risk factors for the development of HCC.[21] In our study 7 patients had serological markers of past HBV infection [HBsAg
(-)/anti-HBc(+)/anti-HBs(+)]
but all of them were cirrhotics. Theoretically, the
hepatocarcinogenicity of HBV remains in patients with resolved HBV
infection (spontaneous seroconversion of HBsAg) mainly through HBV-DNA
integration into the host genome. However, the risk of HCC is
significantly lower in HBsAg negative than in HBsAg positive patients
and the presence of liver cirrhosis is the dominant risk factor for
hepatocarcinogenesis.[25] With respect to chronic HCV
infection, most of our patients were anti-HCV positive (78.5%) of whom
76% had chronic HCV infection. All of them have eventually been treated
and finally achieved SVR at least 12 months before HCC diagnosis.
However, most of them were cirrhotics (79%) at that time. It is well
known that cirrhosis is the main risk factor for HCC in CHC and all
patients with cirrhosis should be closely monitored and followed even
after successful antiviral therapy.[26]
Data
concerning possible factors associated with the survival rate among
patients with TDT who develop HCC remain unclear. It is well
established that the development of HCC is the main factor affecting
the survival of patients with chronic liver disease.[27,28]
The prognostic factors for survival in patients with HCC are related to
tumor status (number and size of nodules, presence of vascular
invasion, extrahepatic spread), liver function and general
tumor-related health status.[18] Previous
population-based data from Italy suggested that in patients with TDT
and HCC, the average survival was 3.5 months in 2004 and rose to 11.5
months in 2014.[3,24] In our cohort,
the analysis, after adjusting for age, has indicated that advanced BCLC
stages were associated with poor survival. To our knowledge, this is
the first real-life study evaluating prognostic factors affecting the
survival of TDT patients with HCC.
The decision of the HCC
treatment method was mainly based on the BCLC algorithm. However, we
have to keep in mind that TDT has been considered as a relative
contraindication for liver transplantation and this therapeutic
approach was not available in our patients with early stage HCC.
Moreover, major operations like surgical HCC resections are limited due
to the problematic peri-operative management of these patients.[29]
Only two of our patients underwent resection of the tumor as initial
treatment but both were relapsed within the next six months and were
subsequently treated with loco-regional therapies. According to BCLC,
systemic algorithm therapy is indicated in the advanced stage of the
disease. In general, these patients bear a poor prognosis, with
expected median survival times of 6–8 months.[30] A
large double-blinded placebo-controlled phase III study showed that the
median overall survival of patients in the sorafenib group was 10.7
months compared to 7.9 months in the placebo group (HR, 0.69; 95% CI
0.55–0.87; p = 0.00058), representing a 31% decrease in the relative
risk of death.[31] In our study, these patients
achieved a median survival period of 5 months (3-12). Patients with
early stage HCC treated with RFA have an overall median survival of
about 36 months which may extend to >5 years after successful
treatment.[30] Patients with intermediate stage HCC
by BCLC treated with TACE have an overall survival of about 16 months
which may extend to >40 months in well selected patients.[32,33]
Our patients who have been treated with TACE/RFA achieved an overall
survival of 12 months (2-96) which is much lower than expected. A
possible explanation is that mortality in these patients may be
influenced by several other complications and also that the biological
activity of HCC in these patients is more aggressive, as can be
hypothesized by the very high αFP levels.
The absence of a
comparable group of patients with TDT without HCC, which would further
provide robustness on the estimation of surveillance of HCC, is the
main limitation of our study. We did demonstrate, however, the
characteristics and risk factors of HCC and the main prognostic factors
of survival in this specific group of patients.
Conclusions
In
conclusion, in patients with TDT, the development of HCC represents
evolving morbidity and mortality in the last years. The main
etiological factors for HCC are chronic iron accumulation in the liver,
chronic HCV infection and cirrhosis. This population with HCC has
discrete characteristics compared to the general population, such as
young age at presentation of HCC and various comorbidities that limit
the therapeutic options particularly transplantation and surgery. Since
the HCC stage seems to be the major prognostic factor of survival, a
personalized approach of surveillance is mandatory taking into
consideration individual patient’s comorbidities. Once a surveillance
test is positive, a more definitive imaging examination is recommended
for noninvasive diagnosis and staging of HCC.
References
- Moukhadder HM, Halawi R, Cappellini MD, Taher AT.
Hepatocellular carcinoma as an emerging morbidity in the thalassemia
syndromes: a comprehensive review. Cancer 2017;123:751-758. https://doi.org/10.1002/cncr.30462 PMid:27911488
- Loukopoulos D. Haemoglobinopathies in Greece: prevention programme over the past 35 years. Indian J Med Res 2011;134:572-6
- Borgna-Pignatti
C, Garani MC, Forni GL, Cappellini MD, Cassinerio E, Fidone C, Spadola
V, Maggio A, Restivo Pantalone G, Piga A, Longo F, Gamberini MR, Ricchi
P, Costantini S, D'Ascola D, Cianciulli P, Lai ME, Carta MP, Ciancio A,
Cavalli P, Putti MC, Barella S, Amendola G, Campisi S, Capra M, Caruso
V, Colletta G, Volpato S. Hepatocellular carcinoma in thalassaemia: an
update of the Italian Registry. Br J Haematol. 2014;167:121-126. https://doi.org/10.1111/bjh.13009 PMid:24992281
- Nikolopoulou G, Zisouli A. Viral hepatitis. Available from: http://www.keelpno.gr/en-us/home.aspx
- Kyriakis
KP, Foudoulaki LE, Papoulia EI, Sofroniadou KE. Seroprevalence of
hepatitis B surface antigen (HBsAg) among first-time and sporadic blood
donors in Greece: 1991-1996. Transfusion Medicine. 2000;10:175-180. https://doi.org/10.1046/j.1365-3148.2000.00257.x PMid:10972911
- Xu
HZ, Liu YP, Guleng B, Ren JL. Hepatitis B Virus-Related Hepatocellular
Carcinoma: Pathogenic Mechanisms and Novel Therapeutic Interventions.
Gastrointest Tumors 2014;1:135-45. https://doi.org/10.1159/000365307 PMid:26676160 PMCid:PMC4645579
- Prati
D, Zanella A, Farma E, De Mattei C, Bosoni P, Zappa M, Picone A, Mozzi
F, Rebulla P, Cappellini MD, Allain JP, Sirchia G. A multicenter
prospective study on the risk of acquiring liver disease in
anti-hepatitis C virus negative patients affected from homozygous
b-thalassemia. Blood. 1998;92:3460-3464. https://doi.org/10.1182/blood.V92.9.3460 PMid:9787188
- Mancuso
A, Rigano P, Renda D, Di Salvo V, Pignatti CB, Guddo F, Buccellato A,
Nicoli N, Maggio A. Hepatocellular carcinoma on cirrhosis-free liver in
a HCV-infected thalassemic. Am J Hematol 2005;78:158-159. https://doi.org/10.1002/ajh.20289 PMid:15682406
- Minola
E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M,
Paggi S, Conte D. Age at infection affects the longterm outcome of
transfusion-associated chronic hepatitis C. Blood 2002;99:4588-4591. https://doi.org/10.1182/blood-2001-12-0192 PMid:12036892
- Papatheodoridis
G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi
H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la
Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo
M, Esteban R, Janssen HL, Lampertico P.PAGE-B predicts the risk of
developing hepatocellular carcinoma in Caucasians with chronic
hepatitis B on 5-year antiviral therapy. J Hepatol 2016;64:800-806. https://doi.org/10.1016/j.jhep.2015.11.035 PMid:26678008
- Morgan
RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication
of hepatitis C virus infection and the development of hepatocellular
carcinoma: a meta-analysis of observational studies. Ann Intern Med
2013;158:329-337. https://doi.org/10.7326/0003-4819-158-5-201303050-00005 PMid:23460056
- Mancuso A. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol 2010;2:171-174. https://doi.org/10.4254/wjh.v2.i5.171 PMid:21160991 PMCid:PMC2999281
- Karageorgos
SA, Stratakou S, Koulentaki M, Voumvouraki A, Mantaka A, Samonakis D,
Notas G, Kouroumalis EA. Long-term change in incidence and risk factors
of cirrhosis and hepatocellular carcinoma in Crete, Greece: a 25-year
study. Ann Gastroenterol 2017;30:357-363. https://doi.org/10.20524/aog.2017.0135 PMid:28469367 PMCid:PMC5411387
- Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 2014;3:31-40. https://doi.org/10.1159/000343856 PMid:24804175 PMCid:PMC3995380
- Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett 2009;286:38-43. https://doi.org/10.1016/j.canlet.2008.11.001 PMid:19081672
- Gandon Y, Olivié D, Guyader D. Non-invasive assessment of hepatic iron stores by MRI. Lancet 2004;363:357-362. https://doi.org/10.1016/S0140-6736(04)15436-6
- Hankins
JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS,
Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the
liver in patients with iron overload. Blood 2009;113:4853-4855. https://doi.org/10.1182/blood-2008-12-191643 PMid:19264677 PMCid:PMC2686136
- Bertuccio
P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E.
Global trends and predictions in hepatocellular carcinoma mortality. J
Hepatol 2017;67:302-309. https://doi.org/10.1016/j.jhep.2017.03.011 PMid:28336466
- Richani
M, Kolly P, Knoepfli M, Herrmann E, Zweifel M, von TenggKobligk H,
Candinas D, Dufour JF. Treatment allocation in hepatocellular
carcinoma: Assessment of the BCLC algorithm. Ann Hepatol 2016;15:82-90 https://doi.org/10.5604/16652681.1184233 PMid:26626644
- Restivo
Pantalone G, Renda D, Valenza F, D'Amato F, Vitrano A, Cassarà F,
Rigano P, Di Salvo V, Giangreco A, Bevacqua E, Maggio A.Hepatocellular
carcinoma in patients with thalassaemia syndromes: clinical
characteristics and outcome in a long term single centre experience. Br
J Haematol. 2010;150:245-247. https://doi.org/10.1111/j.1365-2141.2010.08180.x PMid:20433678
- Balogh
J, Victor D, Asham EH, Burroughs SG, Boktour M,Saharia A, Li X,
Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J
Hepatocell Carcinoma 2016;3:41-53. https://doi.org/10.2147/JHC.S61146 PMid:27785449 PMCid:PMC5063561
- Sekine
S, Ito K, Watanabe H, Nakano T, Moriya K, Shintani Y, Fujie H, Tsutsumi
T, Miyoshi H, Fujinaga H, Shinzawa S, Koike K, Horie T. Mitochondrial
iron accumulation exacerbates hepatic toxicity caused by hepatitis C
virus core protein. Toxicol Appl Pharmacol. 2015;282:237-243. https://doi.org/10.1016/j.taap.2014.12.004 PMid:25545986
- Kew MC, Asare GA. Dietary iron overload in the African and hepatocellular carcinoma. Liver Int 2007;27:735-741. https://doi.org/10.1111/j.1478-3231.2007.01515.x PMid:17617115
- Borgna-Pignatti
C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC,
Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival
and complications in patients with thalassemia major treated with
transfusion and deferoxamine. Haematologica 2004;89:1187-1193.
- Furuta
M, Tanaka H, Shiraishi Y, Unida T, Imamura M, Fujimoto A, Fujita M,
Sasaki-Oku A, Maejima K, Nakano K, Kawakami Y, Arihiro K, Aikata H,
Ueno M, Hayami S, Ariizumi SI, Yamamoto M, Gotoh K, Ohdan H, Yamaue H,
Miyano S, Chayama K, Nakagawa H. Characterization of HBV integration
patterns and timing in liver cancer and HBV-infected livers. Oncotarget
2018;9:25075-25088. https://doi.org/10.18632/oncotarget.25308 PMid:29861854 PMCid:PMC5982772
- EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461-511. https://doi.org/10.1016/j.jhep.2018.03.026 PMid:29650333
- EASL
Clinical Practice Guidelines: Management of hepatocellular carcinoma.
European Association for the Study of the Liver. J Hepatol
2018;69:182-236.
- Cabibbo G, Enea M,
Attanasio M, Bruix J, Craxi A, Camma C. A metaanalysis of survival
rates of untreated patients in randomized clinical trials of
hepatocellular carcinoma. Hepatology 2010;51:1274-1283. https://doi.org/10.1002/hep.23485 PMid:20112254
- Staikou
C, Stavroulakis E, Karmaniolou I. A narrative review of perioperative
management of patients with thalassaemia. Anaesthesia 2014;69:494-510. https://doi.org/10.1111/anae.12591 PMid:24601913
- Forner
A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and
treatment: the BCLC update and future prospects. Semin Liver Dis
2010;30:61-74. https://doi.org/10.1055/s-0030-1247133 PMid:20175034
- Llovet
JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira
AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S,
Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D,
Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP
Investigators Study Group. Sorafenib in advanced hepatocellular
carcinoma. N Engl J Med 2008;359:378-390. https://doi.org/10.1056/NEJMoa0708857 PMid:18650514
- Llovet
JM, Bruix J. Systematic review of randomized trials for unresectable
hepatocellular carcinoma: Chemoembolization improves survival.
Hepatology 2003;37:429-442. https://doi.org/10.1053/jhep.2003.50047 PMid:12540794
- Burrel
M, Reig M, Forner A,Barrufet M, de Lope CR, Tremosini S, Ayuso C,
Llovet JM, Real MI, Bruix J . Survival of patients with hepatocellular
carcinoma treated by transarterialchemoembolisation (TACE) using Drug
Eluting Beads. Implications for clinical practice and trial design. J
Hepatol 2012;56:1330-1335. https://doi.org/10.1016/j.jhep.2012.01.008 PMid:22314428
[TOP]



