In humans, SARS-Cov-2 entry occurs via the host cell surface enzyme angiotensin-converting enzyme 2 (ACE2) receptor.[2] Specifically, downregulation of ACE2 leads to compensatory overproduction of angiotensin II by ACE. Angiotensin II, in turn, stimulates its type Ia receptor, leading to an increased pulmonary vascular permeability. Moreover, both lung injury and symptoms are associated with host response to viremia. Notwithstanding immune response appeared fundamental for SARS infection resolution, SARS-Cov-2 disease present increased levels of plasma pro-inflammatory mediators, as a consequence of an induced dysregulated cytokine storm.[3] Furthermore, Covid-19 patients’ CD4 T-cells arbor enhanced transcription of both IL-6 and GM-CSF favoring symptoms’ duration and disease progression. For these reasons, drugs rebalancing the host immune system, such as pidotimod, could to theoretically useful to prevent SARS-COV-2 clinical worsening.
Pidotimod has been long used both in children and in adults mostly to prevent respiratory tract infections and exacerbations in patients affected by obstructive lung diseases. As a synthetic dipeptide molecule (3-l-pyro- glutamyl-l-thiazolidine-4carboxilic acid) endowing immunomodulatory activity, it affects both innate and adaptive immune responses.[4]
Aim of this study is to evaluate both efficacy and safety of pidotimod in paucisymptomatic SARS-CoV-2 patients without any evidence of concurrent pneumonia.
We enrolled SARS-CoV2 positive patients (Brescia-COVID Respiratory Severity Scale 0), with fever and cough without acute respiratory failure or sign of pneumonia from March to April 2020 at the Infectious Diseases Clinic, University ‘G. d’Annunzio’, SS Annunziata Hospital of Chieti (Italy). None of them required standard therapy regimens or hospitalization.
Twenty SARS-CoV-2 1:1 allocated patients were enrolled and resulted into two groups: Group A (Pidotimod group: Pidotimod 800mg twice a day orally per 10 days) and Group B (Control group: symptomatic regimens). All demographic, epidemiological and clinical data as far as laboratory findings (blood count, serum creatinine, eGFR, D-dimer, LDH, CRP, AST, pO2, pCO2, P/F ratio and lactate) were collected.
All patients were scheduled and interviewed at seven and fourteen days after admission. The study protocol was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The analysis was conducted with IBM SPSS version 20.0 (IBM®, Segrate MI, Italy). Data were reported as absolute numbers (N), percentages (%), means, standard deviations (S.D.s) with their relative 95% confidence interval (95% CI). Statistical differences or correlations between cohorts were evaluated with paired t-test both for categorical and continuous variables. Standard errors (S.E.) and differences (Df) were also reported. A value <0.05 for both was considered significant.
Among twenty patients, 13 (65%) were male and of Caucasian ethnicity. With a mean age of 45.90±10.60 years and a mean time-to-symptoms onset of 8.80±4.27 days, twelve (60%) referred suspected previous exposure. Fatigue and myalgias were the most common non-respiratory symptoms (n = 11, 55%), followed by headache (n=4, 20%) and dysgeusia (n=4, 20%). No patient showed any radiographic sign of pneumonia on admission chest x-ray (Table 1).
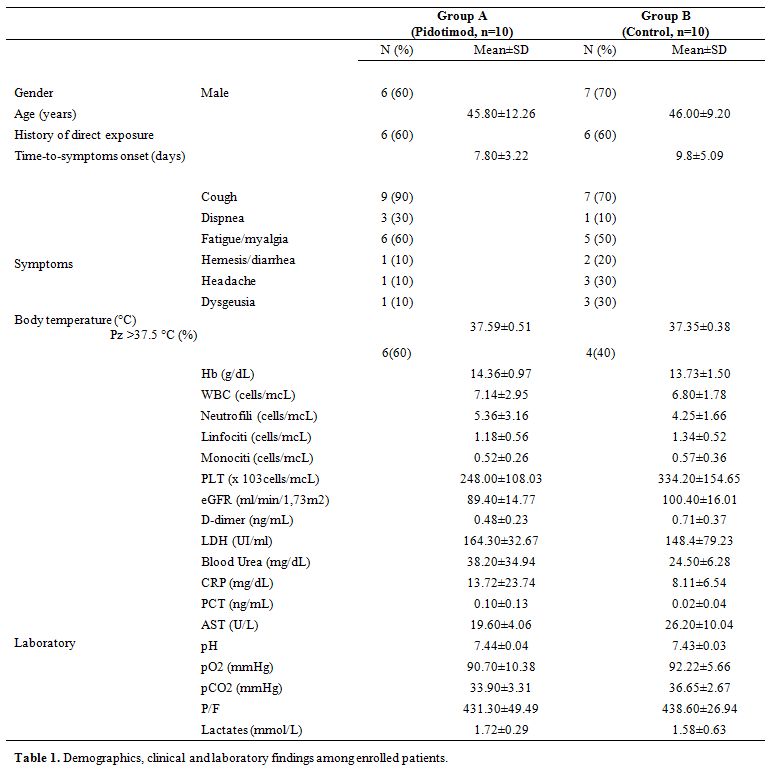 |
Table 1. Demographics, clinical and laboratory findings among enrolled patients. |
In the cohort study, no differences about symptoms’ severity, laboratory and clinical features were found (data not shown), confirming the absence of any selection bias. Concerning with patients’ outcome, Pidotimod group showed an earlier clinical resolution than the control one (4.10±2.18 vs 7.50±2.63 days; 95%CI: 1.13 – 5.67, S.E.: 1.08; p=0.006) (Figure 1). No drug-induced side effects or disease progression during experimental regimen were reported.
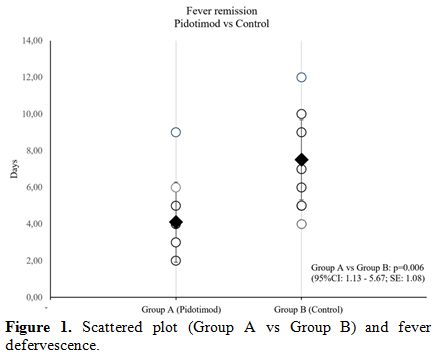 |
Figure 1. Scattered plot (Group A vs Group B) and fever defervescence. |
In our study, Pidotimod administration resulted in a significant reduction of symptoms, fever in particular.
Several studies reported pidotimod regimens as adjuvant therapy in several conditions. In elderly subjects, it enhances cell proliferation and secretion of IFN-γ and decrease of IL-6 production.[4] Similarly, immune host modulation has been reported in decreasing susceptibility to rhinovirus infection, and neutrophil-mediated pulmonary parenchymal injury via TLR-2 upregulation without any IL-8 levels increase.[5] In vitro study demonstrated that pidotimod is able to down-regulate MCP-1, which is a master regulator in the inflammatory response associated with severe recurrent viral bronchiolitis.[6] Finally, pidotimod promoted maturation of mucosal dendritic cells, thus playing a putative role in the expression of HLD-DR and T cells.[7] All these translated effects could represent a new approach on COVID-19 infection management. Recent data report a dysregulated activation of macrophage compartment could contribute to a hyper inflammation state in COVID19 patients,[8] as confirmed by high concentrations of monocyte recruiting chemokines and of mononuclear phagocytes sampled in SARS-CoV-2 bronchoalveolar cytology specimens.
In this setting, the modulation of the host response may play a fundamental role.
Therefore, a lot of immunomodulatory agents are rapidly going into clinical trials as well as already being used routinely in the clinic in an off-label manner.[9] In our study, in the outpatient population affected by SARS-CoV2 infection, pidotimod appears as a valid option to reduce the duration of symptoms in patients, as an earlier defervescence of fever and it could prevent the cytokine cascade activation. Rebalancing the immune status with pidotimod may also have prevented the evolution of the infection in patients. We know about the limitation of this study, such as the small sample size with a not negligible type-II error and the lack of randomization.
This interesting but preliminary results represent the first available results of empiric treatment of inhouse SARS-Cov-2 patients.
In conclusion, in ambulatorial adult patients with SARS-Cov2 infection without pneumonia, pidotimod could be considered an option, well tolerated and associated with a rapid reduction of systemic symptoms of disease. However, further studies are needed to confirm these data.