Swabs were collected for MRSA screening weekly, on at least two occasions before admission and after the insertion of CVC, until discharge and monthly thereafter, for six months from the CVC exit site and another skin site. MRSA confirmed by both oxacillin MIC, and cefoxitin screening was considered for this study.
Patients with a positive nasal/skin swab screening in more than one sample before or within four weeks of admission for transplantation without any signs or symptoms of infection were defined as ‘Colonized’, CABSI was defined as bacteremia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained CVC, clinical manifestations of infection (i.e., fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter. Bloodstream infections with gram-negative organisms colonizing the gut were not considered as CABSI.[6,7]
Both MRSA-positive and MRSA-negative patients were put under barrier nursing precautions according to Centers for Disease Control and Prevention (CDC) guidelines.
As a part of prevention of CRGNB-related sepsis and mortality, pre-emptive granulocyte infusions (pGI) preferably from family members, were administered within 48 hours of the onset of fever if the absolute neutrophil count (ANC) was < 0.5 x 109/l, and on alternate days until the subsidence of fever for greater than 48 hours or neutrophil engraftment as previously described.[8] ABO blood group matched donors received granulocyte colony-stimulating factor (G-CSF) and dexamethasone 8 mg orally, 18-24 hours 4 hours, respectively, before apheresis.[8] The apheresis was carried out on a continuous flow apheresis machine, Spectra Optia® (SPO, Terumo BCT, Lakewood, CO, USA). Granulocytes were collected on the established software-based protocol with an intention to collect at least 1x1010 granulocytes per collection. The product was irradiated and infused within 4 hours of donation, over 8-10 hours.
Conditioning regimens for malignant and non-malignant diseases have been described in detail in our previous publications.[9,10] All patients received post-transplantation cyclophosphamide (PTCy) and CTLA4Ig (Abatacept) along with cyclosporine or sirolimus.[10]
The primary endpoint of the study was the incidence of MRSA-BSI and CABSI. Secondary endpoints were MRSA related mortality and overall non-relapse mortality (NRM). Other outcomes studied were time to engraftment, acute and chronic GVHD, and overall survival (OS). All analyses were performed using a statistical software IBM SPSS Statistics Version 22 (Armonk, NY, USA).
The details of patient and donor characteristics are mentioned in Table 1. The median duration of follow-up for survivors was 24 months (range 12-60 months) in the overall cohort.
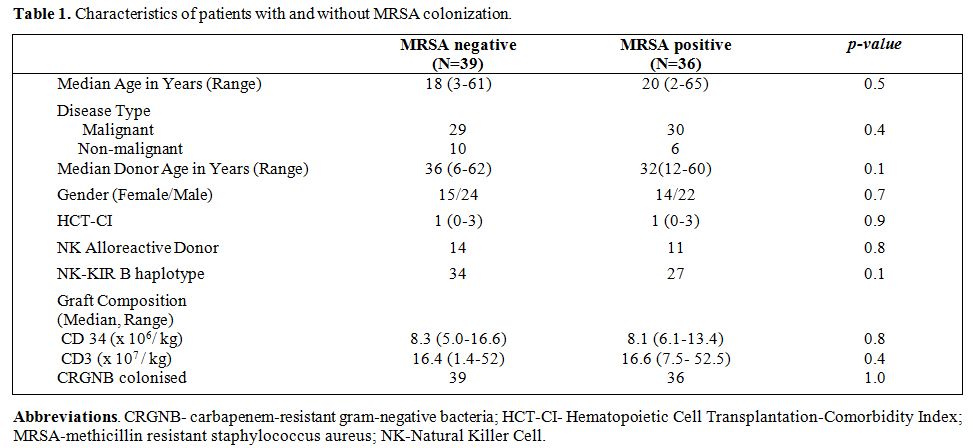 |
Table 1. Characteristics of patients with and without MRSA colonization. |
Thirty-six (48%) out of 75 patients were colonized before HCT. Thirty of these were colonized prior to admission, and the other six were detected within seven days of admission. None of the other 39 patients who were not colonized within 7 days of admission were found to be colonized in the next 6 months. There was no significant difference between the groups in terms of patient or donor age, gender, ABO mismatch, degree of HLA mismatch, NK ligand mismatch, KIR-B haplotype and disease status (Table 1).
All patients in this study were colonized with CRGNB and received pGI as a part of the protocol to prevent CRGNB-related mortality.[8] All developed febrile neutropenia at a median of day +8 (range, -4 to +13). The median number of pGI infusions was 2 (range, 1-7) (Table 2), and that was similar in both groups. The median dose of granulocytes per infusion was 3.7 x 1010 (range, 0.9-6.7), with no significant difference between the groups. There were no significant adverse reactions to granulocyte infusions. There were seven CRGNB-related BSI in each group. CRGNB related mortality was one in each group. There were no instances of MRSA-related BSI in the pre-engraftment period. Thus, the overall incidence of BSI was 18.6% at day +30 with no difference between the groups without any MRSA-BSI or CABSI.
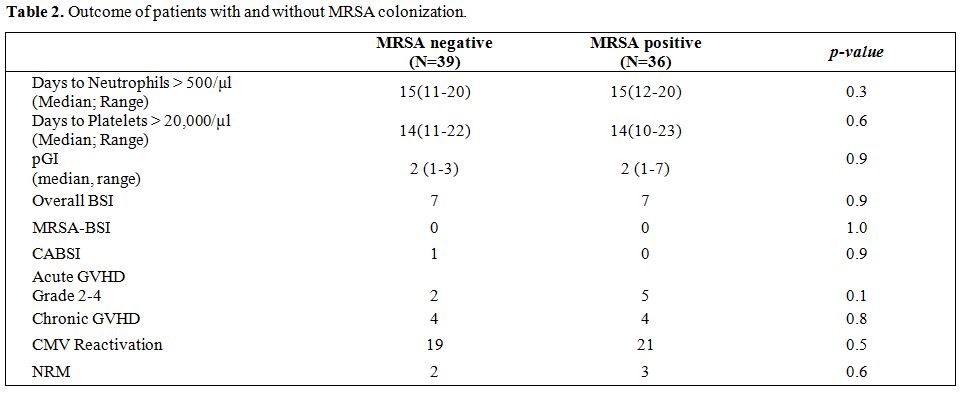 |
Table 2. Outcome of patients with and without MRSA colonization. |
None of the MRSA negative patients was colonized in the post-transplant period as detected on routine surveillance. Two patients in the MRSA positive group and three patients in the MRSA negative group developed CVC associated mild line-site infection (erythema and tenderness) within 7 days of insertion with negative microbiology. All were on levofloxacin prophylaxis and responded to intravenous vancomycin for 5 days. There was no recurrence in any of the patients. None of the other patients received empirical vancomycin during the hospital stay.
Engraftment was similar in the two cohorts (Table 2). There were no additional episodes of MRSA-BSI, CABSI, or line-site infection in either group between day +30 and day +180. However, one patient in the MRSA negative group developed CABSI at day +196 with coagulase-negative staphylococcus. Thus, the overall incidence of CABSI was 3.2% (range, 0.1%-6.3%).
The overall incidence of acute GVHD was 9.6% (range, 6.2-13.0), 14.1% (range, 8.3-19.9) in the MRSA-positive group compared to 5.3% (range,1.6-9.0) in the MRSA-negative group (p=0.1). The overall incidence of chronic GVHD was 14.5% (range, 9.7-19.3), which was similar in the two groups (p=0.8) (Table 1). The overall NRM at 2 years was 6.7% (95% CI, 3.8%-9.6%). None of the deaths was related to MRSA. The OS was 80.6% (95% CI, range 74.0 -87.2%) in the MRSA positive group compared to 81.8% (95% CI, range 75.6 -88.0%) in the MRSA negative group (p= 0.8).
In keeping with the national prevalence,[11] 48% of the patients were detected to be colonized with MRSA before HCT, once the screening was initiated. Despite a high incidence of MRSA colonization in patients undergoing haploidentical HCT, there were no MRSA infections or increased mortality in association with MRSA colonization in our study. This may be attributable to two factors. First, the rigorous implementation and stringent adherence to the preventive measures might have been responsible for the containment of MRSA infection. Secondly, the use of pGI probably played an essential role in the prevention of systemic infection with MRSA, even though it was primarily directed at CRGNB.[12]
There is scant data on the role of granulocyte infusions in the prevention of systemic MRSA infection, as is evident from several randomized or non-randomized studies.[13,14] Unlike in our previous study on the effect of pGI on CRGNB mortality,[12] no control was carried out in the current one. The high incidence (20%) of MRSA-BSI with 30% mortality found in an initial cohort of 15 unscreened patients undergoing HCT at our center induced us to believe not ethical a similar control arm. Considering this limited experience from the cohort of unscreened HCT patients, one might hypothesize that pGI might have played a role in limiting early MRSA-BSI in the study group. However, our study is non-randomized with limited cohort size, so the data should be interpreted with caution.
Several studies have highlighted concerns regarding late-onset MRSA infections related to GVHD.[3,15] We had documented only a single instance of late CABSI with MSSA in the MRSA negative group and none in the MRSA positive cohort. Besides meticulous adherence to the preventive aspect, the low incidence of both acute and chronic GVHD were probably responsible for the absence of late MRSA infections, which, as in most studies, have been related to GVHD and the use of corticosteroids.[3,15] The low incidence of GVHD is primarily attributable to the use of CTLA4Ig- based protocols in our study, which have reduced the incidence of early alloreactivity in our haploidentical HCT program.[9,10,16]
A transplant protocol, which aims at improving outcomes by reducing the progression of the underlying disease, can be considered successful only if sufficient attenuation of infection and GVHD-related morbidity and mortality can be achieved. The low NRM with no deaths being directly or indirectly attributable to MRSA probably underscores the impact of pGI and a reduced incidence of GVHD in the prevention of complications related to MRSA. Further exploration of these strategies in larger cohorts of patients in randomized studies will need to validate these approaches in limiting complications associated with MRSA in patients undergoing alternate donor HCT.