Margherita Mauro1, Giuliana Lo Cascio2, Rita Balter1, Ada Zaccaron1, Elisa Bonetti1, Virginia Vitale1, Matteo Chinello1, Massimiliano De Bortoli1, Paolo Brazzarola3, Costanza Bruno4 and Simone Cesaro1.
1 Pediatric Hematology Oncology, Department of Mother and Child, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
2
Unità Operativa Complessa di Microbiologia e Virologia, Dipartimento di
Patologia e diagnostica, Azienda Ospedaliera Universitaria Integrata,
Verona, Italy.
3 Endocrine Surgery Unit, Department of Surgery and Oncology, University and Hospital Trust of Verona, Verona, Italy.
4 Department of Radiology, Radiology Institute, Verona, Italy.
Correspondence to: Margherita Mauro, M.D. Pediatric Hematology
Oncology, Azienda Ospedaliera Universitaria Integrata, Verona, Italy.
Tel. +39-3408420257. E-mail:
mauro.margherita88@gmail.com
Published: November 1, 2020
Received: September 8, 2020
Accepted: October 17, 2020
Mediterr J Hematol Infect Dis 2020, 12(1): e2020079 DOI
10.4084/MJHID.2020.079
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Invasive mucormycosis is a very aggressive fungal disease among
immunocompromised pediatric patients caused by saprophytic fungi that
belong to the order of the Mucorales.
Case Report: We describe a case of of Lichtheimia corymbifera
infection in a 15-year-old child with B-cell-Non-Hodgkin Lymphoma
(B-NHL) involving lung, kidney and thyroid that initially was diagnosed
as probable aspergillosis delaying the effective therapy for
mucormycosis.
Conclusions:
This case showed that also the intensive chemotherapy for B-NHL may
represent a risk factor for mucormycosis infection. Liposomal
amphotericin B and surgery remain the key tools for
the successful treatment of this aggressive disease.
|
Introduction
Invasive
mucormycosis is a very aggressive fungal disease among
immunocompromised patients caused by saprophytic fungi ubiquitous in
nature. They belong to the order of the Mucorales including Rhizopus species, Mucor, Lichtheimia (previously Absidia), Cunninghamella, Rhizomucor, Apophysomyces and Saksenaea.[1,2]
In
pediatric patients, the risk factors include hematologic malignancy,
hematopoietic stem cell transplant (HSCT), solid organ transplant,
neutropenia, poorly controlled diabetes mellitus, use of
corticosteroids, prematurity and trauma.[3-5]
The
clinical presentations are rhino cerebral, pulmonary,
gastrointestinal, cutaneous, and disseminated disease. All clinical
manifestations progress rapidly because of tissue angioinvasion leading
to thrombosis and tissue necrosis.[6,7] Mortality ranges between 22% and 59% in different reports.[1,2]
We describe a case of Lichtheimia corymbifera
infection in an adolescent with B-cell-Non-Hodgkin lymphoma (B-NHL)
involving lung, kidney and thyroid that initially was diagnosed as
probable aspergillosis, delaying the effective therapy for mucormycosis.
Case Report
A
15-year-old Caucasian boy was diagnosed with B-NHL involving lymph
nodes, mediastinum and bones. The patient was treated according to
B-NHL-BFM (Berlin–Frankfurt–Munster) 1997 protocol combined with
Rituximab.[8]
Seven days after the fifth
chemotherapy course, the patient was hospitalized for fever
(37.9°C), severe cytopenia (white blood cell count 0.15 x 109/L, neutrophils 0 x 109/L, platelet count 23 x 109/L,
Hb 96 g/L), elevated C-reactive protein [C-RP] (92 mg/l), but normal
procalcitonin [PCT] (0.33 ng/ml) and vital signs (blood pressure 124/69
mmHg, heart rate 79 beats per minute [bpm], peripheral oxygen
saturation [Sp02] 97%). After drawing blood cultures from central
line and peripheral vein, an empiric antibiotic treatment with
ceftazidime, amikacin and teicoplanin was promptly initiated together
with granulocyte colony stimulating factor (GCSF), while prophylaxis
with cotrimoxazole and fluconazole was continued. On day 4
tachypnea, dyspnea, hypoxemia (Sp02 80%), tachycardia (heart rate 150
bpm) and left hemithorax acute pain appeared together with an increase
of CRP (440 mg/l) and PCT (3.13 ng/ml). On physical examination, lung
auscultation revealed diminished breath sounds and rales over the left
lung. The first set of blood cultures was negative as well as the
second one and the blood samples for galactomannan (GM) and β-d-glucan
(BDG) antigens. Lung computerized tomography (CT) showed a left
extensive consolidation associated with pleural effusion (Figure 1).
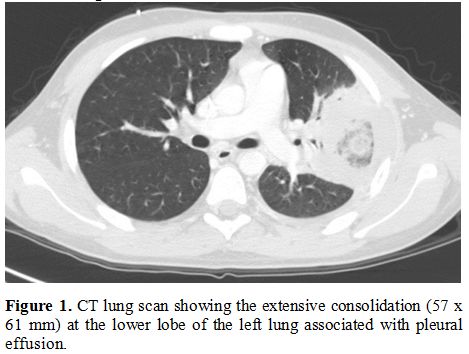 |
Figure 1. CT lung scan
showing the extensive consolidation (57 x 61 mm) at the lower lobe of
the left lung associated with pleural effusion.
|
Considering
a possible fungal origin of pneumonia and the profound
B-lymphocytopenia, micafungin (100 mg/day) was started and, in order to
enhance the innate immunity, a 3-day course of intravenous IgM-enriched
immunoglobulins was administered. On day 6 the fever disappeared while
the neutrophils recovered over 0.5 x 109/L.
On day 7, BDG was markedly positive (> 523 pg/ml) while
GM remained negative. On day 9, high fever reappeared associated
with edema and hyperemia on the left neck. A second lung CT revealed a
slight increment of the left lung consolidation (Figure 2).
A neck ultrasound detected a swelling lesion in left thyroid lobe (19 x
19 mm) and an abdomen ultrasound found a nodule in the middle-superior
part of the right kidney (25 x 22 mm). This nodular lesions, as well as
the lung one, were intensely hypermetabolic on positron emission
tomography (PET)-CT scan proving to have probably the same infectious
origin. We performed a needle aspiration of the thyroid lesion showing
only the presence of a suppurative process. Blood cultures and serum GM
were again negative whereas serum BDG value returned into normal
range.
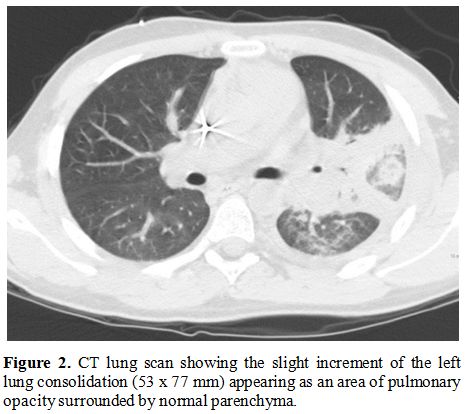 |
Figure
2. CT lung scan
showing the slight increment of the left lung consolidation (53 x 77
mm) appearing as an area of pulmonary opacity surrounded by normal
parenchyma.
|
The
antifungal therapy was enhanced adding voriconazole to micafungin,
while the antibiotic therapy was modified introducing metronidazole and
replacing ceftazidime and amikacin with meropenem. Despite this, the
fever persisted with a CRP rising to 425.8 mg/L on day 11. On day 17
the patient underwent a bronchoalveolar lavage (BAL) that resulted
negative for bacteria, fungi, Mycobacterium tuberculosis, GM and BDG antigen.
The
patient continued to experience evening fever peaks and cough. On day
32 we repeated a CT scan showing unaltered the left lung consolidations
and the left thyroid lobe lesion and an increase of the multiple
nodules at the right kidney. These radiological findings were discussed
in a multidisciplinary meeting with radiologists, infectivologists and
pediatric hematologists discovering that the main lung lesion was
presenting the reverse halo sign from the beginning. Given the
lack of improvement and the severe clinical conditions, on day 36 the
patient underwent left superior lung lobectomy. No postoperative
complications were observed and the patient was discharged home 13 days
later. The histopathological examination was consistent with a fungal
pneumonia for the presence of hyphae. The identification of fungal DNA
was performed by sequencing the internal transcribed spacer (ITS)
domain of the rDNA gene and D1-D2 region of ribosomal sub-unites,
according to the method described by White et al.[9] that revealed the presence of Lichtheimia corymbifera (Figure 3).
Therefore, the patient suspended voriconazole and received antifungal
therapy with liposomal amphotericin B (5 mg/kg three times a week) and
posaconazole (3 x 200 mg/d) for 7 weeks.
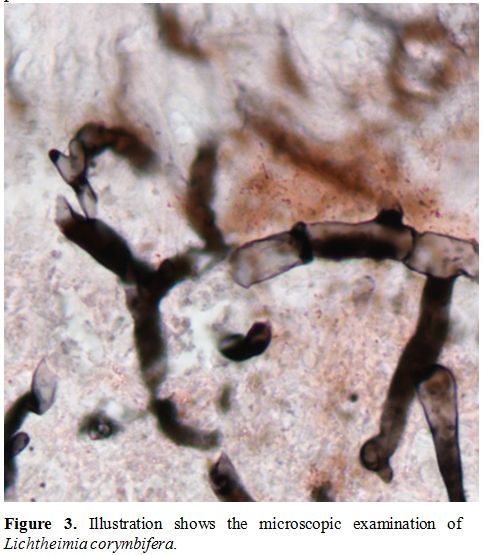 |
Figure 3. Illustration shows the microscopic examination of Lichtheimia corymbifera.
|
Six
days after discharge home the patient developed low grade fever
(37.5°C), painful increase and hyperemia of the left emithyroid (60 x
50 mm), increased of CRP 125 mg/l and PCT 0.13 ng/ml) with normal
blood count. The patient underwent an urgent hemithyroidectomy with
surgical drainage with rapid clinical improvement. The cultural search
for bacteria, fungi and Mycobacteria resulted negative.
The
patient continued a secondary prophylaxis with posaconazole (3 x 200
mg/day) for 18 months. A lung CT, performed 6 months after
surgery, showed only postoperative changes whereas the renal lesion
disappeared after 12 months of posaconazole treatment.
Dicussion
This
case of pulmonary mucormycosis presents two important characteristics
for the clinicians. First, it shows that mucormycosis may occur also in
a patient with a low risk profile. In fact, mucormycosis is a rare
complication in patients with non-Hodgkin lymphoma.[10]
Single cases of mucormycosis have been reported in patients that
received immunosuppressive drug or high dose of steroids and rituximab
for autoimmune disease or renal transplant.[11] This patient received 5 courses of immunochemotherapy where rituximab (375 mg/m2) was combined with high-dose chemotherapy containing dexamethasone (5 x 20 mg/m2/day) and anti-cancer drugs that cause often a profound neutropenia, severe mucositis, and/or enteritis.
Second,
the radiological investigation showed from the beginning, when the
patient was neutropenic, the presence of the reverse halo sign
that was not pointed out initially by the radiologist and described
instead only as lung consolidation. In fact, recent guidelines
recommended strongly the search of halo sign in patients who are
neutropenic or underwent hematopoietic or solid organ transplantations,
together with the number of nodular lesions and the presence of pleural
effusion, to make the differential diagnosis with other fungal
infections.[12,13] The reverse halo sign can be found in several type of infectious pneumonia (bacterial, fungal, mycobacterial, and Pneumocystis jirovecii pneumonia) and in different non-infectious lung diseases (lymphomatoid granulomatosis, radiation pneumonitis).
A
recent study on 70 patients found that the reverse halo sign was
secondary to an infectious bacterial or fungal cause in 66% of patients
with hematological malignancy or who underwent stem cell
transplantation while it was associated with a non-infectious cause in
70% of patients who underwent a solid organ transplantation.
Interestingly, the reverse halo sign was not so specific for
mucormycosis because it was described in 20% of patients with
aspergillosis and 19% of patients with mucormycosis. The
characteristics of halo sign, significantly associated with an
infectious cause, are the presence of neutropenia, the rim thickness,
the central glass attenuation and the pleural effusions.[14]
All these radiological characteristics were present in the case
described but they were initially misinterpreted as possible
aspergillosis. The initial clinical response and the positivity of the
BDG antigen assay contributed to address the clinical suspicion toward
aspergillosis instead of another type of fungal infection. BDG
assay can be found positive in different fungal or bacterial
infections, whereas it is usually absent in cryptococcosis and
mucormycosis. On the other hand, in mucormycosis by Rhizopus spp, BDG antigen assay can be positive.[15] BDG antigen
assay has been added to the EORTC/MSG criteria for the diagnosis of
invasive fungal infections but the data in pediatric populations are
limited.[16] In our case, the transient high
positivity for serum BDG antigen was interpreted as a false-positive,
secondary to the administration of polyvalent IgM-enriched
immunoglobulins.
In the first days of infection, the treatment
was based on empirical antibiotic and antifungal treatment. The
addition of micafungin was directed to cover the patient for Candida and Aspergillus
and the high value of BDG antigen reinforced this therapeutic choice.
Considering that BDG antigen assay cannot indicate the etiology of the
infection and the presence of the reverse halo sign, we agreed
retrospectively that the addition of liposomal amphotericin B would
have been more indicated. Biopsy should be pursued as soon as possible,
because the identification by histopathology, culture, and molecular
tests lead to earlier initiation of antifungal therapy.[6,13]
The
first-line medical treatment of mucormycosis is liposomal Amphotericin
B (L-AmB) at the daily dose of 5-7.5 mg/kg or the combination of L-AmB
with triazoles.[6,7,17]
Surgery
is essential in the treatment of mucormycosis, and the highest levels
of therapeutic success have been achieved when surgery was combined
with medical management especially in pulmonary lesions.[13]
Conclusions
In
conclusion, this case showed that also intensive chemotherapy for B-NHL
may represent a risk factor for mucormycosis infection. Liposomal
amphotericin B and surgery are key tools for a successful treatment of
mucormycosis.
References
- Bassetti M, Bouza E. Invasive mould infections in
the ICU setting: complexities and solutions. J Antimicrob Chemother.
2017;72(Suppl 1):i39‐i47 https://doi.org/10.1093/jac/dkx032 PMid:28355466
- Francis
JR, Villanueva P, Bryant P, Blyth CC. Mucormycosis in children: review
and recommendations for management. J Pediatric Infect Dis Soc.
2017;00:1‐6
- Petrikkos G, Skiada A,
Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and
clinical manifestations of mucormycosis. Clin Infect Dis. 2012;
54(Suppl.1): 23-34 https://doi.org/10.1093/cid/cir866 PMid:22247442
- Roden
MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RI, et
al. Epidemiology and outcome of zygomycosis: a review of 929 reported
cases. Clin Infect Dis. 2005; 41:634-53 https://doi.org/10.1086/432579 PMid:16080086
- Zaoutis
TE, Roilides E, Chiou CC, Buchanan WL, Knudsen TA, Sarkisova TA, et al.
Zygomycosis in children: a systematic review and analysis of reported
cases. Pediatr Infect Dis J. 2007; 26:723-7 https://doi.org/10.1097/INF.0b013e318062115c PMid:17848885
- Otto
WR, Pahud BA, Yin DE. Pediatric Mucormycosis: A 10-Year Systematic
Review of Reported Cases and Review of the Literature. J Pediatric
Infect Dis Soc. 2019; 8:342-350 https://doi.org/10.1093/jpids/piz007 PMid:31181136
- Muggeo
P, Calore E, Decembrino N, Frenos S, De Leonardis F, Colombini A, et
al. Invasive mucormycosis in children with cancer: A retrospective
study from the Infection Working Group of Italian Pediatric Hematology
Oncology Association. Mycoses. 2019;62:165-170 https://doi.org/10.1111/myc.12862 PMid:30338581
- Mussolin
L, Lovisa F, Gallingani I, Cavallaro E, Carraro E, Damanti CC, et al.
Minimal residual disease analysis in childhood mature B-cell
leukaemia/lymphoma treated with AIEOP LNH-97 protocol with/without
anti-CD20 administration. Br J Haematol. 2020; 189(3):e108-e111 https://doi.org/10.1111/bjh.16531 PMid:32080837
- White
TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of
fungal ribosomal RNA genes for phylogenetics. In Innis N, Gelfand J.,
White T, PCR Protocols: A Guide to Methods and Applications. Academic
Press, New York. 1990; p. 315-322 https://doi.org/10.1016/B978-0-12-372180-8.50042-1 PMid:1696192
- Pagano
L, Offidani M, Fianchi L, Nosari A, Candoni A, Picardi M, et al.
Mucormycosis in hematologic patients. Haematologica. 2004;89:207‐214
- Hung
HC, Shen GY, Chen SC, Yeo KJ, Tsao SM, Lee MC, et al. Pulmonary
Mucormycosis in a Patient with Systemic Lupus Erythematosus: A
Diagnostic and Treatment Challenge. Case Rep Infect Dis.
2015;2015:478789 https://doi.org/10.1155/2015/478789 PMid:26185693 PMCid:PMC4491550
- Cornely
OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et
al. ESCMID and ECMM joint clinical guidelines for the diagnosis and
management of mucormycosis 2013. Clin Microbiol Infect. 2014;20 Suppl
3:5‐26 https://doi.org/10.1111/1469-0691.12371 PMid:24479848
- Cornely
OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B
et al. Global guideline for the diagnosis and management of
mucormycosis: an initiative of the European Confederation of Medical
Mycology in cooperation with the Mycoses Study Group Education and
Research Consortium. Lancet Infect Dis. 2019;19(12):e405‐e421
- Thomas
R, Madan R, Gooptu M, Hatabu H, Hammer MM. Significance of the Reverse
Halo Sign in Immunocompromised Patients. AJR Am J Roentgenol.
2019;213:549‐554 https://doi.org/10.2214/AJR.19.21273 PMid:31039026
- Angebault
C, Lanternier F, Dalle F, Schrimpf C, Ruopie AL, Dupuis A, et al.
Prospective Evaluation of Serum β-Glucan Testing in Patients With
Probable or Proven Fungal Diseases. Open Forum Infect Dis.
2016;3:ofw128 https://doi.org/10.1093/ofid/ofw128 PMid:27419189 PMCid:PMC4942764
- De
Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et
al. Revised definitions of invasive fungal disease from the European
Organization for Research and Treatment of Cancer/Invasive Fungal
Infections Cooperative Group and the National Institute of Allergy and
Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.
Clin Infect Dis. 2008;46:1813‐1821
- Groll
AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, et al.
Fourth European Conference on Infections in Leukaemia (ECIL-4):
guidelines for diagnosis, prevention, and treatment of invasive fungal
diseases in paediatric patients with cancer or allogeneic haemopoietic
stem-cell transplantation. Lancet Oncol. 2014;15:e327‐e340 https://doi.org/10.1016/S1470-2045(14)70017-8
[TOP]


