Type of transcript is of interest also in the field of treatment-free remission (TFR), which is the current goal of all hematologists who treat CML, although not all reports on discontinuation take into consideration this variable.
In rare cases of CML, breakpoints on chromosomes 9 and 22 occur in unusual regions, giving rise to atypical fusion transcripts. These transcripts, including e13a3, e14a3, e1a3, e19a2, e8a2, are not amplified by quantitative Real-Time PCR (RT-qPCR), which is the standardized and recommended method of molecular response evaluation. Current recommendations and guidelines consider the possibility to perform RT-qPCR on BCR-ABL1 as one of the criteria to meet to pursue tyrosine kinases inhibitors (TKI) stop both in clinical trials and in everyday practice as well.[6,7]
Nowadays, disease monitoring in atypical transcripts patients is performed routinely by non-quantitative Nested PCR, providing only an idea of their minimal residual disease (MRD) status.
Not having certainties about their biological behavior, due to their rarity, the lack of quantitative information about their molecular response automatically excludes patients with atypical transcripts from prospective protocols on TKI discontinuation.
We retrospectively collected seven patients with chronic-phase CML carrying rare atypical transcripts, identified by Sanger sequencing,[8] who discontinued TKI for various reasons, such as severe comorbidities, toxicity, or patient request (Table 1).
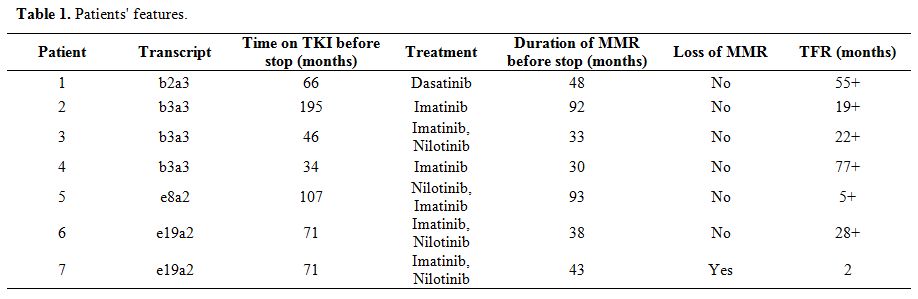 |
Table 1. Patients' features. |
For this study, we defined stable Major Molecular Response (MMR) as an undetectable transcript at nested PCR in all follow-ups in the last 24 months before discontinuation. Molecular monitoring was usually performed every three months during treatment and every month for the first six months after TKI discontinuation, followed by evaluation every six weeks for the remaining six months and every three months after then.[8]
Patients showed a stable MMR, and the median duration of treatment with TKI was 71 months (range: 34-195), the median duration of MMR at nested PCR before discontinuation was 43 months (range: 30-93). Only one patient resumed TKI therapy two months after stopping due to nested PCR positivity in two consecutive controls. The other six patients remained off-treatment at last observation after a median follow-up of 25 months (range: 5-77). Among these, five patients remained negative, with an undetectable transcript in all samples after discontinuation. Patient 3, who stopped the second line Nilotinib for intolerance, showed a fluctuation after stopping TKI between negative PCR and low-level positivity at the second step of nested PCR (2 out of 13 samples). No progressions occurred. All patients, including the one that resumed therapy, are in MMR at the last follow-up.
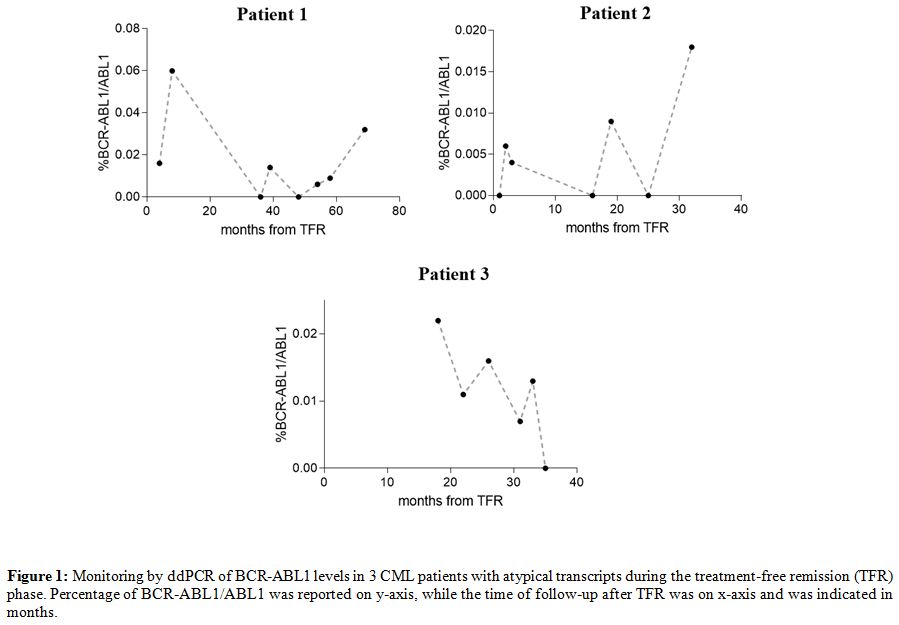
Although nowadays nested PCR represents the only routinely accepted method to monitor molecular response in CML patients with atypical transcripts, the qualitative nature of its results is not enough in an era of quantitative analysis. For this reason, we used recently published droplet digital PCR (ddPCR) assays[9] to quantify the BCR-ABL1 levels in 3 of 6 collected patients in TFR (unfortunately, for 3 of these, RNA samples were not available after routine diagnostic tests). Twenty-one follow-ups were tested after TKI suspension (7 for the patient 1, 8 for the patient 2, and 6 for the patient 3), and results were reported in Figure 1 and Table 2.
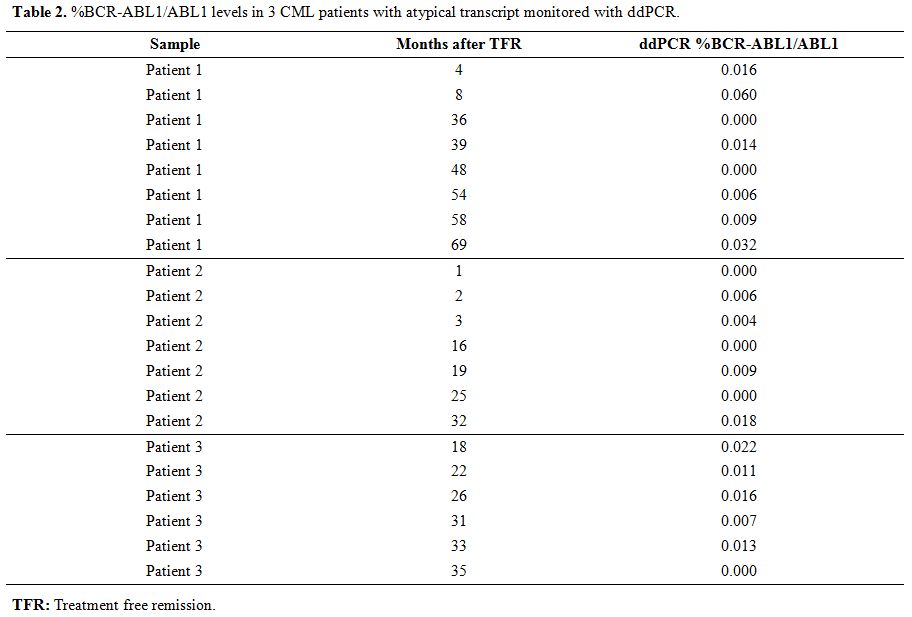 |
Table 2. %BCR-ABL1/ABL1 levels in 3 CML patients with atypical transcript monitored with ddPCR. |
All the tested follow-ups showed a BCR-ABL1/ABL1 percentage lower than 0.1% during all the TFR periods; in some points, %BCR-ABL1/ABL1 achieve values lower than 0.01%, and in 6 follow-ups BCR-ABL1 levels resulted undetectable (0%). Our data in these three patients confirmed with quantitative information the achievement of a stable MMR, previously defined only by qualitative data (nested PCR).
To our knowledge, there are no reports in the literature about patients with atypical transcripts who discontinued therapy. Although current guidelines do not recommend discontinuation for patients lacking a standardized quantitative method for response monitoring, we observed that our small cohort stopped the treatment successfully.
In this particular moment where CML care is focused on TKI discontinuation, it seems rather important to us to raise consciousness on the possibility to extend the policy of withdrawing TKI even in carefully selected patients harboring atypical transcripts. The rapid evolution of molecular technologies in the last years, in particular the use of ddPCR, could help the exploration of TFR opportunity also in these rare cases and could pave the way to study how the atypical transcripts affect treatment response.
In our opinion, this leads to two important matters of debate: first, may qualitative analysis suffice, at least in a specific setting, for MRD monitoring? This could be of interest to all low-income countries that cannot afford to perform RT-qPCR during treatment nor discontinuation. Second, is it plausible to assume that patients who carried the atypical transcript may also have the opportunity to stop treatment? Although our cohort is limited, these patients behave as "standard breakpoints carriers" in terms of survival and progression during therapy. Furthermore, among our cases was also present one patient with fluctuation of BCR-ABL1 levels during the TFR phase, which was not at the end associated with relapse. Although the definition of fluctuation cannot be the same of the A-STIM due to the lacking of the MMR threshold to consider, we observed that, as in the mentioned study, the occurring of this pattern of positive values of BCR-ABL1 did not impair the successfulness of discontinuation.[10]
Although our data are encouraging and represent a preliminary step to consider the possibility of TKI discontinuation also for these patients, further reports are of course needed to make our observations more reliable: the increase in the number of cases we were able to collect, as well as the application of new quantitative technologies, such as digital PCR, for the MRD quantification.
To date, there are no standardized primers and probes set to monitor patients with atypical BCR-ABL1 transcripts with qRT-PCR, thus it is impossible to compare the two methods, and it is difficult to define a priori which is the best technique between qRT-PCR and ddPCR. Based on our experience and literature, ddPCR technology provides absolute quantification of target copies, without the need for standard curves; the massive sample partitioning enables the reliable measurement of small copy numbers of transcript, and error rates are reduced by removing the amplification efficiency reliance of qRT-PCR. Furthermore, recently published works, that compare qRT-PCR and ddPCR methods for the monitoring of canonical BCR-ABL1 fusion transcripts, suggest that ddPCR could be a reliable and promising tool and conclude that ddPCR has a good agreement with qRT-PCR, but it is more precise and reproducible in the quantification of very low BCR-ABL1 transcript levels.[11-14] Lastly, a standardization process of BCR–ABL1 molecular monitoring for CML patients with rare variants by harmonization to an International Scale could be useful to define MRD levels better, compare results, and establish a better therapeutic strategy.