 |
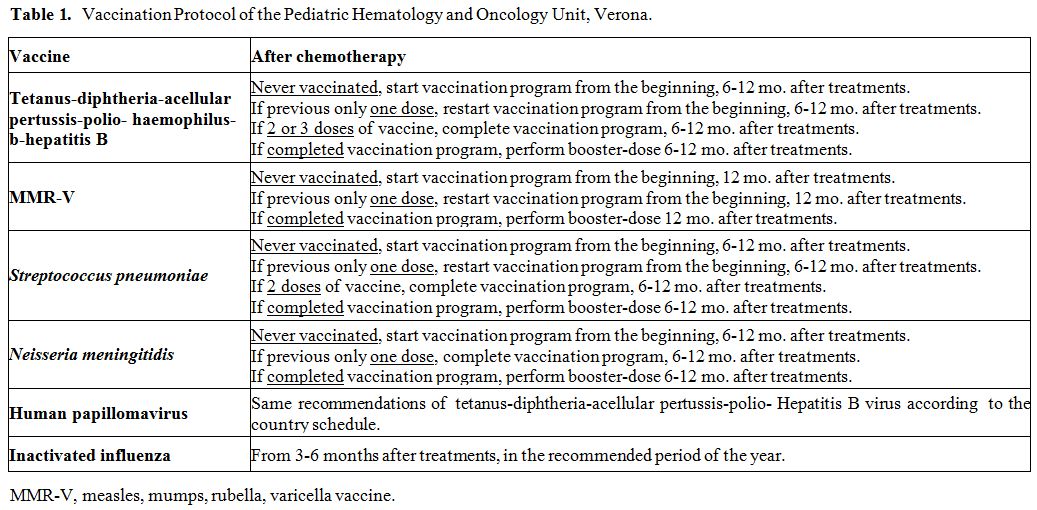
Table 1. Vaccination Protocol of the Pediatric Hematology and Oncology Unit, Verona. |
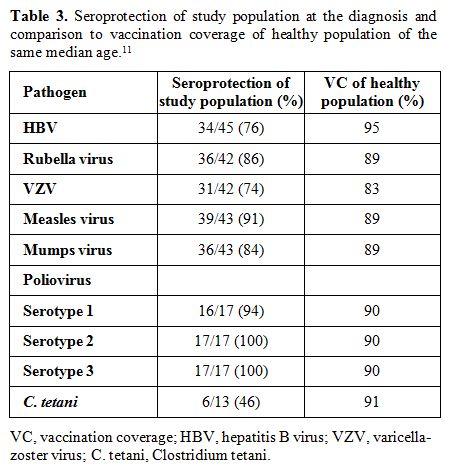
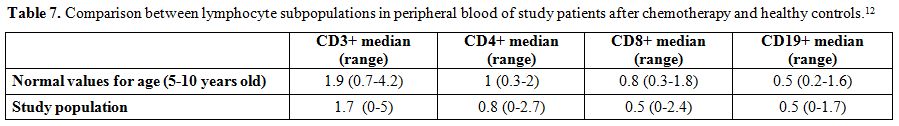
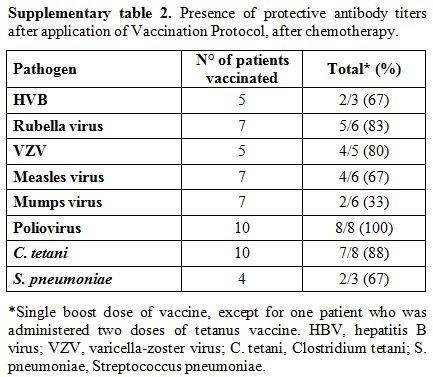
Serologic assays. Antibodies detection for HBV, rubella, VZV, measles, mumps, polio viruses, C. tetani, and S. pneumoniae were conducted on fresh serum samples using commercial kits and following the manufacturer’s instructions. Chemiluminescence technology (CLIA) was used to detect rubella, measles, mumps, VZV IgG (Diasorin S.p.A., Saluggia, Vercelli, Italy) and HBsAg antibodies (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY, US); enzyme immuno assay (EIA) was used for C. tetani IgG (Diasorin S.p.A., Saluggia, Vercelli, Italy) and for S. pneumoniae IgG (The Binding Site Group Ltd, Birmingham, UK); neutralization assay, according to WHO guidelines, was used for poliovirus. Results were interpreted as positive, negative or undetermined according to kit instructions. Positive results were considered as follow: HbsAg antibodies > 12 mUI/ml, rubella IgG > 10 UI/mL, VZV IgG > 135 mUI/mL, measles IgG > 16,5 AU/mL, mumps IgG > 9 AU/mL, C. tetani IgG > 0,51 UI/mL, poliovirus IgG > 1:8. For S. pneumoniae were considered positive results IgG titers >35 mg/L, in accordance with WHO recommendation.[10] Specifically, the kit used measures antibody responses to pneumococcal vaccines incorporating 23 polysaccharides isolated from S. pneumoniae.
Statistical analysis. Collected data were analyzed using descriptive statistics: median and range for continuous variables, absolute frequency, and percentages for categorical or dichotomous variables.
Differences of quantitative variables between groups were texted using the non-parametric Kruskal-Wallis test, whereas categorical variables using the Chi-squared test or Fisher’s exact test.
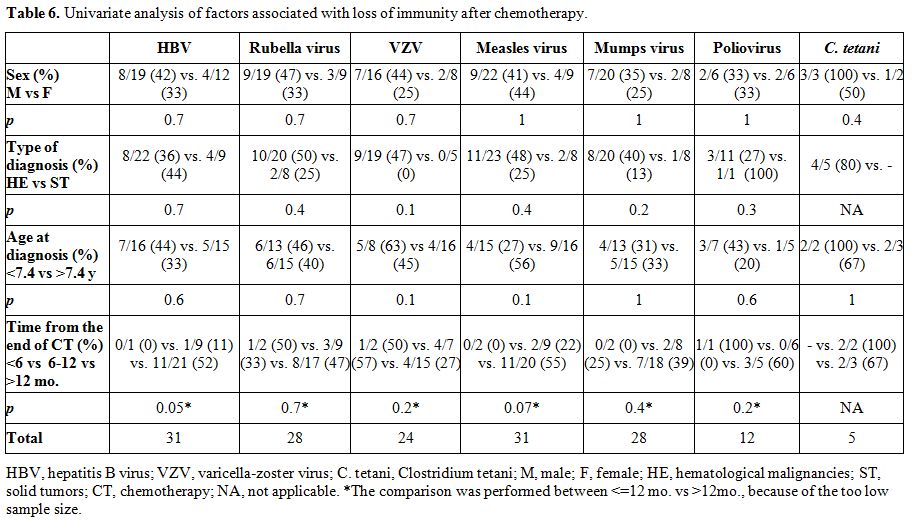
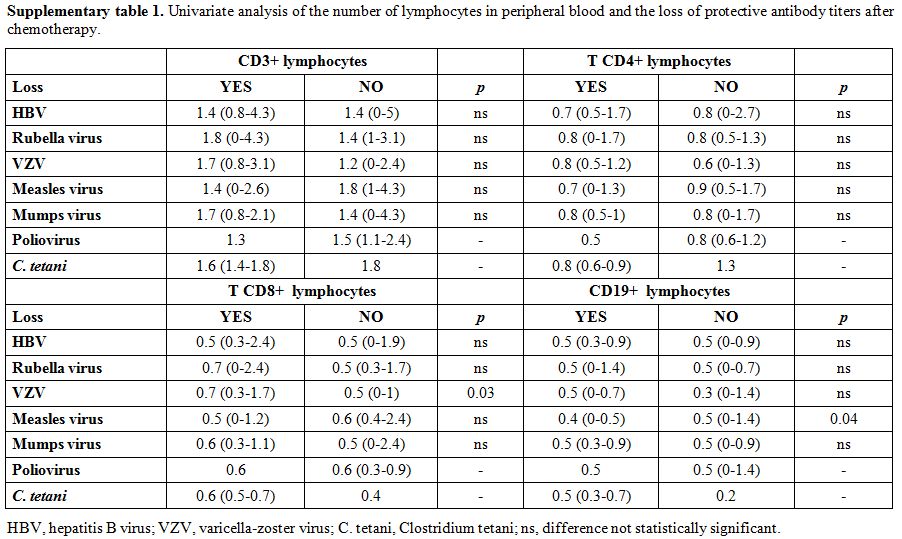
The following variables were tested in univariate analysis for the loss of protective serum antibody titers: sex, type of diagnosis, age at diagnosis, time from the end of chemotherapy.
A P-value of <0,05 was considered statistically significant. Analysis was conducted using the statistical software SAS, 9.4 version (Statistical Analysis Software, SAS Institute Inc.).
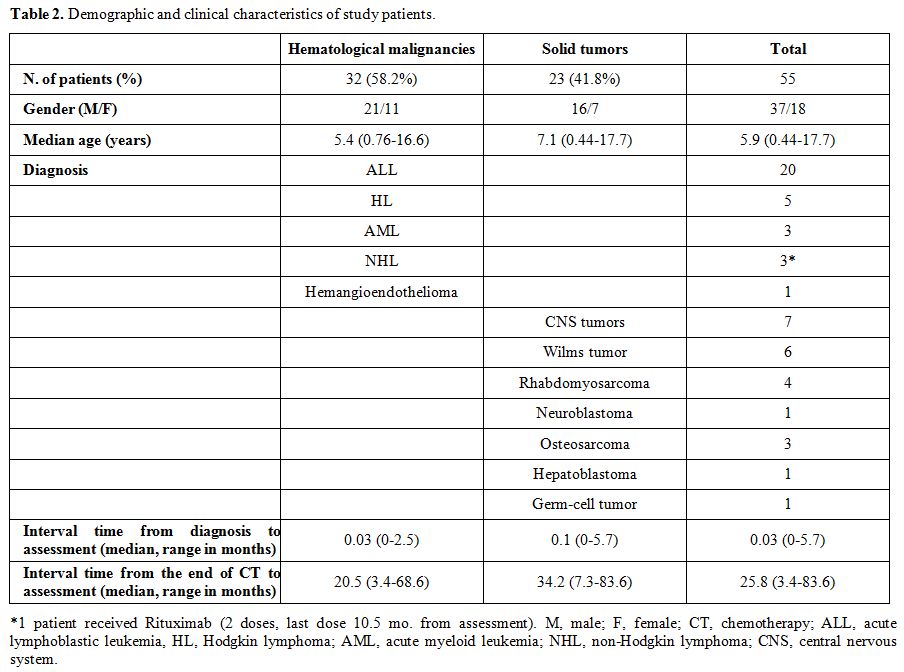
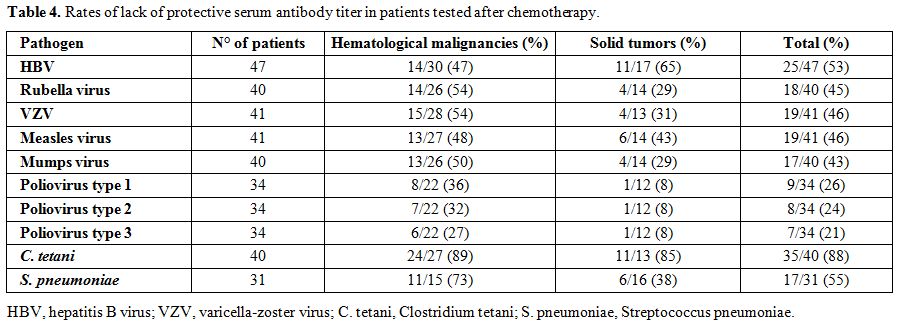
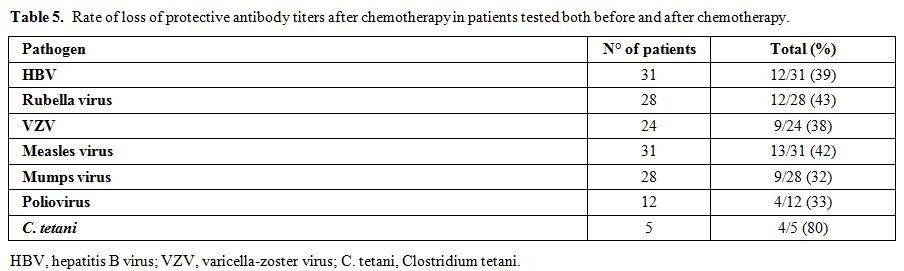
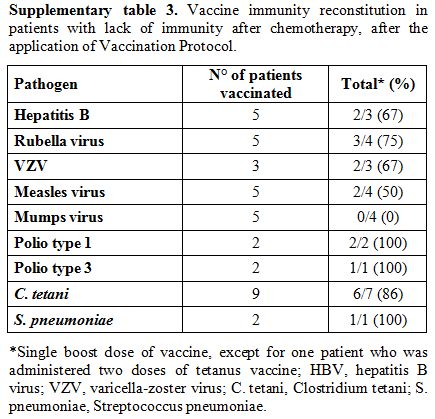
The term lack of immunity was used for patients with non-protective antibody titers if they were tested after chemotherapy. The term loss of immunity was used for patients tested pre- and post- treatment and had a change of the antibody level from positive to negative. Seroprotection was defined by any level of antibody positivity, including a borderline positivity with an undetermined titer.