Xiaoqiang Zheng*1, Hongbing Rui1, Ying Liu2 and Jinfeng Dong1.
1 Department
of Hematology and Rheumatology, The First Affiliated Hospital of Fujian
Medical University, Fuzhou 350000, P.R. China.
2 Department of Liver Medicine, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350000, P.R. China.
Correspondence to: Dr. Xiaoqiang Zheng, Department of Hematology
and Rheumatology, The First Affiliated Hospital of Fujian Medical
University, No.20 Chazhong Road, Fuzhou 350000, P.R. China.
Tel.+86-17712907364. E-mail:
xiaoqiangzheng0207@163.com
Published: November 1, 2020
Received: July 7, 2020
Accepted: October 7, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020073 DOI
10.4084/MJHID.2020.073
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
This
study aimed to explore B-cell lymphoma cells' proliferation and
apoptosis under targeted regulation of FOXO3 by miR-155. We analyzed
the differences between B-cell lymphoma cells and B lymphocytes in
expressions of miR-155 and FOXO3, explored the effects of miR-155 on
proliferation and apoptosis of B-cell lymphoma cells, and relevant
mechanisms, and also analyzed the relationship between expressions of
miR-155 and FOXO3 in 42 patients with diffuse large B-cell lymphoma
(DLBCL) and clinical characteristics of them. B-cell lymphoma cells
showed a higher expression of miR-155 and a low expression of FOXO3
than B lymphocytes (both P<0.05). B-cell lymphoma cells transfected
with miR-155-inhibitor showed significantly decreased expression of
miR-155, significantly weakened cell proliferation ability, and
increased cell apoptosis rate (all P<0.05), and they also showed
upregulated expression of FOXO3 (P<0.05). Dual-luciferase reporter
assay revealed that there were targeted binding sites between miR-155
and FOXO3. Compared with B-cell lymphoma cells transfected with
miR-155-inhibitor alone, those with co-transfection showed lower
expression of FOXO3, higher proliferation and lower cell apoptosis rate
(all P<0.05). The expression of miR-155 in DLBCL tissues was higher
than that in tumor-adjacent tissues (P<0.05), and the expressions of
miR-155 and FOXO3 were closely related to the international prognostic
index (IPI) and the 5-year prognosis and survival of the patients
(P<0.05). miR-155 can promote the proliferation of B-cell lymphoma
cells and suppress apoptosis of them by targeted inhibition of FOCXO3,
and both over-expression of miR-155 and low expression of FOXO3 are
related to poor prognosis of DLBCL patients.
|
Introduction
B-cell lymphoma is a lymphoma from B cells, including Hodgkin's lymphoma and non-Hodgkin's lymphoma.[1]
Non-Hodgkin's lymphoma accounts for about 3/4 of all B-cell lymphomas,
and the most common non-Hodgkin's lymphoma is diffuse large B-cell
lymphoma (DLBCL), which accounts for about 30%-40% of non-Hodgkin's
lymphoma and shows an incidence increasing at a rate of 3% per year.[2,3]
Although therapeutic regimens for DLBCL have made significant progress,
DLBCL patients' prognosis is still not optimistic. For example,
chemotherapy regimens based on anthracycline are only effective for
40%-50% of DLBC patients,[4] so it is of great clinical significance to find a new therapeutic target.
miRNAs
are a short-chain non-coding RNA with a length of about 20-24
nucleotides, which can inhibit the stability and translation of mRNA
and thus regulate proteins' expressions. miRNAs are abnormally
expressed in nearly 400 human diseases, and it is of great significance
to study the mechanism of miRNAs in the diagnosis and treatment of
diseases.[5,6] miR-155 is located in the exon 3
(21q21.3) of the B-cell integration cluster on human chromosome 21. In
recent years, studies have reported that miR-155 is closely related to
the occurrence and development of DLBCL. For example, a study by Zhang
et al. found that miR-155 may affect the metastasis of DLBCL and
prognosis of patients by regulating transcription factor forkhead box
P3,[7] and a study by Huang et al. also found that
miR-155 promoted the growth of DLBCL cells by activating PI3K-AKT
pathway through inhibiting endogenous PIK3R1.[8]
Forkhead-box class O transcription factor (FOXO) is an important tumor
suppressor, which can inhibit tumor cell cycle progression and induce
programmed death of tumor cells.[9] FOXO3 is an
essential member of the FOXO family, able to regulate the proliferation
of immune cells such as B lymphocytes and T lymphocytes.[10] Immune response disorder is an important factor inducting DLBCL,[11]
but there are few studies on FOXO3 in DLBCL. A study by Huang et al.
pointed out that miR-155 could target FOXO3 to suppress apoptosis of
monocytes,[12] and Ling et al. also pointed out that miR-155 could target FOXO3 to regulate proliferation and invasion of gliomas[13]
and that may be another mechanism of miR-155 in DLBCL. This study
explored B lymphocytes' proliferation and apoptosis under targeted
regulation of FOXO3 by miR-155 to find a new therapeutic target for
DLBCL.
Materials and Methods
Cell culture.
Human B-cell lymphoma cells DOHH2 and OCI-LY10 (BNCC338032 and
BNCC337742) and human B lymphocyte AHH-1 (ATCC No. CRL-8146) were
purchased from BeNa Culture Collection and ATCC core collection,
respectively. AHH-1 was collected from the peripheral blood of a 33
years old human of Caucasian ethnicity. DOHH2 was cultured in 90%
high-sugar Dulbecco's modified eagle medium (DMEM) containing 4mmL of
glutamine and sodium pyruvate and 10% fetal bovine serum (FBS), and
AHH-1and OCI-LY10 were cultured in 90% Roswell Park Memorial
Institute-1640 (RPMI-1640) containing 10% FBS. The cells were all
cultured under 95% air + 5% carbon dioxide at 37℃. The purchased cells
were used after 2-3 times of passage. Cells at the logarithmic growth
phase were collected and lysed with TRIzol lysate, and then the total
RNA was extracted from the cells with chloroform, isopropanol, and
ethanol in order. The purity, concentration, and integrity of the total
RNA were determined using ultraviolet spectrophotometry and agarose gel
electrophoresis. It was required that the ratio of these factors at 28s
to these factors at 18s was larger than or equal to 2, and the ratio of
A260/A280 was between 1.8 and 2.1.
Source of patients sample.
The patients' inclusion criteria were as follows: Patients confirmed
based on histopathology, patients without other lymph node diseases,
and patients with detailed case data and follow-up data. The
researchers followed the Declaration of Helsinki.
The patients' exclusion criteria were as follows: patients with other
tumors or history of tumors; patients with severe diseases in heart,
brain, liver, kidney or vessel, or with a severe infection such as
sepsis, pregnant women, or patients with cardiovascular diseases or
hepatorenal diseases. This study was approved by the Ethics Committee
of The First Affiliated Hospital of Fujian Medical University, and the
patients and their families signed an informed consent form based on
our consultation by telephone or letter. Tumor tissues and normal
tumor-adjacent tissues were collected from the tissues of 42 DLBCL
patients (30-80 years old) stored from March 2010 to May 2014. Total
RNA was extracted using Qiazol reagent and RNAeasy Mini Kit (Qiagen,
Hombrechtikon, Switzerland) according to the manufacturer's
instructions. Total RNA, 250 ng, was reverse transcribed, and the same
RNA samples were used for qPCR, as described in the following section.
For western blot, proteins from biopsy tissue were extracted using the
RIPA lysis method. The total proteins' concentration was determined
using the BCA method and adjusted to 4μg/μL.
Main reagents and instruments.
Lipofectamine TM2000 transfection kit (Invitrogen Company, United
States, item number: 35050); TRIzol kit (Invitrogen Company, United
States, item number: 15596018); EasyScript One-Step RT-PCR SuperMix kit
(Beijing TransGen Biotech, China, item number: AE411-02); RIPA kit,
bicinchoninic acid (BCA) protein kit, and electrochemiluminescence
(ECL) kit (Thermo Scientific™, item numbers: 89901, 23250, and 35055);
rabbit anti-FOXO3 polyclonal antibody and goat anti-rabbit
immunoglobulin G (IgG) secondary antibody (monoclonal antibody) (Abcam
Company, United States, item numbers: ab58518 and ab6721); cell
counting kit-8 (CCK8) kit (Beijing Beyotime Biotechnology, China, item
number: C0037); Annexin V-FITC/PI apoptosis determination kit
(Invitrogen Company, United States, item number: V35113).
Construction of expression vectors and transfection.
All expression vectors were designed by Thermo Fisher Scientific
(China), and the expression vectors included FOXO3 low expression
vector (si-FOXO3), the miR-155 low expression vector
(miR-155-inhibitor), miR-155 over-expression vector (miR-155-mimic),
blank vector miR-NC, blank vector si-NC, pMiR-miR-155-3UTR wild type
(Wt), pMiR- miR-155-3UTR Mutant type (Mut) and blank vector pMiR-NC.
Cells at the logarithmic growth phase were collected, digested with
trypsin, and then resuspended. Subsequently, the cells were seeded into
a 96-well plate and transfected with expression vectors when the fusion
degree was up to about 80%. The specific operation steps were carried
out by referring to the instructions of the kit. The cells were
cultured in an incubator with 5% CO2
at 37℃ for 48h, and the culture medium was replaced every 6h.
Quantitative real-time polymerase chain reaction (qRT-PCR) and Western
blot assay were employed to analyze the transfection results. Cells
that did not receive any intervention were taken as a blank group.
qRT-PCR.
This study carried out one-step RNA amplification in a total of 20μl of
total reaction volume containing 1μg of RNA Template, 0.4μl of Forward
GSP (10μM), 0.4μl of Reverse GSP (10μM), 10μl of 2*One-Step Reaction
Mix, 0.4μl of EasyScript One-Step Enzyme Mix, and RNase-free water to
adjust the volume. The reaction conditions were as follows: 40℃ for 30
min, 94℃ for 5 min, 94℃ for 30 s, 60℃ for 30 s, 72℃ at 2kb/min, 72℃ for
10 min, a total of 42 cycles. In order to normalize the target and
target gene expression, U6 was used as an internal reference gene
control. The data collected was analyzed as per 2-ΔΔCt method and
expressed as folds over experimental control groups. These experiments
were performed in three biological replicates. The primer sequences are
shown in Table 1.
 |
Table 1. Primer sequences.
|
Western blot.
The total protein concentration in each sample was determined using the
BCA method and normalized to 4μg/μL. The total protein was separated
through 12% polyacrylamide gel electrophoresis. The initial voltage was
90V, and then the voltage was increased to 120V to move the sample to
an appropriate position of the separation gel. After electrophoresis,
the protein was transferred to a membrane under 100V constant voltage
for 100min and blocked at 37℃ for 60 min. Subsequently, the membrane
was blocked with 5% skim milk powder for future immune response. The
membrane was incubated with primary antibody (1:1000) at 4℃ for one
night, then washed with warm PBS three times, 5min each time. After
washing, the membrane was incubated with secondary antibody (1: 1000)
at room temperature for one h. After incubation, the protein was
developed and fixed with an ECL agent. The expression of the U6 gene
was used as an internal reference control. The scanned protein band was
analyzed using Quantity One software, and the relative protein
expression level = the gray value of the band/gray value reference.
Cell proliferation detection by CCK-8 assay.
Cells at the logarithmic growth phase were collected, digested with
trypsin, and resuspended. A total of 100μL of cells were seeded into a
96-well plate after the concentration was adjusted to 2*104/ml.
The cells were added with 200μL of CCK8 mixed solution (10: 1) at 24h,
48h, 72h, and 96h after culturing, and after the 4-time points, the
cells were cultured for 3h again, and then the optical density (OD) of
each well at 450nm was determined.
Cell apoptosis determination.
The cells were digested with 0.25% trypsin. After digestion, the cells
were washed with PBS two times, then added with 100μL of binding buffer
to prepare 1*106 cells /mL
suspension. The suspension was added with AnnexinV-FITC and PI in
order, incubated at room temperature for 5min in the dark, and finally
detected using the CytoFLE S flow cytometer system. The experiment was
repeated three times, and the average value was taken.
Dual-luciferase reporter assay.
Human embryonic kidney cell 293T (BeNa Culture Collection, BNCC100530)
were cultured to the logarithmic growth phase and then transfected with
pmirGLO-FOXO3-3'UTR wild type (Wt), pmirGLO-FOXO3-3'UTR mutant type
(Mut), miR-155-mimic, and miR-NC. At 48h after transfection, the cells'
fluorescence intensity was determined using the dual-luciferase
determination system (CytoFLEX flow cytometer). The sequences were
designed by Thermo Fisher Scientific (China).
Statistical analysis.
SPSS 19.0 (Asia Analytics Formerly SPSS China) was adopted in this
study. Measurement data were expressed by mean ± standard deviation
(mean ± sd), and comparison between groups was analyzed using the
independent-samples T-test. Receiver operating characteristic (ROC)
curves were adopted for diagnostic value evaluation. Pearson
correlation analysis was adopted to analyze correlation. P<0.05
indicated a significant difference. Separate biological replicates
trails were conducted thrice, and the experimental data is showcased
here as average and standard deviation.
Results
Expressions of miR-155 and FOXO3 in B-cell lymphoma cell lines.
The qRT-PCR assay revealed that the expression of miR-155 in DOHH2 and
OCI-LY10 cells was higher than that in AHH-1cells (P<0.05), while
the expression of FOXO3 in DOHH2 and OCI-LY10 cells was lower than that
in AHH-1cells (P<0.05) (Figure 1a).
In Tumor tissues and normal tumor-adjacent tissues samples of patients
showed similar trends for the expression of miR-155 and FOXO3 (Figure 1b)
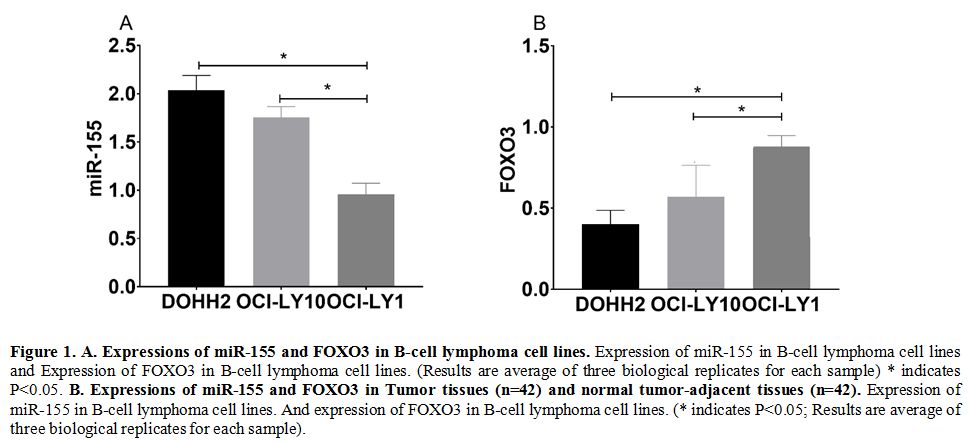 |
Figure
1. A. Expressions of miR-155 and FOXO3 in B-cell lymphoma cell lines.
Expression of miR-155 in B-cell lymphoma cell lines and Expression of
FOXO3 in B-cell lymphoma cell lines. (Results are average of three
biological replicates for each sample) * indicates P<0.05. B. Expressions of miR-155 and FOXO3 in Tumor tissues (n=42) and normal tumor-adjacent tissues (n=42).
Expression of miR-155 in B-cell lymphoma cell lines. And expression of
FOXO3 in B-cell lymphoma cell lines. (* indicates P<0.05; Results
are average of three biological replicates for each sample).
|
Effects of inhibiting miR-155 on proliferation and apoptosis of B-cell lymphoma cells. DOHH2 and OCI-LY10 cells transfected with miR-155-inhibitor showed significantly decreased expression of miR-155 (Figure 2a and 2b), significantly decreased cell proliferation ability (Figure 2c and 2d), and increased cell apoptosis rate (Figure 2e) (all P<0.05).
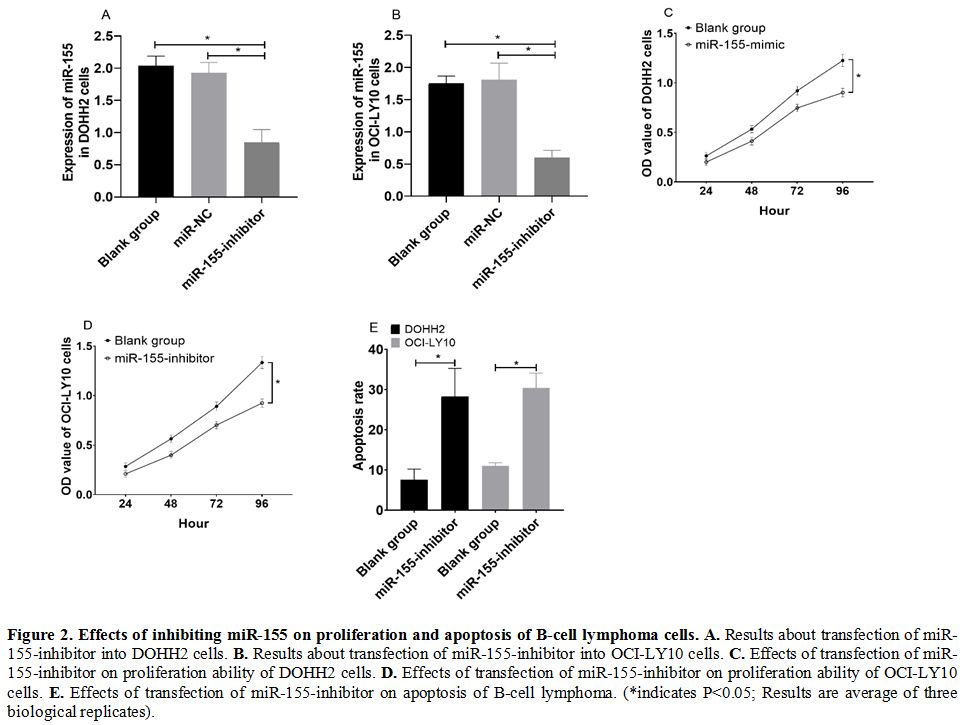 |
Figure 2. Effects of inhibiting miR-155 on proliferation and apoptosis of B-cell lymphoma cells. A. Results about transfection of miR-155-inhibitor into DOHH2 cells. B. Results about transfection of miR-155-inhibitor into OCI-LY10 cells. C. Effects of transfection of miR-155-inhibitor on proliferation ability of DOHH2 cells. D. Effects of transfection of miR-155-inhibitor on proliferation ability of OCI-LY10 cells. E.
Effects of transfection of miR-155-inhibitor on apoptosis of B-cell
lymphoma. (*indicates P<0.05; Results are average of three
biological replicates).
|
Effects of inhibiting miR-155 on expression of FOXO3 in B-cell lymphoma cells. In western blot results, DOHH2 and OCI-LY10 cells transfected with miR-155-inhibitor showed increased expression of FOXO3 (Figure 3a) (P<0.05). Relative expression of miR-155 was more in miR-155-mimic, while FOXO3 was comparable in both (Figure 3b).
The conclusion of predication by TargetScanHuman 7.2 indicated that
there were targeted binding sites between miR-155 and FOXO3.
Dual-luciferase reporter assay revealed that after transfection with
miR-155-mimic, the fluorescence intensity of cells in the
pmirGLO-FOXO3-3'UTR Wt group was significantly lower than that in cells
in the pmirGLO-FOXO3-3'UTR Mut (P<0.05). (Figure 3c)
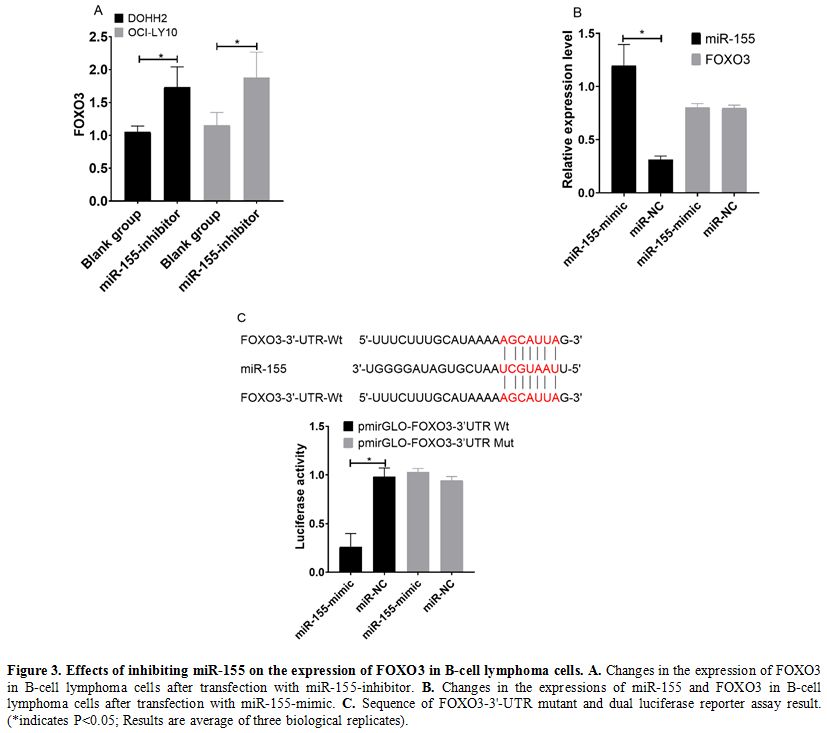 |
Figure 3. Effects of inhibiting miR-155 on the expression of FOXO3 in B-cell lymphoma cells. A. Changes in the expression of FOXO3 in B-cell lymphoma cells after transfection with miR-155-inhibitor. B. Changes in the expressions of miR-155 and FOXO3 in B-cell lymphoma cells after transfection with miR-155-mimic. C. Sequence
of FOXO3-3'-UTR mutant and dual luciferase reporter assay result.
(*indicates P<0.05; Results are average of three biological
replicates).
|
Effects of inhibiting FOXO3 on tumor-promoting action of miR-155.
DOHH2 and OCI-LY10 cells co-transfected with miR-155-inhibitor +
si-FOXO3 were not different from those transfected with
miR-155-inhibitor alone (P>0.05) (Figure 4a), but showed significantly lower expression of FOXO3 than those transfected with miR-155-inhibitor alone (P<0.05) (Figure 4b).
In addition, DOHH2 and OCI-LY10 cells co-transfected with
miR-155-inhibitor + si-FOXO3 showed stronger cell proliferation ability
(Figure 4c and 4d) and lower cell apoptosis rate than those transfected with miR-155-inhibitor alone (both P<0.05) (Figure 4f).
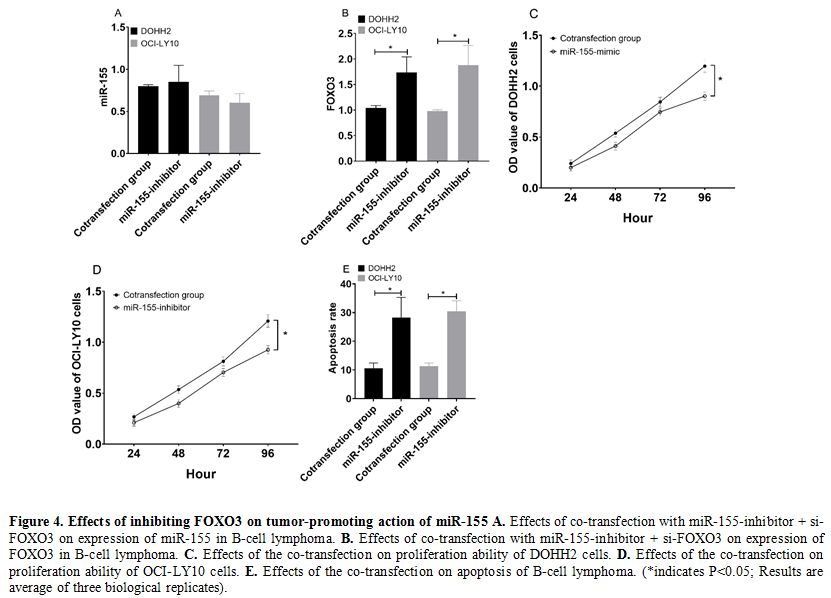 |
Figure 4. Effects of inhibiting FOXO3 on tumor-promoting action of miR-155 A. Effects of co-transfection with miR-155-inhibitor + si-FOXO3 on expression of miR-155 in B-cell lymphoma. B. Effects of co-transfection with miR-155-inhibitor + si-FOXO3 on expression of FOXO3 in B-cell lymphoma. C. Effects of the co-transfection on proliferation ability of DOHH2 cells. D. Effects of the co-transfection on proliferation ability of OCI-LY10 cells. E. Effects
of the co-transfection on apoptosis of B-cell lymphoma. (*indicates
P<0.05; Results are average of three biological replicates).
|
Correlation of miR-155 and FOXO3 with clinicopathological features of DLBCL patients.
DLBCL tissues also showed increased expression of miR-155 and decreased
expression of FOXO3 (both P<0.05). Analysis of the correlation of
miR-155 and FOXO3 with clinicopathological features of DLBCL patients
revealed that miR-155 and FOXO3 were closely related to the
international prognostic index (IPI) score and 5-year prognosis and
survival of the patients (P<0.05). ROC analysis revealed that the
area-under-the-curve (AUC) of miR-155 for evaluating IPI score and
5-year prognosis and survival of DLBCL patients was 0.710 and 0.824,
respectively, and the AUC of FOXO3 for them was 0.786 and 0.768,
respectively. (Table 2 and Figure 5)
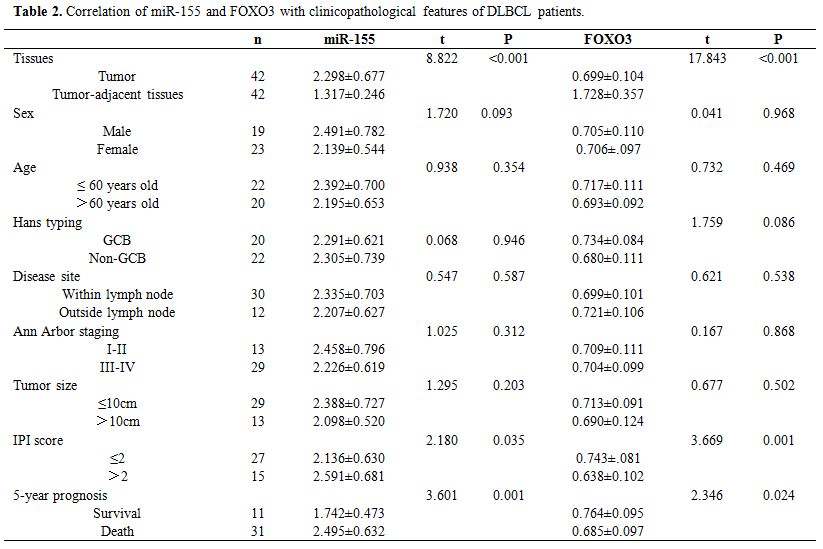 |
Table 2. Correlation of miR-155 and FOXO3 with clinicopathological features of DLBCL patients. |
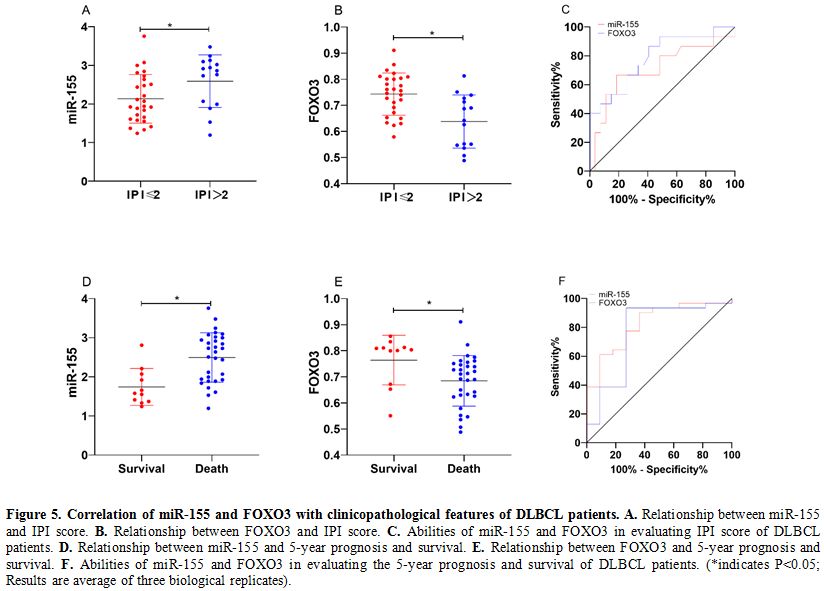 |
Figure 5. Correlation of miR-155 and FOXO3 with clinicopathological features of DLBCL patients. A. Relationship between miR-155 and IPI score. B. Relationship between FOXO3 and IPI score. C. Abilities of miR-155 and FOXO3 in evaluating IPI score of DLBCL patients. D. Relationship between miR-155 and 5-year prognosis and survival. E. Relationship between FOXO3 and 5-year prognosis and survival. F. Abilities
of miR-155 and FOXO3 in evaluating the 5-year prognosis and survival of
DLBCL patients. (*indicates P<0.05; Results are average of three
biological replicates). |
Discussion
MiR-155 plays a role in various physiological and pathological processes.[8,9] Exogenous molecular control in vivo of miR-155 expression may inhibit malignant growth[9,11] viral infections,[12] and enhance the progression of cardiovascular diseases.[13]
MiR-155 is a microRNA that, in humans, is encoded by the MIR155 host
gene or MIR155HG. The MIR155HG was initially identified as a
transcriptionally activated gene by promoter insertion; its RNA
transcript does not contain a long open reading frame (ORF); however,
it does include an imperfectly base-paired stem-loop that is conserved
across species.[15] Once miR-155 pri-miRNA is
transcribed, this transcript is cleaved by the nuclear microprocessor
complex, of which the core components are the RNase III type
endonuclease Drosha and the DiGeorge critical region 8 (DGCR8) protein,
to produce a 65-nucleotide stem-loop precursor miRNA (pre-mir-155).[16,17]
The 23-nucleotide single-stranded miR-155, which is harbored in exon 3,
is subsequently processed from the parent RNA molecule.[14]
Very few studies have investigated the expression levels of miR-155-3p,
Landgraf et al. established that expression levels of this miRNA were
very low in hematopoietic cells. Additionally, PCR analyses found that
while miR-155-3p was detectable in many human tissues, the expression
levels of this miRNA were 20–200 fold less compared to miR-155-5p
levels.[24,25] In previous studies, many scholars had
reported on the correlation of miR-155, FOXO3 with tumors as follows:
The expression of miR-155 was upregulated in tumors and played a role
in promoting cancer,[14,15] while the expression of FOXO3 was down-regulated, and could inhibit the occurrence and development of tumors.[16,17] Recent studies have indicated that miR-155 and FOXO families are related to B-cell lymphoma's occurrence and diffusion.[18,19]
However, the role of FOXO3 in B-cell lymphoma is still under
investigation, and whether there was a regulatory relationship between
miR-155, FOXO3, and B-cell lymphoma is also under investigation.
This
study analyzed the roles of miR-155 and FOXO3 in two B-cell lymphoma
cell lines, compared with normal B lymphocytes; B-cell lymphoma cells
showed significantly increased expression of miR-155, which was
consistent with previous studies, showing tumor-promoting effects on
B-cell lymphoma.[20] In our study, we also found that
inhibiting the expression of miR-155 in B-cell lymphoma cells led to
significantly decreased proliferation ability and increased apoptosis
rate of B-cell lymphoma cells, and it also led to decreased expression
of FOXO3 in them. However, we also found that inhibiting the expression
of miR-155 led to increased expression of FOXO3 in B-cell lymphoma
cells. It indicated that FOXO3 might play a similar role in B-cell
lymphoma as FOXO3 in breast cancer and pancreatic cancer by inhibiting
tumor development.[20,21] In order to verify this
hypothesis, we co-transfected inhibition vectors of miR-155 and FOXO3
into B-cell lymphoma cells, finding the following situations: B-cell
lymphoma cells co-transfected with inhibition vectors were not
different from those transfected with inhibition vector of miR-155
alone in the expression of miR-155, but showed lower expression of
FOXO3 than those transfected with inhibition vector of miR-155 alone.
In addition, B-cell lymphoma cells co-transfected with inhibition
vectors showed higher cell proliferation ability and lower cell
apoptosis rate than those transfected with miR-155-inhibitor. It
suggested that FOXO3 could suppress the tumor-promoting action of
miR-155 in B-cell lymphoma cells. Dual-luciferase reporter assay
revealed that there were targeted binding sites between miR-155 and
FOXO3. Based on the above results, we preliminarily verified that
miR-155 promoted B-cell lymphoma cells' proliferation ability and
inhibited apoptosis by targeted inhibition of FOCXO3.
In recent
years, some studies have also pointed out that miR-155 plays a
regulatory role in tumors by targeting FOXO3. For example, a study by
Kim et al. reported that miR-155 suppressed glucose uptaking and
metabolism of breast cancer cells and inhibited tumor growth through
the PIK3R1-FOXO3a-cMYC signal axis.[22] A study by
Zhang et al. also indicated that miR-155 could target inhibition of
FOCXO3 and promote proliferation and metastasis of non-small cell lung
cancer cells and inhibit apoptosis.[23] A study by Ji
et al. pointed out that miR-155 could promote proliferation, colony
formation, migration, invasion of renal clear cell carcinoma by
targeted inhibition of FOCXO3, and suppress block and apoptosis of the
cells in the G1 phase.[24] The role of FOXO3 against
B-cell lymphoma is related to the immune function regulation by FOXO3.
As we all know, the occurrence of B-cell lymphoma is bound up with both
immune dysfunction.[25] FOXO3 was reportedly able to promote the proliferation of T lymphocytes and B lymphocytes.[26,27] However, in this study, we have not conducted in vivo cell experiments to verify it in future studies.
We
analyzed the correlation of miR-155 and FOXO3 with clinicopathological
features of DLBCL patients, finding that miR-155 and FOXO3 were closely
related to IPI score and 5-year prognosis and survival of the patients,
which indicated that miR-155 and FOXO3 were strongly linked to the
prognosis of DLBCL patients. A study by Hanne et al. pointed out that
overexpression of miR-155 was related to poor prognosis of patients
with B-cell lymphoma,[28] and a study by Ahmadvand et al. also drew similar conclusions.[29]
It may be related to the effects of miR-155 and FOXO3 on chemotherapy
to B-cell lymphoma. miR-155 can regulate the sensitivity of tumor cells
to radiotherapy by targeting FOXO3. A study by Khoshinani et al.
revealed that miR-155 reduced colorectal cancer cells' sensitivity to
radiotherapy by targeted inhibition of FOCXO3.[30] It
provides a direction for our future research. Namely, targeted
inhibition of FOXO3 by miR-155 may also affect B-cell lymphoma cells'
sensitivity to radiotherapy and chemotherapy. To sum up, miR-155 can
promote the proliferation of B-cell lymphoma cells and suppress
apoptosis of them by targeted inhibition of FOCXO3, and both
over-expression of miR-155 and low expression of FOXO3 are related to
poor prognosis of DLBCL patients.
References
- Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, Wang
X, Zhang B, Zhang Q, Chen L, et al: Genetic landscape of hepatitis B
virus-associated diffuse large B-cell lymphoma. Blood 131: 2670-2681,
2018. https://doi.org/10.1182/blood-2017-11-817601 PMid:29545328 PMCid:PMC6063049
- Swerdlow
SH: WHO classification of tumours of haematopoietic and lymphoid
tissues. WHO classification of tumours 22008: 439, 2008.
- Horwitz
SM, Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH,
Andreadis CB, Bartlett N, Byrd JC, Fayad LE, et al: NCCN guidelines
insights:non-Hodgkin's lymphomas, version 3.2016. J Natl Compr Canc
Netw 14: 1067-1079, 2016. https://doi.org/10.6004/jnccn.2016.0117 PMid:27587620
- Lenz
G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam
LT, Shaffer AL, Xiao W, et al: Molecular subtypes of diffuse large
B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci
U S A 105: 13520-13525, 2008. https://doi.org/10.1073/pnas.0804295105 PMid:18765795 PMCid:PMC2533222
- Gonçalves
OSL, Wheeler G, Dalmay T, et al: Detection of miRNA cancer biomarkers
using light activated Molecular Beacons. Rsc Advances 9: 12766-12783,
2019. https://doi.org/10.1039/C9RA00081J
- Mullany
LE, Herrick JS, Sakoda LC, Samowitz W, Stevens JR, Wolff RK and
Slattery ML: miRNA involvement in cell cycle regulation in colorectal
cancer cases. Genes Cancer 9: 53, 2018. https://doi.org/10.18632/genesandcancer.167 PMid:29725503 PMCid:PMC5931252
- Zhang
J, Wei B, Hu H, Liu F, Tu Y, Zhao M and Wu D: Preliminary study on
decreasing the expression of FOXP3 with miR-155 to inhibit diffuse
large B-cell lymphoma. Oncol Lett 14: 1711-1718, 2017. https://doi.org/10.3892/ol.2017.6345 PMid:28789399 PMCid:PMC5529978
- Huang
X, Shen Y, Liu M, Bi C, Jiang C, Iqbal J, McKeithan TW, Chan WC, Ding
SJ, Fu K: Quantitative proteomics reveals that miR-155 regulates the
PI3K-AKT pathway in diffuse large B-cell lymphoma. Am J Pathol 181:
26-33, 2012. https://doi.org/10.1016/j.ajpath.2012.03.013 PMid:22609116 PMCid:PMC3388146
- Coomans
de Brachène A and Demoulin JB: FOXO transcription factors in cancer
development and therapy. Cell Mol Life Sci 73: 1159-1172, 2016. https://doi.org/10.1007/s00018-015-2112-y PMid:26686861
- Obrador-Hevia
A, Serra-Sitjar M, Rodríguez J, Villalonga P and Fernández de Mattos S:
The tumour suppressor FOXO3 is a key regulator of mantle cell lymphoma
proliferation and survival. Br J Haematol 156: 334-345, 2012. https://doi.org/10.1111/j.1365-2141.2011.08951.x PMid:22107151
- He
B, Yan F and Wu C: Overexpressed miR-195 attenuated immune escape of
diffuse large B-cell lymphoma by targeting PD-L1. Biomed Pharmacother
98: 95-101, 2018. https://doi.org/10.1016/j.biopha.2017.11.146 PMid:29247952
- Huang
J, Jiao J, Xu W, Zhao H, Zhang C, Shi Y and Xiao Z: MiR-155 is
upregulated in patients with active tuberculosis and inhibits apoptosis
of monocytes by targeting FOXO3. Mol Med Rep 12: 7102-7108, 2015. https://doi.org/10.3892/mmr.2015.4250 PMid:26324048
- Ling
N, Gu J, Lei Z, Li M, Zhao J, Zhang HT and Li X: microRNA-155 regulates
cell proliferation and invasion by targeting FOXO3a in glioma. Oncol
Rep 30: 2111-2118, 2013. https://doi.org/10.3892/or.2013.2685 PMid:23970205
- Van
Roosbroeck K, Fanini F, Setoyama T, Ivan C, Rodriguez-Aguayo C,
Fuentes-Mattei E, Xiao L, Vannini I, Redis RS, D'Abundo L, et al:
Combining anti-miR-155 with chemotherapy for the treatment of lung
cancers. Clin Cancer Res 23: 2891-2904, 2017. https://doi.org/10.1158/1078-0432.CCR-16-1025 PMid:27903673 PMCid:PMC5449263
- Zargar
S, Tomar V, Shyamsundar V, Vijayalakshmi R, Somasundaram K and
Karunagaran D: A feedback loop between microRNA 155 (miR-155),
programmed cell death 4, and activation protein 1 modulates the
expression of miR-155 and tumorigenesis in tongue cancer. Mol Cell Biol
39: e00410-18, 2019. https://doi.org/10.1128/MCB.00410-18 PMid:30617160 PMCid:PMC6399668
- Yao
S, Fan LY and Lam EW: The FOXO3-FOXM1 axis:A key cancer drug target and
a modulator of cancer drug resistance[C]//Seminars in cancer biology.
Semin Cancer Biol 50: 77-89, 2018. https://doi.org/10.1016/j.semcancer.2017.11.018 PMid:29180117 PMCid:PMC6565931
- Kumazoe
M, Takai M, Bae J, Hiroi S, Huang Y, Takamatsu K, Won Y, Yamashita M,
Hidaka S, Yamashita S, et al: FOXO3 is essential for CD44 expression in
pancreatic cancer cells. Oncogene 36: 2643, 2017. https://doi.org/10.1038/onc.2016.426 PMid:27893718
- Slezak-Prochazka
I, Kluiver J, de Jong D, Smigielska-Czepiel K, Kortman G, Winkle M,
Rutgers B, Koerts J, Visser L, Diepstra A, et al: Inhibition of the
miR-155 target NIAM phenocopies the growth promoting effect of miR-155
in B-cell lymphoma. Oncotarget 7: 2391, 2016. https://doi.org/10.18632/oncotarget.6165 PMid:26497687 PMCid:PMC4823043
- Ushmorov
A and Wirth T: FOXO in B-cell lymphopoiesis and B cell
neoplasia[C]//Seminars in cancer biology. Semin Cancer Biol 50:
132-141, 2018. https://doi.org/10.1016/j.semcancer.2017.07.008 PMid:28774833
- Zhang
L, Cai M, Gong Z, Zhang B, Li Y, Guan L, Hou X, Li Q, Liu G, Xue Z, et
al: Geminin facilitates FoxO3 deacetylation to promote breast cancer
cell metastasis. J Clin Invest 127: 2159-2175, 2017. https://doi.org/10.1172/JCI90077 PMid:28436938 PMCid:PMC5451250
- Kumazoe
M, Takai M, Hiroi S, Takeuchi C, Kadomatsu M, Nojiri T, Onda H, Bae J,
Huang Y, Takamats u K, et al: The FOXO3/PGC-1β signaling axis is
essential for cancer stem cell properties of pancreatic ductal
adenocarcinoma. J Biol Chem 292: 10813-10823, 2017. https://doi.org/10.1074/jbc.M116.772111 PMid:28507102 PMCid:PMC5491768
- Kim
S, Lee E, Jung J, Lee JW, Kim HJ, Kim J, Yoo HJ, Lee HJ, Chae SY, Jeon
SM, et al: microRNA-155 positively regulates glucose metabolism via
PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene 37: 2982, 2018. https://doi.org/10.1038/s41388-018-0124-4 PMid:29527004 PMCid:PMC5978802
- Zhang
Y, Zhao H and Zhang L: Identification of the tumor suppressive function
of circular RNA FOXO3 in non small cell lung cancer through sponging
miR 155. Mol Med Rep 17: 7692-7700, 2018. https://doi.org/10.3892/mmr.2018.8830
- Ji
H, Tian D, Zhang B, Zhang Y, Yan D and Wu S: Overexpression of miR 155
in clear cell renal cell carcinoma and its oncogenic effect through
targeting FOXO3a. Exp Ther Med 13: 2286-2292, 2017. https://doi.org/10.3892/etm.2017.4263 PMid:28565840 PMCid:PMC5443202
- Andor
N, Simonds EF, Czerwinski DK, Chen J, Grimes SM, Wood-Bouwens C, Zheng
GXY, Kubit MA, Greer S, Weiss WA, et al: Single-cell RNA-Seq of
follicular lymphoma reveals malignant B-cell types and coexpression of
T-cell immune checkpoints. Blood 133: 1119-1129, 2019. https://doi.org/10.1182/blood-2018-08-862292 PMid:30591526 PMCid:PMC6405336
- Togher
S, Larange A, Schoenberger SP and Feau S:FoxO3 is a negative regulator
of primary CD8+ T‐cell expansion but not of memory formation. Immunol
Cell Biol 93: 120-125, 2015. https://doi.org/10.1038/icb.2014.78 PMid:25245112 PMCid:PMC4324096
- Ottens
K, Hinman RM, Barrios E, Skaug B, Davis LS, Li QZ, Castrillon DH and
Satterthwaite AB: Foxo3 Promotes Apoptosis of B Cell
Receptor-Stimulated Immature B Cells, Thus Limiting the Window for
Receptor Editing. J Immunol 201: 940-949, 2018. https://doi.org/10.4049/jimmunol.1701070 PMid:29950509 PMCid:PMC6057821
- Due
H, Svendsen P, Bødker JS, Schmitz A, Bøgsted M, Johnsen HE, El-Galaly
TC, Roug AS and Dybkær K: miR-155 as a Biomarker in B-Cell
Malignancies. Biomed Res Int 2016: 1-14, 2016. https://doi.org/10.1155/2016/9513037 PMid:27294145 PMCid:PMC4884835
- Ahmadvand
M, Eskandari M, Pashaiefar H, Yaghmaie M, Manoochehrabadi S, Khakpour
G, Sheikhsaran F and Montazer Zohour M: Over expression of circulating
miR-155 predicts prognosis in diffuse large B-cell lymphoma. Leuk Res
70: 45-48, 2018. https://doi.org/10.1016/j.leukres.2018.05.006 PMid:29807272
- Khoshinani
HM, Afshar S, Pashaki AS, Mahdavinezhad A, Nikzad S, Najafi R, Amini R,
Gholami MH, Khoshghadam A and Saidijam M: Involvement of miR-155/FOXO3a
and miR-222/PTEN in acquired radioresistance of colorectal cancer cell
line. Jpn J Radiol 35: 664-672, 2017. https://doi.org/10.1007/s11604-017-0679-y PMid:28879560
[TOP]






