Xinchen Fang1, Xiaoyu Zhu2, Baolin Tang2, Kaidi Song2, Wen Yao2, Xiang Wan2, Huilan Liu2, Jun Peng1 and Zimin Sun2.
1 Department
of Hematology, Qilu Hospital, Cheeloo College of Medicine, Shandong
University, No. 27 Shanda South Road, Jinan, Shandong, 250012, China.
2
Department of Hematology, The First Affiliated Hospital of University
of Science and Technology of China, Hefei, Anhui, 230001, China.
Correspondence to: Jun
Peng, Ph.D., Department of Hematology, Qilu Hospital, Cheeloo College
of Medicine, Shandong University, No. 27 Shanda South Road, Jinan,
Shandong, 250012, China. Tel.: +86 53182169114, Fax: +86 53182169114.
E-mail:
junpengfxc@163.com
Published: January 1, 2021
Received: August 13, 2020
Accepted: December 3, 2020
Mediterr J Hematol Infect Dis 2021, 13(1): e2021005 DOI
10.4084/MJHID.2021.005
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Donor natural killer (NK) cell alloreactivity in umbilical cord bone
marrow transplantation (UCBT) can lead to leukemic relapse. However, NK
cell function is calibrated by interaction with human leukocyte
antigens (HLAs). This study aimed to investigate graft-resistant
leukemia after transplantation and compared specific genotypes of
killer immunoglobulin-like receptors (KIRs) in donors and human
leukocyte antigen ligands in patients.
Methods:
We retrospectively analyzed 232 patients with acute leukemia from a
single center. Patients had undergone UCBT with myeloablative
conditioning and without anti-thymocyte globulin. We identified the KIR
genotypes of cord blood donors using polymerase chain reaction with
sequence-specific primers. All of the donors contained KIR3DL1.
Results:
The patients were divided into three groups according to the HLA-B
locus. The donor KIR3DL1 and recipient HLA-Bw4-80I combination was
predictive of being highly educated and was associated with a lower
relapse (P = 0.006) and better overall survival (probability of relapse = 0.13, P
< 0.001) than the uneducated group. We found no significant increase
in the incidence of acute or chronic graft-versus-host disease.
Conclusions:
Our data suggest that the donor KIR3DL1/receptor and HLA-Bw4-80I
combination in UCBT results in stronger graft-versus-leukemia effects
and improved outcomes in patients with acute leukemia.
|
Introduction
Umbilical cord blood transplantation (UCBT) is widely used for treating hematological malignancies.[1]
Natural killer (NK) cells are an important component of umbilical cord
blood stem cells and are the fastest recovering cells in the early
stage after UCBT. Therefore, NK cells are an essential component of the
graft-versus-leukemia (GVL) response and are critical for positive
outcomes after UCBT.[2,3] NK cells have a highly
specific and complex target-cell recognition receptor system. NK cells
are regulated by many inhibitory and activating receptors and trigger
cytotoxicity and secretion of chemokines and cytokines.[4,5] Killer immunoglobulin-like receptors (KIRs) are essential for the development and function of human NK cells,[6]
which is achieved through a process called education. Education is
governed by the interaction between NK cell receptors and major
histocompatibility complex proteins.[7]
Hematopoietic
stem cell transplantation provides an opportunity for NK cells to
re-develop. Different combinations of KIRs and their ligands result in
different NK education levels, through which NK cells enhance
cytotoxicity against Human leukocyte antigen (HLA) class I molecular
tumors compared with unlicensed cells.[8] The most
typical KIR HLA ligand pair is KIR3DL1 and HLA-B. HLA-B is classified
into non-binding (Bw6) and binding (Bw4) types. Bw4 is further
classified into Bw4-80I and Bw4-80T according to whether the amino acid
at position 80 is isoleucine or threonine. The KIR3DL1 and HLA-Bw4-80I
pair has the most potent educational ability,[9-11]
which means that allogeneic proliferating NK cells combined with donor
KIR3DL1 and recipient HLA-Bw4-80I more effectively reduce the
recurrence of leukemia. Therefore, this study aimed to determine
whether specific combinations of KIR receptors and HLA ligands in
patients undergoing UCBT have a better clinical outcome.
Materials and Methods
Patients and transplant protocols.
All participants in the study provided written informed consent.
Participants included patients with lympho-and myeloproliferative
malignancies who received UCBT at the Center of Hematology, Anhui
Provincial Hospital between Jul 31, 2012, and Dec 31, 2017. Donor
sources were unrelated from a cord blood bank and matched at alleles of
HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 loci. All patients
underwent allogeneic transplantation after having undergone either
myeloablative or reduced-intensity regimens.
The primary outcomes
included the following: 1) probability of relapse (PR), which was
defined as any morphologically proven recurrence of leukemia occurring
after the allograft; 2) overall survival (OS), which was defined as the
time from transplantation to death; and 3) disease-free survival (DFS),
which was the time from transplantation to relapse.
Secondary
outcomes included engraftment, hematopoietic chimerism, and acute or
chronic graft-versus-host disease (GVHD). Recovery of neutrophils was
defined by a neutrophil count of a least 0.5 × 109/L
for three consecutive days. Graft failure was defined as no sign of
neutrophil recovery, as well as transient engraftment of donor cells
within 60 days after transplantation. The platelet recovery was defined
by a count of a least 20,000/µl for three consecutive days within 120
days after transplantation. Full donor chimerism was defined as the
presence of > 95% of the donor cells. Acute GVHD (aGVHD) was defined
as the development of grade II to IV GVHD during the first 100 days
post-transplantation. Severe aGVHD involved the development of grade
III to IV GVHD. Chronic GVHD (cGVHD) occurred over 100 days
post-transplantation.
HLA typing.
Genomic DNA was extracted from patients' whole blood and cord blood
with the QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). HLA
classes I and II alleles were hybridized with the LAB Type SSO kit (One
Lambda, Hannover, GERMANY). HLA sequences were read with a LAB Scan 200
(Luminex, Texas, USA) and computer-assisted HLA Fusion software.
Patients were divided into the following three groups according to the
HLA-B locus of donors: HLA-Bw6, HLA-Bw4-80T, and HLA-Bw4-80I.
KIR genotyping.
KIR genotyping was performed using polymerase chain reaction with the
KIR typing kit (BAG Healthcare, Lich, Germany), according to the
manufacturer's instructions. The KIR genotype of cord blood donors was
detected by the sequence-specific primer method. The KIR genotype of
cord blood donors all contained KIR3DL1.
Statistical analysis.
The Kaplan–Meier method was used to calculate probabilities of
relapse-free, OS, and DFS, including the 95% confidence interval (CI).
The nonparametric test was used for comparing outcomes by three
different HLA-B groups. Finally, Cox regression models were constructed
to assess HLA groups' effect on the outcome variables while controlling
for demographic and other covariates that showed an association with
the primary outcomes. The cumulative incidence was used to estimate
non-recurring mortality (NRM), neutrophil and platelet recovery, and
aGVHD and cGVHD. Calculations were performed using SPSS version 17.0.
Results
The
ages of the 232 AL and MDS patients ranged from 2 to 45 with a median
of 13 years, contains 110 women and 122 men. All the patients were
diagnosed with acute myeloid leukemia (AML, n = 112), acute lymphocyte
leukemia (ALL, n = 104), and myelodysplastic syndrome (MDS, n = 16).
All patients were Chinese. One hundred sixteen patients were at first
remission, 52 patients at second/third remission, 64 patients were not
in remission when transplantation. All the patients received UCBT. 23
pairs were 6/6 allele matched at HLA-A, -B, -C, -DRB1, and -DQB1; the
rest were 1(n = 98) or >=2 HLA allele (n = 101) mismatched. Most of
them (n= 180) received reduced-intensity conditioning (RIC), which
contains fludarabine (Flu), busulfan (BU), and cyclophosphamide (CY);
some of them (n = 47) received conditioning total body irradiation
(TBI), cytarabine (Ara-c) and CY, 5 of them received conditioning
Ara-c, BU and CY. There were no significant differences in other
clinical variables. We classified patients according to the presence of
genes encoding recipient HLA-B ligands for donor inhibitory KIRs. None
of the patients received rabbit anti-thymocyte globulin. GvHD
prophylaxis regimens for UCBT included cyclosporine A and mycophenolate
mofetil. We classified patients according to the presence of genes
encoding recipient HLA-B ligands. The characteristics of each HLA-B
group are shown in Table 1.
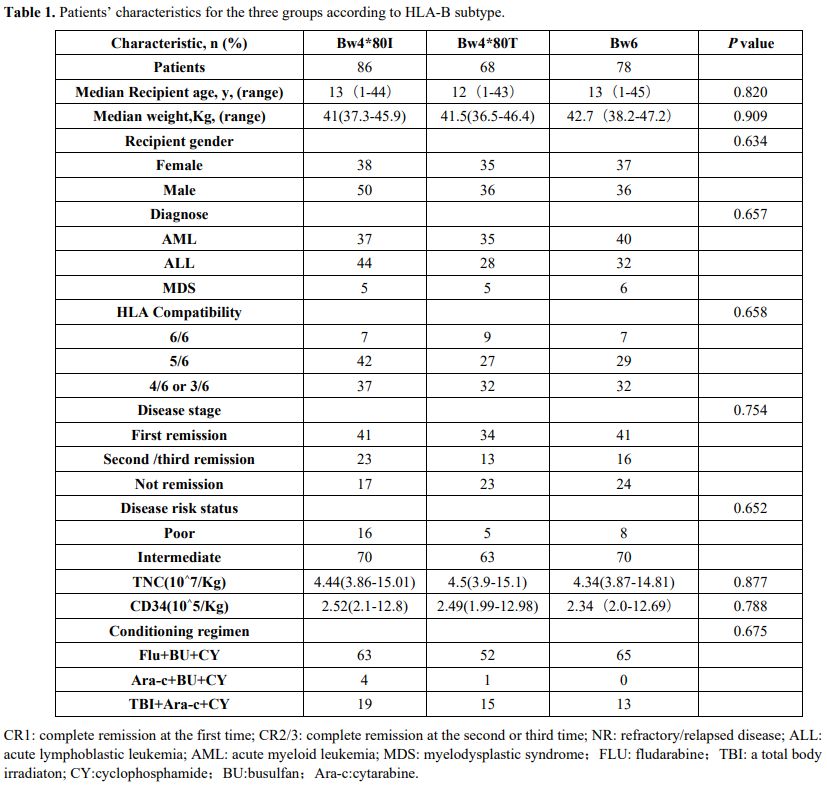 |
Table 1. Patients’ characteristics for the three groups according to HLA-B subtype.
|
Table 2
shows the comparison of the transplantation results of the three
groups. Only nine of the total patients had primary graft failure. The
median recovery time of neutrophils in the Bw6, Bw4-80T, and Bw4-80I
groups was 16(14-20) days, 17(14-21) days, and 17(14-19) days,
respectively. The engraftment rate in the Bw6, Bw4-80T, and Bw4-80I
groups was 96.5%, 95.5%, and 96.2%, respectively (P = 0.202). The
median recovery time of platelet recovery in Bw6, Bw4-80T, and Bw4-80I
groups was 36(29-47) days, 38(29-60) days, and 38(31-45) days. The days
of neutrophils and platelet recovery showed no significant difference
among the three groups. The cumulative incidence of recovery of
neutrophils by day 42 in the three groups was 96.5% (95% CI, 89.4% to
98.8%), 95.6% (95% CI, 86.6% to 98.5%), and 94.8%, respectively (95%
CI, 86.7% to 98%; P = 0.81).
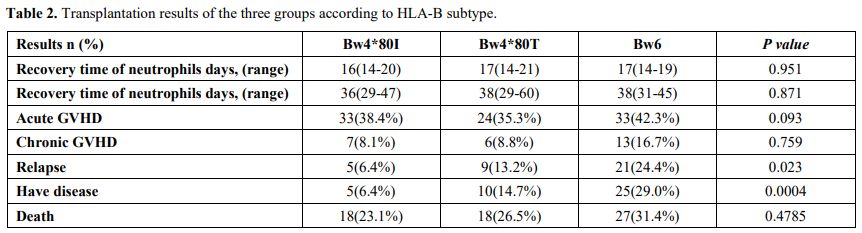 |
Table
2. Transplantation results of the three groups according to HLA-B subtype.
|
Within
100 days after transplantation, the incidence of grades II to IV aGVHD
in the Bw6, Bw4-80T, and Bw4-80I groups was 38.4% (95% CI, 25.1% to
48.5%), 35.3% (95% CI, 24.1% to 46.7%), and 42.3% (95% CI, 31.2% to
53.0%), respectively (P = 0.68). The cumulative incidence of severe
aGVHD (grades III and IV) in the three groups was 15.1% (95% CI, 8.5%
to 23.6%), 22.1% (95% CI, 13.0% to 32.6%), and 24.7% (95% CI, 15.7% to
34.8%), respectively (P = 0.38). Among the patients who survived for
longer than 100 days, the cumulative incidence of cGVHD at 2 years
showed a tendency to be higher than that in Bw4-80I group. The
cumulative incidence of cGVHD at 2 years after transplantation in the
Bw6, Bw4-80T, and Bw4-80I groups was 10.6% (95% CI, 4.4% to 19.9%),
10.6 % (95% CI, 4.1% to 20.5%), and 20.6% (95% CI, 10.5% to 33.0%),
respectively (P = 0.18).
The cumulative incidence of relapse two
years after transplantation in the Bw4-80I group was significantly
lower than that in the other two groups. In the Bw4-80I group, only 5
cases (6.4%) relapsed, while in the Bw6 group, 21 cases (24.4%)
relapsed. In the univariate analysis, the HLA-B subtype was a
significant risk factor for relapse (P
= 0.02). At 2 years after transplantation, the DFS in the Bw4-80I group
(91.7%, 95% CI, 81.7% to 96.5%) was significantly higher than that in
the other 2 groups (Bw6 group: 60.2%, 95% CI, 81.1% to 96.5%; Bw4-80T
group: 79.3%, 95% CI, 64.2% to 88.5%, P = 0.002; Figure 1).
Multivariate analysis was performed for variables, including age,
receptor weight, HLA matching, diagnosis, stage, conditioning regimen,
and HLA-B subtype, to identify risk factor in the three groups (P
= 0.0003).TRM occurred in 12 of 86 recipients in the Bw6 group, in 15
of 68 recipients in the Bw4-80T group, and in 13 of 78 recipients in
the Bw4-80I group. The main cause of death was a severe infection
caused by bone marrow failure after recurrence; the first type of
infection was a fungal infection. The cumulative incidence of TRM by 2
years was 14.6% (95% CI, 6.6% to 21.9%), 22.2% (95% CI, 11.6% to
31.4%), and 16.9% (95% CI, 8.1% to 24.8%) in the three groups,
respectively (P
= 0.45). OS at 2 years was 64.6% (95% CI, 51.9% to 74.7%), 73.4% (95%
CI, 61.2% to 83.4%), and 76% (95% CI, 64.9% to 84.4%) in the Bw6,
Bw4-80T, and Bw4-80I groups, respectively (P = 0.53), with no significant difference between the groups (Figure 2).
In multivariate analysis, including HLA-B difference and other factors,
OS at two years after transplantation in the Bw4-80I group (hazard
ratio = 0.13, P = 0.0001) was significantly higher than that in the Bw6 group. HLA-B difference was a risk factor for OS (P = 0.0003).
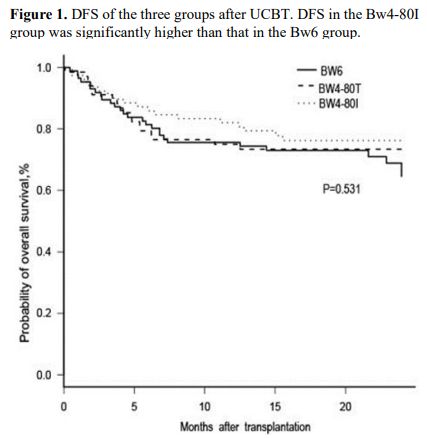 |
Figure
1. DFS of the three groups after UCBT. DFS in the Bw4-80I group was significantly higher than that in the Bw6 group. |
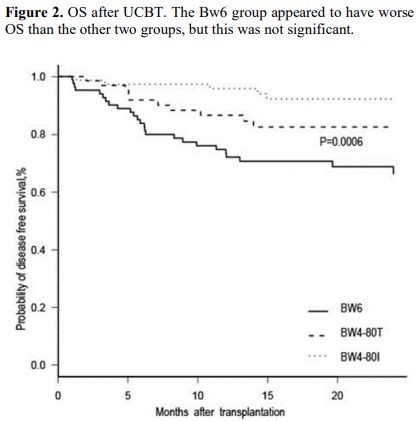 |
Figure 2. OS after UCBT. The Bw6 group appeared to have worse OS than the other two groups, but this was not significant. |
Discussion
The
objective of this study was to investigate the effect of donor KIR and
recipient ligands their effect on DFS. HLA-B subtype was a significant
on clinical outcomes after UCBT. We retrospectively analyzed data from
patients with hematological malignancies who received T cell-repleted
UCBTs (with neither ex vivo nor in vivo
T cell depletion) at a single Chinese center. We found that the PR
after UCBT was significantly lower in recipients whose HLA-B was
Bw4-80I with allografts from KIRs containing 3DL1 donors than in
recipients whose HLA-B was Bw6.
The role of KIRs in early
reporting and their ligands in UCBT is not consistent. NK cell
alloreactivity in a transplantation setting was first recognized in
patients with acute myeloid leukemia in the absence of T cells with
HLA-haploidentical donors and grafts.[12] However,
the traditional view is that KIR mismatch of donors and recipients
should be accepted in transplantation. Ruggeri and colleagues[13]
first reported that KIRs not matching their ligand or ligand loss could
reduce NK cell inhibition, and therefore, they were easier to activate,
which resulted in enhanced GVL effects and a reduced post-transplant
recurrence rate of leukemia. However, this previous study mainly
focused on depleting T cells in vitro before transplantation.[13] Recent reports have continued to focus on the efficacy of better transplantation associated with the activating KIR gene.[14,15,16]
A limitation of these studies is that they only considered KIR–ligand
mismatch, without consideration for the role of NK licensing. Our study
assessed the effect of NK cell licensing and education. The higher GVL
effects in the donor KIR3DL1/receptor Bw4-80I group can be explained by
the more active cytolytic function of alloreactivity in donor NK cells
because of interaction between the Bw4-80I ligand and donor NK cells.
Conversely, the donor KIR3DL1/receptor Bw6 group could not educate NK
cells. Therefore, a high recurrence of leukemia was observed in this
group. The KIR3DL1/receptor Bw4-80T group also educated NK cells, while
its lower education conferred a mild improvement in DFS, PR, and OS. We
observed that the different education results did not affect single
factor analysis of OS. Conversely, in multi-factor analysis with the
regression model, the effect of other confounding factors was adjusted,
and it revealed the effect of each factor on the dependent variable.
The
number of UCBT cases in most transplant centers has a limited
investigation of the role of KIRs in UCBT. Therefore, how donor NK
cells enter the recipient after cord blood transplantation and how they
differentiate, educate, or play a role in the killing are unclear. Many
studies have focused on the combination of KIR2DL1/2/3 and HLA-C.
However, our previous findings indicated that, although the inhibitory
KIR2DL1/2/3 family members' binding affinity to ligands and diversity
of surface expression were observed, these differences were smaller
than those in the KIR3DL1 and HLA-B ligand pair.[17,18] Therefore, we consider that focusing on the KIR3DL1-Bw pair is more meaningful.
Previous
studies have shown that higher GVL effects are associated with a higher
probability of GVHD, but our study did not show that aGVHD of the
KIR3DL1/receptor Bw4-80I group was increased. There were no significant
differences in the II-IV GVHD and III-IV GVHD in group Bw4-80I.
Although group Bw4-80I showed a trend for a higher incidence of cGVHD,
this difference was not significant. We also found that different KIR
and donor groups did not significantly affect the neutrophil and
platelet implantation rate, consistently with other studies.[19,20]
Conclusions
Our
data show that the donor KIR3DL1/receptor and recipient Bw4-80I
combination may affect the incidence of the PR and DFS in T
cell-repleted UCBT in Chinese patients. Therefore, close monitoring of
the residual disease status may be recommended in patients with HLA-Bw6
receiving KIR3DL1 cord blood. Further studies are required to clarify
the relationship between the education of NK cells and clinical
outcomes of UCBT. Examination of a larger cohort is also required to
develop confident recommendations.
Acknowledgments
This
study was funded by grants from the National Natural Science Foundation
of China (No. 81470350) and the Fundamental Research Funds for the
Central Universities (No. WK9110000001).
Alexandra H. Marshall
(Marshall Medical Communications) edited the manuscript. We also thank
Ellen Knapp, PhD, from Liwen Bianji, Edanz Group China
(www.liwenbianji.cn/ac), for editing the English text of a draft of
this manuscript.
References
- Pasquini M, Wang Z, Horowitz MM, Gale RP. 2013
report from the Center for International Blood and Marrow Transplant
Research (CIBMTR): current uses and outcomes of hematopoietic cell
transplants for blood and bone marrow disorders. Clin Transpl. 2013:
187-97.
- Horowitz MM, Gale RP, Sondel PM,
Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions
after bone marrow transplantation. Blood. 1990; 75: 555-62 https://doi.org/10.1182/blood.V75.3.555.555 PMid:2297567
- Farag
SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer
cell receptors: new biology and insights into the graft-versus-leukemia
effect. Blood. 2002; 100: 1935-47 https://doi.org/10.1182/blood-2002-02-0350 PMid:12200350
- Ruggeri
L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al.
Effectiveness of donor natural killer cell alloreactivity in mismatched
hematopoietic transplants. Science. 2002; 295: 2097-100. https://doi.org/10.1126/science.1068440 PMid:11896281
- Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010; 129: 8-19. https://doi.org/10.1111/j.1365-2567.2009.03208.x PMid:20028428 PMCid:PMC2807482
- McQueen
KL, Parham P. Variable receptors controlling activation and inhibition
of NK cells. Curr Opin Immunol. 2002 Oct; 14(5): 615-21. https://doi.org/10.1016/S0952-7915(02)00380-1
- Anfossi
N, André P, Guia S, Falk CS, Roetynck S, Stewart CA et al. Human NK
cell education by inhibitory receptors for MHC class I. Immunity. 2006
Aug; 25(2): 331-42. Epub 2006 Aug 10. https://doi.org/10.1016/j.immuni.2006.06.013 PMid:16901727
- Moesta
AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic
polymorphism at two positions distal to the ligand-binding site makes
KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008 Mar
15; 180(6): 3969-79. https://doi.org/10.4049/jimmunol.180.6.3969 PMid:18322206
- O'Connor,
G.M. and McVicar, D. The Yin-Yang of KIR3DL1/S1: molecular mechanisms
and cellular function. Crit.Rev. Crit Rev Immunol. 2013; 33(3): 203-18.
https://doi.org/10.1615/CritRevImmunol.2013007409 PMid:23756244 PMCid:PMC3741655
- Boudreau
JE, Giglio F, Gooley TA, Stevenson PA, Le Luduec JB, Shaffer BC et al.
KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after
hematopoietic cell transplantation. J Clin Oncol. 2017 Jul 10; 35(20):
2268-2278. https://doi.org/10.1200/JCO.2016.70.7059 PMid:28520526 PMCid:PMC5501362
- Bern
MD, Beckman DL, Ebihara T, Taffner SM, Poursine-Laurent J, White JM et
al. Immunoreceptor tyrosine-based inhibitory motif-dependent functions
of an MHC class I-specific NK cell receptor. Proc Natl Acad Sci U S A.
2017 Oct 3; 114(40): E8440-E8447. https://doi.org/10.1073/pnas.1713064114 PMid:28923946 PMCid:PMC5635927
- Farag
SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer
cell receptors: new biology and insights into the graft-versus-leukemia
effect. Blood. 2002; 100: 1935-47. https://doi.org/10.1182/blood-2002-02-0350 PMid:12200350
- Ruggeri
L, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T et al. NK
cell alloreactivity and allogeneic hematopoietic stem cell
transplantation. Blood Cells Mol Dis. 2008 Jan-Feb; 40(1): 84-90. https://doi.org/10.1016/j.bcmd.2007.06.029 PMid:17964828
- Stringaris
K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN et al. Donor KIR Genes
2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia
relapse after HLA identical sibling stem cell transplantation for acute
myeloid leukemia but not other hematologic malignancies. Biol Blood
Marrow Transplant. 2010; 16(9): 1257-1264 https://doi.org/10.1016/j.bbmt.2010.03.004 PMid:20302958 PMCid:PMC3801172
- Sahin
U, Dalva K, Gungor F, Ustun C, Beksac M. Donor-recipient killer
immunoglobulin like receptor (KIR) genotype matching has a protective
effect on chronic graft versus host disease and relapse incidence
following HLA-identical sibling hematopoietic stem cell
transplantation. Ann Hematol. 2018 Jun; 97(6): 1027-1039. https://doi.org/10.1007/s00277-018-3274-0 PMid:29549412
- Hoseinian
SA, Jafari D, Mahmoodi M, Alimoghaddam K, Ostadali M, Talebzadeh
Bonakdar A, et al. The impact of donor and recipient KIR genes and KIR
ligands on the occurrence of acute graft-versus-host disease and graft
survival after HLA-identical sibling hematopoietic stem cell
transplantation. Turk J Med Sci. 2018 Aug 16; 48(4): 794-804. https://doi.org/10.3906/sag-1712-75
- Moesta
AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic
polymorphism at two positions distal to the ligand-binding site makes
KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008 Mar
15; 180(6): 3969-79. https://doi.org/10.4049/jimmunol.180.6.3969 PMid:18322206
- Dunphy
SE, Guinan KJ, Chorcora CN, Jayaraman J, Traherne JA, Trowsdale J et
al. 2DL1, 2DL2 and 2DL3 all contribute to KIR phenotype variability on
human NK cells. Genes Immun. 2015 Jul-Aug; 16(5): 301-10. https://doi.org/10.1038/gene.2015.15 PMid:25950617
- Cooley
S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT et al.
Donors with group B KIR haplotypes improve relapse-free survival after
unrelated hematopoietic cell transplantation for acute myelogenous
leukemia. Blood. 2009; 113(3): 726-732. https://doi.org/10.1182/blood-2008-07-171926 PMid:18945962 PMCid:PMC2628378
- Erbe
AK, Wang W, Reville PK, Carmichael L, Kim K, Mendonca EA et al.
HLA-Bw4-I-80 isoform differentially influences clinical outcome as
compared to HLA-Bw4-T-80 and HLA-A-Bw4 isoforms in rituximab or
dinutuximab-based cancer immunotherapy. Front Immunol. 2017 Jun 12; 8:
675. https://doi.org/10.3389/fimmu.2017.00675 PMid:28659916 PMCid:PMC5466980
[TOP]



