Shaimaa Sahmoud1, Mostafa S. Ibrahim2, Eman A. Toraih3,4, Noha Kamel5, Manal S. Fawzy6,7* and Samar Elfiky1.
1 Pediatric Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
2 Diagnostic Radiology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
3 Genetics Unit, Histology and Cell Biology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
4 Department of Surgery, Tulane University, School of Medicine, New Orleans, Louisiana, USA.
5 Clinical Pathology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
6 Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
7 Biochemistry Department, Faculty of Medicine, Northern Border University, Arar, Saudi Arabia.
Published: July 1, 2020
Received: March 31, 2020
Accepted: June 4, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020037 DOI
10.4084/MJHID.2020.037
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
The reduced rate of bone formation despite the availability of vitamin
D has been reported in β-thalassemia. Genetic factors, together with
environmental ones, could be implicated in this condition. Since
vitamin D binding protein (VDBP) maintains bioavailability of vitamin D
which binds to vitamin D receptor (VDR)-retinoid X receptor alpha
(RXRA) heterodimer to exert its molecular actions, we speculated that
vitamin D metabolic-axis expression signature and variants could be
potential molecular candidates for bone turnover/disease in
thalassemia. To this end, this study aims to analyze VDR/RXRA expression signature, and two VDBP variants in a pilot sample of Egyptian β-thalassemia children in correlation with bone mineral density (BMD).
Patients and methods:
Forty-four well-chelated β-thalassemia children and 40 unrelated
controls were enrolled. The serum bone chemistry profile was measured.
Peripheral blood mononuclear cells (PBMN) VDR/RXRA expression levels were quantified by Real-Time quantitative reverse transcription-polymerase chain reaction (qRT-PCR). VDBP
rs7041 and rs4588 variants were identified by Real-Time allelic
discrimination assay. All patients were subjected to lumbar-spine
Dual-energy X-ray absorptiometry (DEXA).
Results: VDR/RXRA expressions were significantly higher in β-thalassemia children compared to controls (P
= 0.001 and <0.001, respectively) and showed higher values in
β-thalassemia major relative to β-thalassemia intermedia. Expression
levels of both genes were not associated with sex or BMD. However, VDBP rs4701 genotyping revealed lower BMD-L4 and a higher frequency of osteoporosis.
Conclusions: β-Thalassemia children had higher expression levels of PBMN VDR/RXRA. VDBP
rs4701 variant was associated with osteoporosis in our β-thalassemia
patients on vitamin D supplementation. Further large-scale studies in
other ethnic populations are warranted.
|
Introduction
As
an emerging global health burden, carriers of hemoglobin disorders
approach 7% worldwide, and nearly 50,000-100,000 children with beta
(β)-thalassemia major die each year in low- and middle-income
countries.[1] In Egypt, β-thalassemia is considered the most common monogenic disorder with a carrier rate of almost 5.3 to 9%,[2] representing the most common genetically determined chronic hemolytic anemia (85.1%).[3]
Vitamin D deficiency has been reported to be prevalent among children and adolescents with thalassemia[4] in several countries,[5-7] including upper Egypt.[8]
Vitamin D is essential for calcium hemostasis and bone mineralization,
and 25 (OH) vitamin D is considered the major circulating vitamin D
metabolite and the best indicator of vitamin D deficiency.[9]
The main carrier protein which supports the bioavailability of
circulating vitamin D and its metabolites is vitamin D binding protein
(VDBP).[10] By maintaining the serum levels of the bioactive 1,25(OH)2D, VDBP could impact vitamin D levels under different physiologic and pathologic conditions,[11,12] contributing jointly or independently to a variety of adverse health outcomes.[13]
Vitamin D binding protein is encoded by the GC (group-specific component) gene located at 4q11-13.[14]
The two most common single nucleotide polymorphisms (SNP) associated
with approximately 80% of the variation in levels of VDBP are rs7041
and rs4588, which have been identified in the coding region of exon 11
of this gene.[15] These variants have been associated with both circulating vitamin D levels and their function[16,17] and show different allele frequencies based on ethnic variations.[18]
Vitamin
D exerts most of its biological activities by binding to a specific
high-affinity receptor, the vitamin D receptor (VDR). This receptor
binds target DNA sequences as a heterodimer with retinoid X receptor
alpha (RXRA) to regulate transcription.[19] This
heterodimer receptor belongs to the superfamily of nuclear receptors
for steroid hormones and regulates gene expression by acting as a
ligand-dependent transcription factor.[19,20] VDR activation and expression are necessary for the effects of vitamin D, in which several SNPs have been identified.[21]
The
vitamin D metabolic axis could be implicated in many aspects of bone
mineral density (BMD) in β thalassemia. To our knowledge, the
association of VDR/RXR expression and VDBP
variants with BMD in β-thalassemia children has not been studied
before. In this sense, the current study aimed to evaluate the
association between VDR/RXR expression levels, as well as VDBP polymorphisms (rs7041 and rs4588) with BMD in a sample of Egyptian pediatric β-thalassemia on vitamin D supplementation.
Patients nd Methods
Study participants.
A total of forty-four children with beta-thalassemia and forty age- and
sex-matched healthy controls were enrolled in the study. All cases were
prepubertal children aged 2-12 years who were followed up in the
Hematology clinic, Suez Canal University Hospital, Ismailia, Egypt. All
thalassemic children were receiving the daily requirement of vitamin D2. None of them had ever been on Vitamin D3 therapy, while only 70% of the controls were on vitamin D2
supplements. Healthy children who were attending the pediatric clinics
for general check-up were assigned as controls. Children with chronic
renal or liver disease, clinically diagnosed rickets, or using
medications influencing bone mineral metabolism (as glucocorticoids or
antiepileptic drugs), were excluded. The study was approved by the Suez
Canal University Ethical Committee (Approval no. 3125). Written
informed consent was taken from all participants' parents.
Clinical assessment of patients.
All participants were subjected to history taking, thorough
examination, and data collection by screening the hospital medical
records, including socio-demographic data and course of thalassemia
(age at diagnosis, transfusion therapy, drug therapy, presence of
complications). Weight and height were plotted on the Center for
Disease Control and Prevention (CDC) curves, and puberty staging was
assessed using Tanner staging.
Blood biochemical profile.
The following laboratory workup was performed on all participants: (a)
Complete blood picture using fully automated hematology analyzer
(HORIBA ABX Micros 60, France) with blood film examination; (b) Serum
calcium, phosphorus, alkaline phosphatase, liver enzymes using
commercially available kits (Cobas 6000 analyzer, USA); (c) Serum
ferritin using electrochemiluminescence technology on immunoassay
analyzer Cobas 411 (Roche Diagnostics, Japan); (d) Parathyroid hormone
assay immunoassay analyzer Cobas 411 (Roche Diagnostics, Japan).
Serum vitamin D level quantification.
Total 25 (OH) Vitamin D was assessed for all participants by a
commercially available ELISA kit (EIA-5396, DRG International Inc.,
USA). The procedure and the quality control measurements were performed
according to the manufacturer's instructions. The detection limit was
3.2-120 ng/mL, the interassay coefficient of variation (CV) was around
3.7%, and the interassay CV was 7.1%. Vitamin D status was defined
sufficient at a level of ≥ 20 ng/mL, insufficient between 10 and 19
ng/mL, and deficient <10 ng/mL.[22]
Dual Energy X-ray absorptiometry (DEXA).
Dual-energy X-ray absorptiometry (DXA) is the most widely used method
for evaluating bone mineral content and BMD in patients of all ages.[23]
BMD was measured using a DEXA densitometer (GE Lunar DPX NT, USA) with
dedicated pediatric software (GE enCORE, USA) at the lumbar spine
(L1–L4) in the AP projection. The instrument was calibrated daily
according to the manufacturer's instructions. Reproducibility was
calculated as a CV obtained by weekly measurements of a standard
phantom on the instrument. The CV of the current instrument was 0.5%
with the standard phantom, and the in vivo precision of the BMD measurement at the L1–L4 region was 1.2%. BMD data were expressed as g/cm2 and as Z scores after being compared with BMD values of healthy subjects of the same age.
The
results were expressed as absolute values with a Z- Score (difference
in SD of healthy age and sex-matched subjects) (Figure S1). BMD Z-score
≤ -2.0 was considered as osteoporosis, according to the International
Society for Clinical Densitometry (Official Position 2013 available at https://www.iscd.org/official-positions/2013-iscd-official-positions-pediatric/).
Expression profiling.
RNA extraction was carried out from the separated peripheral
mononuclear cells (PMNCs) by Ficoll-Paque as a density-gradient medium
using ABIOpure Total RNA (AllianceBio, Catalog no. M541RP50-B)
following the protocol supplied by the manufacturer. Nucleic acid
concentration and purity at the "absorbance ratio 260/280 nm" were
determined by the NanoDrop ND-1000 spectrophotometer (NanoDrop Tech.,
USA). High Capacity cDNA Reverse Transcription Kit (Applied Biosystems,
P/N 4368814) was used to convert RNA into cDNA. RT was carried out in
T-Professional Basic, Biometra PCR System (Biometra, Goettingen,
Germany). Gene expression of RXRA and VDR
genes were quantified in accordance with the Minimum Information for
Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines
using SYBR Green qPCR analysis and compared to GAPDH using the following primers (Table 1).
The reaction mixture and PCR thermal conditions were applied in
StepOne™ Real-Time PCR System (Applied Biosystems) with an annealing
temperature of 58°C for GAPDH, 62°C for RXRA, and 70°C for VDR.
Melting curve analysis confirmed the specificity of the amplicons,
using appropriate negative controls; the fold change was calculated
using the delta threshold cycle equation.[24].
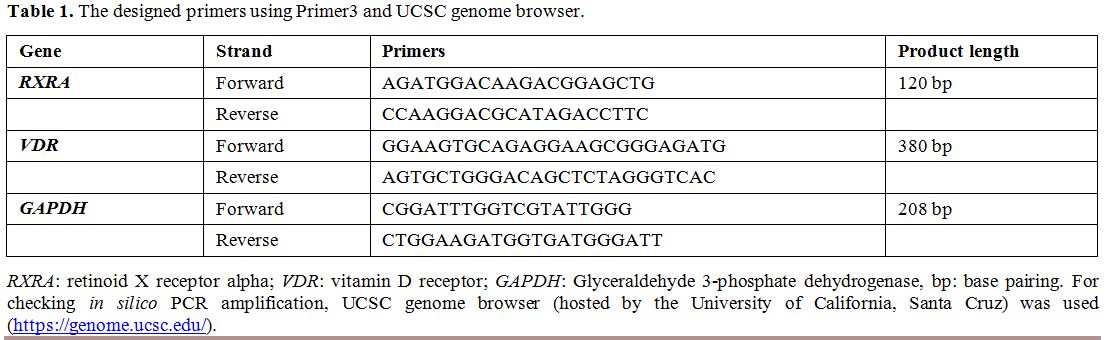 |
Table
1. The designed primers using Primer3 and UCSC genome browser. |
SNP identification.
Genomic DNA was isolated from whole blood using ABIOpureTM Total DNA
(AllianceBio, Catalog no. M501DP100) following the instructions
supplied with the kits. DNA assessment was executed using NanoDrop
ND-1000 (NanoDrop Tech., Inc. Wilmington, DE, USA). Samples were
genotyped for VDBP polymorphisms (rs7041 and rs4588) using Real-Time
polymerase chain reaction allelic discrimination technology. PCR
reaction was carried out in a 25-µL reaction volume containing 12.5 μL
2x Taqman® genotyping Master Mix and 1.25 µL TaqMan® SNP Genotyping
Assay Mix (Applied Biosystems) with 40 ng genomic DNA. Appropriate
controls were used. PCR amplification was performed on StepOne™
Real-Time PCR System (Applied Biosystems, USA) in duplicates with 100%
concordance using the conditions as described in an earlier
publication.[25]
Statistical analysis.
Statistical analysis was managed using the R software version 3.3.2,
GraphPad prism 7.0, and "Statistical Package for the Social Sciences
(SPSS) for Windows" software, version 23. Online software, (http://www.oege.org/software/hwe-mr-calc.shtml)
was used for calculating Hardy–Weinberg equilibrium. Chi-square,
Fisher's exact, Student's t-, Mann-Whitney U (MW), and Kruskal-Wallis
(KW) tests were used. Genotype and allele frequencies were estimated
for each group to calculate the odds ratios (ORs) and 95% confidence
intervals (CIs) for multiple genetic association models.[26] Logistic regression was employed to adjust confounder parameters. A two-tailed p P < 0.05 was considered statistically significant.
Results
Characteristics and biochemical profile of the study groups. Table 2
demonstrates the baseline characteristics and biochemical profile of
thalassemia children and controls. Although the height Z score was
significantly reduced in patients with thalassemia compared to controls
(P= 0.005), BMI Z score was also reduced in the patient group compared to controls, but not reach statistical significance (P = 0.929). Thalassemia patients exhibited higher levels of serum 25 (OH) vitamin D (41.6 ± 30.1 versus 18.7 ± 5.8, P < 0.001), serum calcium (9 ± 0.7 and 8.2 ± 0.6, P < 0.01), and alkaline phosphatase (158.6 ± 57.9 versus 126.8 ± 29.8, P = 0.003), and lower levels of parathyroid hormone (22.2 ± 13.1 versus 38.4 ± 21.2, P
< 0.001) compared with controls. Serum ferritin among thalassemia
children was 1000 + 241µg/L, and iron overload was not correlated with
25 (OH) vitamin D level or bone density (P = 0.143, and 0.211, respectively).
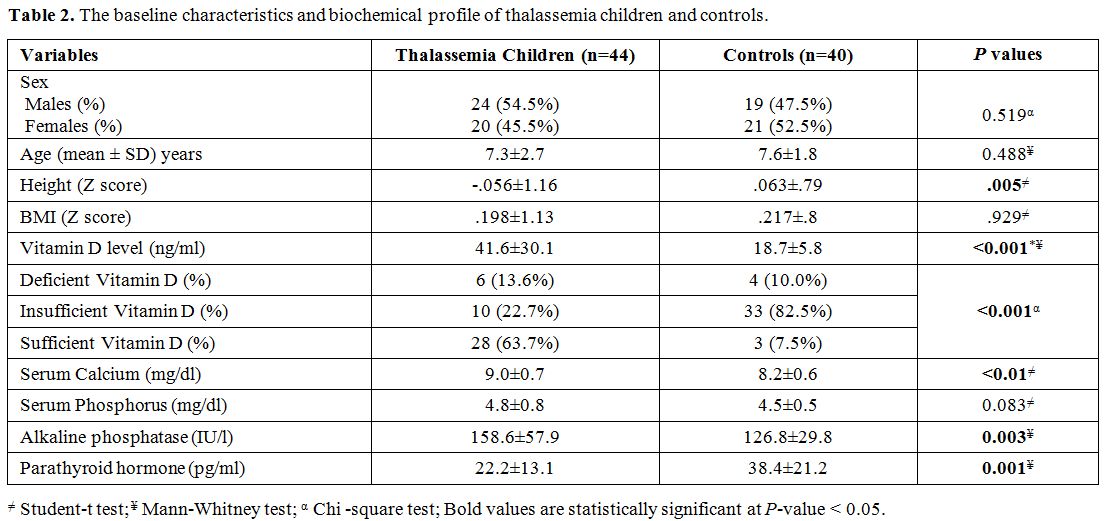 |
Table 2. The baseline characteristics and biochemical profile of thalassemia children and controls.. |
Gene expression profiling. VDR and RXRA mRNAs were significantly higher in thalassemia children compared to controls (P = 0.001 and < 0.001) (Figure 1).
Additionally, significantly higher expression values of both
transcripts were observed in thalassemia major cases compared to
thalassemia intermedia children (P = 0.003 and < 0.001, respectively). Expression levels of VDR and RXRA genes were not associated with sex (P = 0.786 and 0.548) or bone density (P = 0.208 and 0.176, respectively).
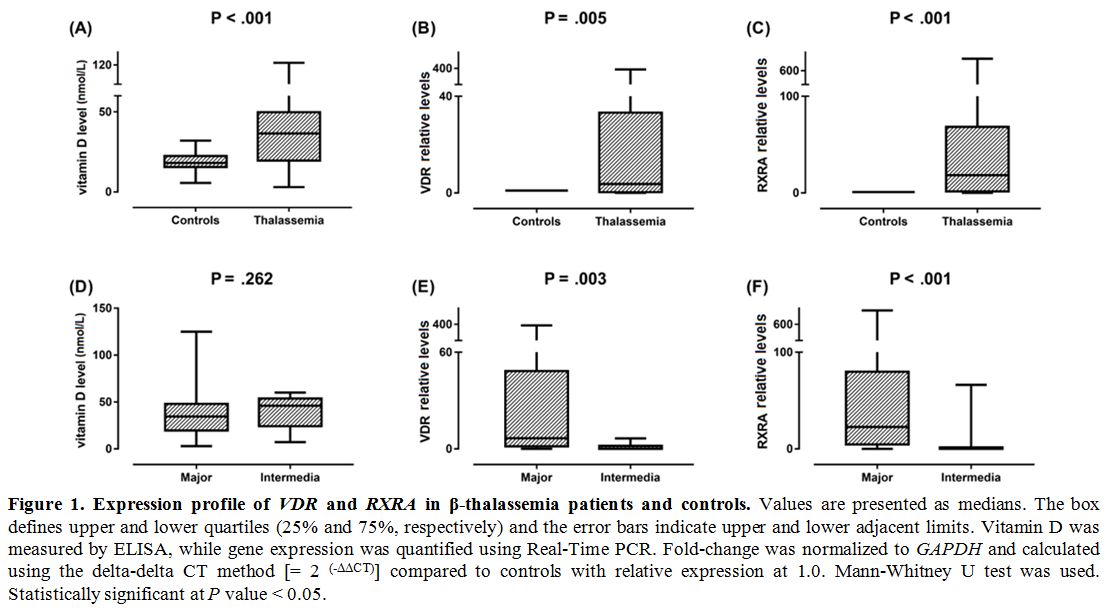 |
Figure
1. .Expression profile of VDR and RXRA
in β-thalassemia patients and controls. Values are presented as
medians. The box defines upper and lower quartiles (25% and 75%,
respectively) and the error bars indicate upper and lower adjacent
limits. Vitamin D was measured by ELISA, while gene expression was
quantified using Real-Time PCR. Fold-change was normalized to GAPDH and
calculated using the delta-delta CT method [= 2 (-ΔΔCT)] compared to controls with relative expression at 1.0. Mann-Whitney U test was used. Statistically significant at P value < 0.05. |
Genotype analysis of VDBP polymorphisms.
Genotype frequencies in both patients and controls were found in
accordance with those expected by the Hardy Weinberg equilibrium. VDBP rs4701 GG shows borderline association with thalassemia under recessive model [OR 95% CI: 3.62 (0.9-14.2); P
= 0.053]. Otherwise, the genotyping of both variants revealed no
significant difference between patients and controls under all genetic
association models (Table 3).
The frequency of T*rs7041 was 0.70 in patients, and 0.66 in the
controls and that of C*rs4588 was 0.74 in patients, and 0.78 in the
controls, being these alleles the most common in our population.
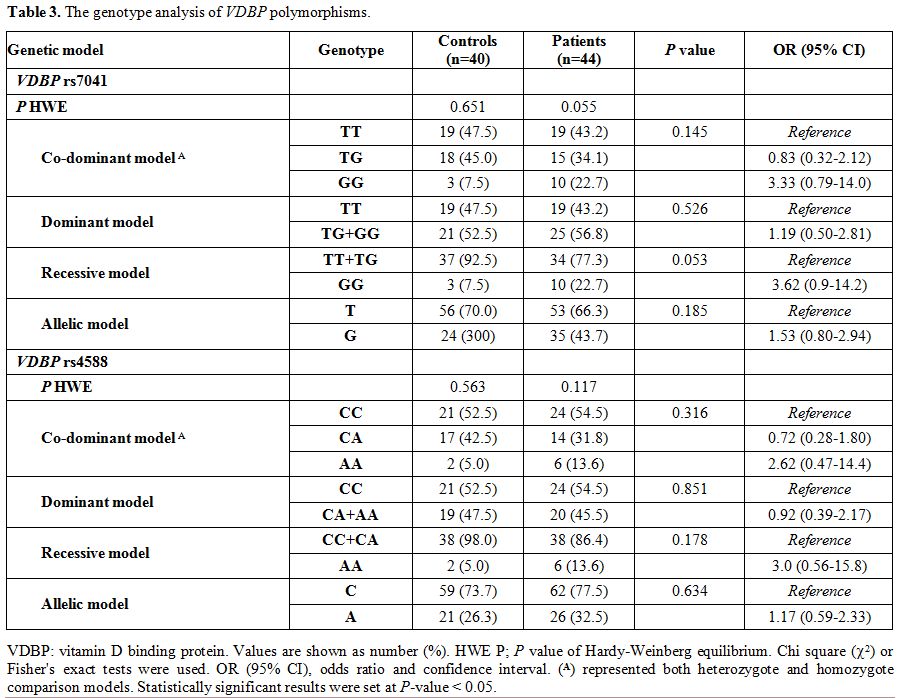 |
Table 3. The genotype analysis of VDBP polymorphisms. |
Association of clinical and biochemical features with VDBP polymorphisms. Disease characteristics of patients, according to VDBP rs4701 and rs4588 genotypes, are demonstrated in Table 4. The heterozygote form of the rs4701 variant was associated with higher weight in thalassemia patients (P = 0.031). The same TG genotype showed a higher frequency of osteoporosis among thalassemia patients (P = 0.023), while the homozygote state (GG) was associated with lower BMD than other genotypes (TT and TG) (P = 0.021).
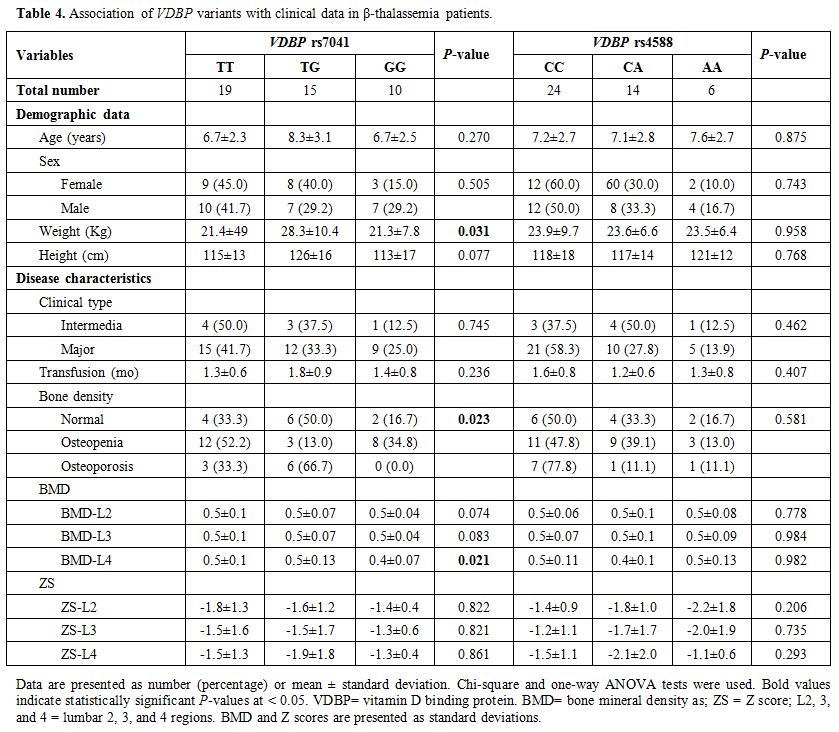 |
Table 4. Association of VDBP variants with clinical data in β-thalassemia patients. |
Discussion
Recent evidence supports the prevalence of low BMD in β-thalassemia pediatric patients despite vitamin D supplementation.[27] Osteoporosis had been observed among adult and pediatric thalassemia,[28] and vitamin D deficiency has been reported by many previous studies.[8,28-30] However, El-Edel et al.[31] could not find a significant difference in 25-hydroxy vitamin D level between pediatric thalassemia and healthy children.
The
present study revealed that vitamin D status and mineral concentrations
were normal in β-thalassemia children and controls. Apart from that,
the included patients were on continuous vitamin D supplementation; it
is worth noting that they were also on deferasirox chelation therapy
for at least being 2 years with adequate control of iron overload. A
previous study similarly concluded a significant improvement of BMD
after long term deferasirox chelation therapy.[32]
The
controversial outcomes observed in the studies mentioned above,
including the present one, could be related to the multifactorial
etiology of bone disorders in thalassemia; probably due to defective
liver hydroxylation, iron overload, the use of iron chelation therapy,
and the contribution of different genetic elements in this context.[32-35]
The
active vitamin D exerts most of its biological activities by binding to
a high-affinity receptor; VDR that forms a heterodimer with the RXRA
receptor, with subsequent interaction with several vitamin D response
elements, initiating a transcriptional signal on multiple effector
RNAs.[36,37] By this way, VDR/RXRA activation could
be implicated in transcriptional control of hundreds of genes related
to the diversity of vitamin D effects,[20,38] including regulation of the intestinal calcium uptake,[34] cytokine signaling, immune cells function, hematopoietic cells differentiation and proliferation,[39] and the final stages of monocyte and granulocyte colony-forming lines differentiation,[40] among others (reviewed in details previously).[41] The results of the present study have revealed a significant increase in peripheral VDR and RXRA
expression in thalassemic children compared to controls. To the best of
the authors̛ knowledge, the expression level of these receptors has not
been tested previously in thalassemia. However, the authors cannot
exclude the effect of the exogenous supplementation of vitamin D on the
circulating receptor upregulation as confirmed by previous experimental
studies that reported increased B cells VDR mRNA expression on exposure
to the biologically active vitamin D compared to cells in the resting
state.[42,43] In this sense, further expression
studies in newly diagnosed cases of β-thalassemia with no history of
receiving any type of medications are warranted to validate this
finding.
Accumulating evidence has suggested various factors could
affect circulating vitamin D levels with subsequent bone mineral
metabolism (e.g., ethnicity, gender, binding proteins, several variants
in VDR and VDBP, and other pharmacogenetic factors in vitamin D
metabolic pathways).[44,45] As VDR variants have been extensively studied in β-thalassemia,[21,46,47]
and up to the authors̛ knowledge, no study uncovered the association of
VDBP polymorphisms with BMD in β-thalassemia, the authors were
interested in exploring for the first time the impact of two most
common variants of VDBP gene;
rs7041 and rs4588 in the coding region of exon 11, on BMD in pediatric
β-thalassemia cases. These variants have been reported to be associated
with approximately 80% of the VDBP level variations.[48] In addition, they have been associated with vitamin D function,[16] and show different allele frequencies based on ethnic variations.[18]
Our
in silico analysis revealed that the exonic rs7041 and rs4588 variants
are located in the forward strand of the chromosome 4, positions:
71762617 and 71752606, respectively. The former variant consists of two
alleles, T and G, where T is the ancestral form. This single-nucleotide
variation is a missense one that leads to the substitution of Aspartate
by Glutamate at amino acid number 432. The later one included three
alleles C, A, and T, where the ancestral allele is C, and the minor
allele is A/T. Its missense variation changes Threonine to
Lysine/Methionine at amino acid number 436.[45]
Currently,
both study variants showed comparable frequencies in β-thalassemia
children and controls. Interestingly, rs4701 GG and TG genotypes showed
significant associations with lower BMD at level-L4 and a higher
frequency of osteoporosis (P = 0.021 and 0.023, respectively) (Table 4).
It is worth noting that the reflected phenotypic presentation of the
combined effect of both study variants will change VDBP availability
and affinity to vitamin D with subsequent impact on BMD.[49]
The three phenotypic variations from these variants include "GC1F,
GC1S, and GC2", which are sorted by their different VDBP levels in
homozygote states and affinity for 25-hydroxy vitamin D[48] with some controversy for these associations remain.[50]
As the GG genotype of the rs4701 variant represents the GC1S phenotype,
which is known by its intermediate affinity to vitamin D, this could,
in part, explain the observed association of this genotype with a high
frequency of osteoporosis in the present pediatric thalassemia cases.
Several previous studies confirmed the association of vitamin D status and BMD, according to VDBP genotypes.[17,51,52] Johnsen et al.,[51]
also, have reported that the correlations of the bio-available forms of
25-hydroxy vitamin D with bone density were stronger after adjusting
for the study variants. Similarly, other studies found that the
specified variants could be associated with either VDBP lower plasma
concentration or lower affinity to the total serum levels of 25-hydroxy
vitamin D and 1,25 dihydroxy vitamin D in cases of GC2 for rs4588, or
GC1F for rs7041, respectively.[53-56] However, Sinotte et al.[54] confirmed that VDBP
variants could explain only 2% or less of the variation in circulating
vitamin D levels, similar to the amount explained by vitamin D intake.
The latter finding can support the previously emerged conclusion by
Bhan[57] in that "the genetic variant could impact
the non-vitamin D binding activities of VDBP, including potential
effects on macrophage and osteoclast activation, so the effects on
vitamin D biology may not be the only relevant factor to explain the
changes in BMD".
It is worth noting that our findings with that of Abbassy et al.,[27] who found associations of some VDR genetic variants (i.e., BsmI bb, FokI
Ff, and ff) with BMD changes and occurrence of osteoporosis in the same
type of population, confirm and support vitamin D metabolic-axis
genetic variants implication in BMD of pediatric Egyptian β-thalassemia
patients.
Although the present study could be limited by the small
sample size and including β-thalassemia children on vitamin D
supplementation that warrant further large-scale studies on newly
diagnosed β-thalassemia cases in different ethnicities, an essential
element of the potential reliability of our study is its agreement with
HWE in both study groups, particularly the controls which ensures
population representation, excluding any guided sample selection by the
authors. Also, as explained previously, the external intake of vitamin
D could explain ≤ 2% of circulating vitamin D levels, which supports the significant implications of other factors.
Conclusions
The present study has reported an increase of circulating VDR and RXRA expressions in pediatric well-chelated β-thalassemia patients on vitamin D supplementation, and a significant association of VDBP
rs4701 variant with BMD-L4 and a higher frequency of osteoporosis in
the study population. These findings suggest that the genetic
background of pediatric β-thalassemia could be potentially implied in
BMD pathogenesis in β-thalassemia, but it is worth noting that the
simultaneous testing of multiple variants may be optimal for
determining the contribution of the genetic background on BMD, at least
in some populations. Further large-scale studies are warranted as
stated above to verify the current conclusions for future improvement
in the management of osteoporosis in this devastating disorder.
Acknowledgments
The
authors would like to thank all study participants and the Oncology
Diagnostic Unit, and Center of Excellence in Molecular and Cellular
Medicine; Suez Canal University, Egypt, for providing the facilities to
perform the current work.
References
- Weatherall DJ. The inherited diseases of hemoglobin
are an emerging global health burden. Blood. 2010;115(22):4331-6. Epub
2010/03/18. doi: 10.1182/blood-2010-01-251348. PubMed PMID: 20233970;
PubMed Central PMCID: PMCPMC2881491. https://doi.org/10.1182/blood-2010-01-251348 PMid:20233970 PMCid:PMC2881491
- El-Beshlawy
A, Youssry I. Prevention of hemoglobinopathies in Egypt. Hemoglobin.
2009;33 Suppl 1:S14-20. Epub 2009/12/17. doi:
10.3109/03630260903346395. PubMed PMID: 20001619. https://doi.org/10.3109/03630260903346395 PMid:20001619
- Shawky
RM, Kamal TM. Thalassemia intermedia: An overview. Egyptian Journal of
Medical Human Genetics. 2012;13(3):245-55. doi:
10.1016/j.ejmhg.2012.03.006. https://doi.org/10.1016/j.ejmhg.2012.03.006
- Soliman
AT. Vitamin D Status in Thalassemia Major: An Update. Mediterranean
Journal of Hematology and Infectious Diseases. 2013;5(1). doi:
10.4084/mjhid.2013.057. https://doi.org/10.4084/mjhid.2013.057 PMid:24106607 PMCid:PMC3787712
- Fung
EB, Aguilar C, Micaily I, Haines D, Lal A. Treatment of vitamin D
deficiency in transfusion-dependent thalassemia. American Journal of
Hematology. 2011;86(10):871-3. doi: 10.1002/ajh.22117. https://doi.org/10.1002/ajh.22117 PMid:21818763
- Singh
K, Kumar R, Shukla A, Phadke SR, Agarwal S. Status of 25-hydroxyvitamin
D deficiency and effect of vitamin D receptor gene polymorphisms on
bone mineral density in thalassemia patients of North India.
Hematology. 2013;17(5):291-6. doi: 10.1179/1607845412y.0000000017. https://doi.org/10.1179/1607845412Y.0000000017 PMid:22971535
- Nakavachara
P, Viprakasit V. Children with hemoglobin E/beta-thalassemia have a
high risk of being vitamin D deficient even if they get abundant sun
exposure: a study from Thailand. Pediatr Blood Cancer.
2013;60(10):1683-8. Epub 2013/06/05. doi: 10.1002/pbc.24614. PubMed
PMID: 23733667. https://doi.org/10.1002/pbc.24614 PMid:23733667
- Fahim
FM, Saad K, Askar EA, Eldin EN, Thabet AF. Growth Parameters and
Vitamin D status in Children with Thalassemia Major in Upper Egypt. Int
J Hematol Oncol Stem Cell Res. 2013;7(4):10-4. Epub 2014/02/08. PubMed
PMID: 24505537; PubMed Central PMCID: PMCPMC3915427.
- Root A. Disorders of calcium metabolism in the child and adolescent. Pediatric endocrinology. 2002.
- Christakos
S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism.
Endocrinol Metab Clin North Am. 2010;39(2):243-53, table of contents.
Epub 2010/06/01. doi: 10.1016/j.ecl.2010.02.002. PubMed PMID: 20511049;
PubMed Central PMCID: PMCPMC2879391. https://doi.org/10.1016/j.ecl.2010.02.002 PMid:20511049 PMCid:PMC2879391
- Yousefzadeh
P, Shapses SA, Wang X. Vitamin D binding protein impact on
25-hydroxyvitamin D levels under different physiologic and pathologic
conditions. International journal of endocrinology. 2014;2014. https://doi.org/10.1155/2014/981581 PMid:24868205 PMCid:PMC4020458
- Chun
RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D
and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol
Biol. 2014;144 Pt A:132-7. Epub 2013/10/08. doi:
10.1016/j.jsbmb.2013.09.012. PubMed PMID: 24095930; PubMed Central
PMCID: PMCPMC3976473. https://doi.org/10.1016/j.jsbmb.2013.09.012 PMid:24095930 PMCid:PMC3976473
- Malik
S, Fu L, Juras DJ, Karmali M, Wong BY, Gozdzik A, et al. Common
variants of the vitamin D binding protein gene and adverse health
outcomes. Crit Rev Clin Lab Sci. 2013;50(1):1-22. Epub 2013/02/23. doi:
10.3109/10408363.2012.750262. PubMed PMID: 23427793; PubMed Central
PMCID: PMCPMC3613945. https://doi.org/10.3109/10408363.2012.750262 PMid:23427793 PMCid:PMC3613945
- Cooke
NE, Willard HF, David EV, George DL. Direct regional assignment of the
gene for vitamin D binding protein (Gc-globulin) to human chromosome
4q11-q13 and identification of an associated DNA polymorphism. Hum
Genet. 1986;73(3):225-9. Epub 1986/07/01. doi: 10.1007/bf00401232.
PubMed PMID: 3015768. https://doi.org/10.1007/BF00401232 PMid:3015768
- Speeckaert
M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of
the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin
Chim Acta. 2006;372(1-2):33-42. Epub 2006/05/16. doi:
10.1016/j.cca.2006.03.011. PubMed PMID: 16697362. https://doi.org/10.1016/j.cca.2006.03.011 PMid:16697362
- Agnello
L, Scazzone C, Lo Sasso B, Bellia C, Bivona G, Realmuto S, et al. VDBP,
CYP27B1, and 25-Hydroxyvitamin D Gene Polymorphism Analyses in a Group
of Sicilian Multiple Sclerosis Patients. Biochem Genet.
2017;55(2):183-92. Epub 2016/12/03. doi: 10.1007/s10528-016-9783-4.
PubMed PMID: 27904983. https://doi.org/10.1007/s10528-016-9783-4 PMid:27904983
- Carpenter
TO, Zhang JH, Parra E, Ellis BK, Simpson C, Lee WM, et al. Vitamin D
binding protein is a key determinant of 25-hydroxyvitamin D levels in
infants and toddlers. J Bone Miner Res. 2013;28(1):213-21. Epub
2012/08/14. doi: 10.1002/jbmr.1735. PubMed PMID: 22887780; PubMed
Central PMCID: PMCPMC3511814. https://doi.org/10.1002/jbmr.1735 PMid:22887780 PMCid:PMC3511814
- Fu
L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants
of the vitamin D binding protein (DBP) predict differences in response
of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation.
Clin Biochem. 2009;42(10-11):1174-7. Epub 2009/03/24. doi:
10.1016/j.clinbiochem.2009.03.008. PubMed PMID: 19302999. https://doi.org/10.1016/j.clinbiochem.2009.03.008 PMid:19302999
- Barsony
J, Prufer K. Vitamin D receptor and retinoid X receptor interactions in
motion. Vitam Horm. 2002;65:345-76. Epub 2002/12/17. doi:
10.1016/s0083-6729(02)65071-x. PubMed PMID: 12481554. https://doi.org/10.1016/S0083-6729(02)65071-X
- Huang
P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor
superfamily: insights into physiology and therapeutics. Annu Rev
Physiol. 2010;72:247-72. Epub 2010/02/13. doi:
10.1146/annurev-physiol-021909-135917. PubMed PMID: 20148675; PubMed
Central PMCID: PMCPMC3677810. https://doi.org/10.1146/annurev-physiol-021909-135917 PMid:20148675 PMCid:PMC3677810
- Elhoseiny
SM, Morgan DS, Rabie AM, Bishay ST. Vitamin D Receptor (VDR) Gene
Polymorphisms (FokI, BsmI) and their Relation to Vitamin D Status in
Pediatrics betaeta Thalassemia Major. Indian J Hematol Blood Transfus.
2016;32(2):228-38. Epub 2016/04/12. doi: 10.1007/s12288-015-0552-z.
PubMed PMID: 27065588; PubMed Central PMCID: PMCPMC4789011. https://doi.org/10.1007/s12288-015-0552-z PMid:27065588 PMCid:PMC4789011
- Braegger
C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin
D in the healthy European paediatric population. J Pediatr
Gastroenterol Nutr. 2013;56(6):692-701. Epub 2013/05/28. doi:
10.1097/MPG.0b013e31828f3c05. PubMed PMID: 23708639. https://doi.org/10.1097/MPG.0b013e31828f3c05 PMid:23708639
- Bianchi
ML, Baim S, Bishop NJ, Gordon CM, Hans DB, Langman CB, et al. Official
positions of the International Society for Clinical Densitometry (ISCD)
on DXA evaluation in children and adolescents. Pediatr Nephrol.
2010;25(1):37-47. Epub 2009/07/16. doi: 10.1007/s00467-009-1249-z.
PubMed PMID: 19603190. https://doi.org/10.1007/s00467-009-1249-z PMid:19603190
- Livak
KJ, Schmittgen TD. Analysis of relative gene expression data using
real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
Methods. 2001;25(4):402-8. Epub 2002/02/16. doi:
10.1006/meth.2001.1262. PubMed PMID: 11846609. https://doi.org/10.1006/meth.2001.1262 PMid:11846609
- Toraih
EA, Fawzy MS, Mohammed EA, Hussein MH, El-Labban MM. MicroRNA-196a2
Biomarker and Targetome Network Analysis in Solid Tumors. Mol Diagn
Ther. 2016;20(6):559-77. Epub 2016/06/28. doi:
10.1007/s40291-016-0223-2. PubMed PMID: 27342110. https://doi.org/10.1007/s40291-016-0223-2 PMid:27342110
- Hussein
MH, Sobhy KE, Sabry IM, El Serafi AT, Toraih EA. Beta2-adrenergic
receptor gene haplotypes and bronchodilator response in Egyptian
patients with chronic obstructive pulmonary disease. Adv Med Sci.
2017;62(1):193-201. Epub 2017/03/23. doi: 10.1016/j.advms.2016.07.008.
PubMed PMID: 28327457. https://doi.org/10.1016/j.advms.2016.07.008 PMid:28327457
- Abbassy
HA, Elwafa RAA, Omar OM. Bone Mineral Density and Vitamin D Receptor
Genetic Variants in Egyptian Children with Beta Thalassemia Major on
Vitamin D Supplementation. Mediterr J Hematol Infect Dis.
2019;11(1):e2019013. Epub 2019/01/24. doi: 10.4084/MJHID.2019.013.
PubMed PMID: 30671219; PubMed Central PMCID: PMCPMC6328042. https://doi.org/10.4084/mjhid.2019.013 PMid:30671219 PMCid:PMC6328042
- Mirhosseini
NZ, Shahar S, Ghayour-Mobarhan M, Banihashem A, Kamaruddin NA, Hatef
MR, et al. Bone-related complications of transfusion-dependent beta
thalassemia among children and adolescents. Journal of Bone and Mineral
Metabolism. 2013;31(4):468-76. doi: 10.1007/s00774-013-0433-1. https://doi.org/10.1007/s00774-013-0433-1 PMid:23475127
- Sultan
S, Irfan SM, Ahmed SI. Biochemical Markers of Bone Turnover in Patients
with beta-Thalassemia Major: A Single Center Study from Southern
Pakistan. Adv Hematol. 2016;2016:5437609. Epub 2016/03/24. doi:
10.1155/2016/5437609. PubMed PMID: 27006658; PubMed Central PMCID:
PMCPMC4783526. https://doi.org/10.1155/2016/5437609 PMid:27006658 PMCid:PMC4783526
- Isik
P, Yarali N, Tavil B, Demirel F, Karacam GB, Sac RU, et al.
Endocrinopathies in Turkish Children with Beta Thalassemia Major:
Results from a Single Center Study. Pediatric Hematology and Oncology.
2014;31(7):607-15. doi: 10.3109/08880018.2014.898724. https://doi.org/10.3109/08880018.2014.898724 PMid:24854890
- El-Edel
RH, Ghonaim MM, Abo-Salem OM, El-Nemr FM. Bone mineral density and
vitamin D receptor polymorphism in beta-thalassemia major. Pak J Pharm
Sci. 2010;23(1):89-96. Epub 2010/01/14. PubMed PMID: 20067873.
- Voskaridou
E, Terpos E. New insights into the pathophysiology and management of
osteoporosis in patients with beta thalassaemia. British Journal of
Haematology. 2004;127(2):127-39. doi: 10.1111/j.1365-2141.2004.05143.x.
https://doi.org/10.1111/j.1365-2141.2004.05143.x PMid:15461618
- Casale
M, Citarella S, Filosa A, De Michele E, Palmieri F, Ragozzino A, et al.
Endocrine function and bone disease during long-term chelation therapy
with deferasirox in patients with beta-thalassemia major. Am J Hematol.
2014;89(12):1102-6. Epub 2014/09/10. doi: 10.1002/ajh.23844. PubMed
PMID: 25197009. https://doi.org/10.1002/ajh.23844 PMid:25197009
- Tantawy
AA, El Kholy M, Moustafa T, Elsedfy HH. Bone mineral density and
calcium metabolism in adolescents with beta-thalassemia major. Pediatr
Endocrinol Rev. 2008;6 Suppl 1:132-5. Epub 2009/04/11. PubMed PMID:
19337166.
- Gaudio A, Morabito N, Xourafa
A, Curro M, Caccamo D, Ferlazzo N, et al. Role of genetic pattern on
bone mineral density in thalassemic patients. Clin Biochem.
2010;43(10-11):805-7. Epub 2010/05/07. doi:
10.1016/j.clinbiochem.2010.04.070. PubMed PMID: 20444423. https://doi.org/10.1016/j.clinbiochem.2010.04.070 PMid:20444423
- Bunce
C, Brown G, Hewison M. Vitamin D and hematopoiesis. Trends in
Endocrinology and Metabolism. 1997;8(6):245-51. doi:
10.1016/s1043-2760(97)00066-0. https://doi.org/10.1016/S1043-2760(97)00066-0
- O'Kelly
J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal
myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor
knockout mice. Journal of Clinical Investigation. 2002;109(8):1091-9.
doi: 10.1172/jci0212392. https://doi.org/10.1172/JCI0212392 PMid:11956247
- Ryan
JW, Anderson PH, Morris HA. Pleiotropic Activities of Vitamin D
Receptors - Adequate Activation for Multiple Health Outcomes. Clin
Biochem Rev. 2015;36(2):53-61. Epub 2015/08/01. PubMed PMID: 26224895;
PubMed Central PMCID: PMCPMC4504155.
- Studzinski
GP, Harrison JS, Wang X, Sarkar S, Kalia V, Danilenko M. Vitamin D
Control of Hematopoietic Cell Differentiation and Leukemia. J Cell
Biochem. 2015;116(8):1500-12. Epub 2015/02/20. doi: 10.1002/jcb.25104.
PubMed PMID: 25694395. https://doi.org/10.1002/jcb.25104 PMid:25694395
- Taschner
S, Koesters C, Platzer B, Jorgl A, Ellmeier W, Benesch T, et al.
Down-regulation of RXRalpha expression is essential for neutrophil
development from granulocyte/monocyte progenitors. Blood.
2007;109(3):971-9. Epub 2006/10/05. doi: 10.1182/blood-2006-04-020552.
PubMed PMID: 17018855. https://doi.org/10.1182/blood-2006-04-020552 PMid:17018855
- Medrano
M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: Effect on
Haematopoiesis and Immune System and Clinical Applications. Int J Mol
Sci. 2018;19(9). Epub 2018/09/13. doi: 10.3390/ijms19092663. PubMed
PMID: 30205552; PubMed Central PMCID: PMCPMC6164750. https://doi.org/10.3390/ijms19092663 PMid:30205552 PMCid:PMC6164750
- Morgan
JW, Kouttab N, Ford D, Maizel AL. Vitamin D-mediated gene regulation in
phenotypically defined human B cell subpopulations. Endocrinology.
2000;141(9):3225-34. Epub 2000/08/31. doi: 10.1210/endo.141.9.7666.
PubMed PMID: 10965893. https://doi.org/10.1210/endo.141.9.7666 PMid:10965893
- Chen
S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of
1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol.
2007;179(3):1634-47. Epub 2007/07/21. doi: 10.4049/jimmunol.179.3.1634.
PubMed PMID: 17641030. https://doi.org/10.4049/jimmunol.179.3.1634 PMid:17641030
- Fawzy
MS, Beladi FIA. Association of Circulating Vitamin D, VDBP, and Vitamin
D Receptor Expression with Severity of Diabetic Nephropathy in a Group
of Saudi Type 2 Diabetes Mellitus Patients. Clin Lab.
2018;64(10):1623-33. Epub 2018/10/20. doi:
10.7754/Clin.Lab.2018.180401. PubMed PMID: 30336516. https://doi.org/10.7754/Clin.Lab.2018.180401
- Fawzy
MS, Elgazzaz MG, Ibrahim A, Hussein MH, Khashana MS, Toraih EA.
Association of group-specific component exon 11 polymorphisms with
bronchial asthma in children and adolescents. Scand J Immunol.
2019;89(3):e12740. Epub 2018/12/15. doi: 10.1111/sji.12740. PubMed
PMID: 30548492. https://doi.org/10.1111/sji.12740 PMid:30548492
- Tayel
SI, Soliman SE, Elsayed HM. Vitamin D deficiency and vitamin D receptor
variants in mothers and their neonates are risk factors for neonatal
sepsis. Steroids. 2018;134:37-42. Epub 2018/03/14. doi:
10.1016/j.steroids.2018.03.003. PubMed PMID: 29530503. https://doi.org/10.1016/j.steroids.2018.03.003 PMid:29530503
- Dimitriadou
M, Christoforidis A, Fidani L, Economou M, Perifanis V, Tsatra I, et
al. Fok-I gene polymorphism of vitamin D receptor in patients with
beta-thalassemia major and its effect on vitamin D status. Hematology.
2011;16(1):54-8. Epub 2011/01/29. doi:
10.1179/102453311X12902908411878. PubMed PMID: 21269569. https://doi.org/10.1179/102453311X12902908411878 PMid:21269569
- Powe
CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin
D-binding protein and vitamin D status of black Americans and white
Americans. N Engl J Med. 2013;369(21):1991-2000. Epub 2013/11/22. doi:
10.1056/NEJMoa1306357. PubMed PMID: 24256378; PubMed Central PMCID:
PMCPMC4030388. https://doi.org/10.1056/NEJMoa1306357 PMid:24256378 PMCid:PMC4030388
- Bhan
I. Vitamin d binding protein and bone health. Int J Endocrinol.
2014;2014:561214. Epub 2014/07/06. doi: 10.1155/2014/561214. PubMed
PMID: 24987416; PubMed Central PMCID: PMCPMC4058579.
- Lauridsen
AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin
D-binding protein (Gc globulin) is related to the Gc phenotype in
women. Clin Chem. 2001;47(4):753-6. Epub 2001/03/29. PubMed PMID:
11274031. https://doi.org/10.1093/clinchem/47.4.753 PMid:11274031
- Johnsen
MS, Grimnes G, Figenschau Y, Torjesen PA, Almas B, Jorde R. Serum free
and bio-available 25-hydroxyvitamin D correlate better with bone
density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest.
2014;74(3):177-83. Epub 2014/01/05. doi: 10.3109/00365513.2013.869701.
PubMed PMID: 24383929. https://doi.org/10.3109/00365513.2013.869701 PMid:24383929
- Nimitphong
H, Sritara C, Chailurkit LO, Chanprasertyothin S, Ratanachaiwong W,
Sritara P, et al. Relationship of vitamin D status and bone mass
according to vitamin D-binding protein genotypes. Nutr J. 2015;14:29.
Epub 2015/04/19. doi: 10.1186/s12937-015-0016-1. PubMed PMID: 25890042;
PubMed Central PMCID: PMCPMC4389666. https://doi.org/10.1186/s12937-015-0016-1 PMid:25890042 PMCid:PMC4389666
- Lauridsen
AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et
al. Plasma concentrations of 25-hydroxy-vitamin D and
1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin
D-binding protein): a cross-sectional study on 595 early postmenopausal
women. Calcif Tissue Int. 2005;77(1):15-22. Epub 2005/05/04. doi:
10.1007/s00223-004-0227-5. PubMed PMID: 15868280. https://doi.org/10.1007/s00223-004-0227-5 PMid:15868280
- Sinotte
M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of
the vitamin D binding protein and plasma concentrations of
25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr.
2009;89(2):634-40. Epub 2009/01/01. doi: 10.3945/ajcn.2008.26445.
PubMed PMID: 19116321. https://doi.org/10.3945/ajcn.2008.26445 PMid:19116321
- Lauridsen
AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin
D-binding protein (Gc globulin) is related to the Gc phenotype in
women. Clinical chemistry. 2001;47(4):753-6. https://doi.org/10.1093/clinchem/47.4.753 PMid:11274031
- Wang
TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al.
Common genetic determinants of vitamin D insufficiency: a genome-wide
association study. The Lancet. 2010;376(9736):180-8. doi:
10.1016/s0140-6736(10)60588-0. https://doi.org/10.1016/S0140-6736(10)60588-0
- Bhan
I. Vitamin D Binding Protein and Bone Health. International Journal of
Endocrinology. 2014;2014:1-5. doi: 10.1155/2014/561214. https://doi.org/10.1155/2014/561214 PMid:24987416 PMCid:PMC4058579
Supplementary Files
 |
Figure S1 Some results of lumbar spine
Dual-energy X-ray absorptiometry (DEXA) for study pediatric
β-thalassemia cases. A patient no. 5 (male; 6 year-old), B patient no.
17 (male; 7 year-old), C patient no. 28 (female; 15 3/12 year-old), D
patient no. 34 (female; 8 year-old). |
 |
Figure S2. Association of VDBP
polymorphisms (rs 7041 and rs4588) with biochemical data in pediatric
β-Thalassemia cases. Kruskal Wallis test followed by Dunn's multiple
comparison tests were used. |
[TOP]






