Stefano Botti1, Nicola Serra2, Fausto Castagnetti3, Sabina Chiaretti4, Nicola Mordini5, Gianpaolo Gargiulo6 and Laura Orlando7.
1 Hematology Unit, Azienda USL-IRCCS Reggio Emilia, Viale Risorgimento 80, 42123 Reggio Emilia, Italy.
2
Department of Molecular Medicine and Medical Biotechnology, University
Federico II of Naples, via S. Pansini 80131 Naples, Italy.
3
Department of Experimental, Diagnostic and Specialty Medicine, S.
Orsola-Malpighi Hospital, University of Bologna, via G. Massarenti 9,
40138 Bologna, Italy.
4 Heamatology, Department of Precision and Translational Medicine, Sapienza University, piazzale A. Moro 5, 00185 Rome, Italy.
5 Hematology Division, AO S. Croce e Carle, via M. Coppino 26, 12100 Cuneo, Italy.
6
Hematology and Hematopoietic Stem Cell Transplantation Centre, Federico
II University Hospital of Naples, via Pansini 80131 Naples, Italy.
7 Hemato-Oncology Unit, Istituto Oncologico della Svizzera Italiana (IOSI), via A. Gallino 12, 6500 Bellinzona, Switzerland.
Published: January 1, 2021
Received: September 21, 2020
Accepted: December 11, 2020
Mediterr J Hematol Infect Dis 2021, 13(1): e2021011 DOI
10.4084/MJHID.2021.011
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Italy has been one of the first European countries hit by the COVID-19
pandemic, with many patients dying from severe respiratory issues,
especially frail subjects. Hematology patients are generally thought to
be at high risk of developing severe COVID-19-associated complications.
The aim of this work was to describe the infection control measures
adopted in Italian hematology settings to protect patients and
health-care professionals.
Materials and Methods:
On behalf of the Nursing Campus in Hematology Group, a nationwide
nursing survey was conducted. Questionnaire items included general
information, infection control measures, patient and health-care
professional protection, information management, and participants'
opinion on critical issues. Data have been analyzed by center location
(Northern, Central, or Southern Italy) and by patient age (adult vs
pediatric).
Results:
Forty-four Italian hematology centers participated, representing 52.4%
of those invited. Patients underwent nasopharyngeal swabs (93.2%)
generally the day before admission (43.2%), though less frequently in
Southern centers (p = 0.0377). Visitor restrictions were implemented in
all centers: 65.9% barred all visitors, while 25.0% allowed visitors
only for patients with specific conditions, especially in Central
Italy. Deficiency of personal protective equipment, including masks
(45.5%) and gloves (22.7%), was reported, although the nurses' opinion
was that the emergency was nevertheless well managed to protect
patients and professionals. Almost all health-care institutions (97.7%)
provided recommendations on emergency management. No significant
differences were found between adult and pediatric centers in terms of
infection prevention and control.
Discussion:
Low variability in patient protection strategies was observed, meaning
that national recommendations were effective. However, some critical
issues emerged regarding the management of infected health-care
professionals and their contacts.
|
Introduction
Most
individuals infected with the novel coronavirus disease (COVID-19) will
experience mild to moderate respiratory illness and recover without
requiring special treatment. Older people and those with underlying
medical problems, like cardiovascular disease, diabetes, chronic
respiratory disease, and cancer, are more likely to develop a serious
form of the COVID-19 disease named Severe Acute Respiratory Syndrome
Coronavirus 2 (SARS-CoV-2).[1,2,3,4]
In Italy, the number of
COVID-19 cases began to increase exponentially in the second half of
February 2020.[5] However; it has been hypothesized that the virus was
already circulating in the population in late January.[6]
Over
the weeks following the initial outbreak, clusters of SARS-CoV-2
infection began to appear in Northern Italy,[7] and many people
developed life-threatening conditions that required admission to an
intensive care unit (ICU).[8] Despite several actions undertaken by the
Italian health authorities, a rapid spread of the virus was observed
throughout March, leading the Italian Government to issue strict
containment measures to limit individuals' free circulation throughout
the country.[9,10,11,12] Considering the number of infected cases and
the mortality rate, Italy was initially one of the worst-hit countries
in Europe.[13]
Initially, COVID-19 mortality, expressed as
fatality rate (FR), was higher in Italy than in other European
countries and in China due to various factors, such as demographic
aging, screening, and testing strategies, and the definition of death
adopted.[6] Examining the cohort of patients who died before March 30,
2020, a higher prevalence was observed in people over age 70 years (FR
= 23.8%) and in males (FR 13.3% vs. 7.4%). The presence of
comorbidities appears to be associated with mortality, especially among
older patients,[14] as documented by previous publications.[15,16,17]
Although
there are few and controversial data on COVID-19 patients with
malignant hematological conditions,[18,19] it is reasonable to consider
them at a high risk of death because they are
immunocompromised.[20,21,22,23,24] The Italian Society of
Hematology (SIE) and the Italian Group for Bone and Marrow
Transplantation (GITMO), the Italian Bone Marrow Donor Registry
(IBMDR), the Italian Blood Center (CNS), and the National Italian
Transplant Center (CNT) have all published several suggestions and
recommendations[25,26,27,28] on how to manage hematological
patients in different settings.
However, the practical
application of those suggestions and recommendations may have fallen
short due to the still limited knowledge of the virus's transmission
mechanisms,[29,30] the lack of reliable screening tests and
strategies,[15,16] and the high speed of contagion.[31] Hospital
departments and wards had very little time to put emergency measures in
place to contain the spread of infection. A proactive approach adopting
strict isolation precautions as well as surveillance and control
strategies appeared to be the best method to prevent the spread of the
virus, thereby protecting and ensuring hematology patients'
safety.[32,33]
This paper describes the main organizational and
contextual issues in Italian hematology units and how they have
protected their patients and staff during the COVID-19 pandemic.
The
aims of the survey were: a) to explore real-life practices for
containing the spread of SARS-CoV-2 in hematology settings; b) to
analyze significant differences according to each center's location
(Northern, Central, Southern Italy) and patient age (adult, pediatric);
c) to investigate nurses' opinions on how well his/ her local health
authority managed the crisis.
Materials and Methods
A
cross-sectional study was conducted from April 17 to May 8, 2020, on
behalf of the Nursing Campus in Hematology (NCH) project, a cooperative
network of nurses working in Italy's hematology settings. Data focused
on different clinical settings of hematology were previously provided
by our organization without concerning nursing issues.[34,35,36,37]
Hematology
nurses involved in our group's various activities were invited to
participate via email and were provided with a link to the online
survey. Participation was voluntary, and consent was assumed upon
completion of the questionnaire. Data were collected anonymously. Given
the exploratory objectives of the survey, only one questionnaire per
center was required.
The questionnaire was developed by the
multidisciplinary NCH team composed of hematology nurses, physicians,
and research methodologists, who took into account the suggestions
provided in the available literature and focused on the major areas of
interest for practice. The questionnaire was tested for clarity and
comprehension in 8 centers before the formal start of the study.
The
nurse survey consisted of 39 items: 5 investigating details of the
hematology center, the remaining 34 covering general information
(organization, local epidemiology), infection control measures adopted,
patient and Health Care Professionals (HCPs) protection (COVID-19
testing strategies, safety behaviors, etc.), information management,
and nurses' perception of infection control management (evaluated with
a 10-point Likert scale).
Statistical analysis.
The adopted strategy for data analysis was based on the epidemiology
items, i.e., the spread of infection throughout the geographical areas
(Northern, Central, Southern Italy) and the variable incidence in
different age groups (Adult, Pediatric). Clustering analysis was done
to explore any behavioural differences due to the virus' spread
characteristics and / or timing and the different regional health care
organizational systems.
The statistical analysis was
performed by Matlab statistical toolbox version 2008 (MathWorks,
Natick, MA, USA) for Windows at 32 bites, on a sample of 44 different
centers.
Data are presented as numbers and percentages for
categorical variables, and continuous data are expressed as the mean ±
standard deviation (SD) unless otherwise specified. The chi-square test
and Fisher's exact test were performed to evaluate significant
differences in proportions or percentages between groups. A binomial
test was performed to compare two mutually exclusive proportions or
percentages. The multiple comparison chi-square test was used to define
significant differences between percentages for unpaired data; if the
chi-square test was positive (p-value less than 0.05), then post-hoc
with Z-test was performed to locate the highest or lowest significant
presence. Multiple comparison Cochran's Q tests were used to compare
the differences between percentages for paired data, considering the
null hypothesis that there were no differences between the variables or
modalities. When the Cochran's Q test was positive (p<0.05), a
minimum required difference for a significant difference between two
proportions was calculated using the minimum required differences
method with Bonferroni p-value corrected for multiple comparisons.
One-way ANOVA test was used in multiple comparisons between means; a
post-hoc Scheffe test for pairwise comparison of subgroups was
performed. The Kolmogorov-Smirnov test was conducted to test normal
distribution, with Lilliefors significance correction. When the one-way
ANOVA was not adapted (non-normality), the Kruskal-Wallis test was
performed to compare three or more independent samples; if the
Kruskal-Wallis test was significant (p-value < 0.05), the post-hoc
Dunn test for pairwise comparison of subgroups was performed. The
Mann-Whitney test, the alternative to the independent samples t-test
when the samples' distribution is non-normal, was used to test the
significance of the difference between two independent samples. All
tests with p-value (p) <0.05 were considered significant.
Results
Eighty-four
hematology centers were invited to complete the survey; 44 centers
(52.4%) completed the survey, 26 of which (59.1%) were in the North of
Italy (NIT), 6 (13.6%) in the Center of Italy (CIT), and 12 (27.3%) in
the South of Italy and Islands (SITI).
Thirty-one centers (70.4%)
provide care to adult patients, 7 (15.9%) to pediatric patients, and 6
(13.6%) to both pediatric and adult patients. Only two adult centers (1
in the North and 1 in the South) did not perform stem cell transplants.
As is known, the SARS-CoV-2 virus has spread throughout Italy,
starting from the North to the South, with different characteristics of
severity.[38] Furthermore, the incidence and severity of clinical
manifestations in adult and pediatric patients appear to differ.[39,40]
We performed our analysis assuming differences in patient protection
strategies by geographic area and patient age.
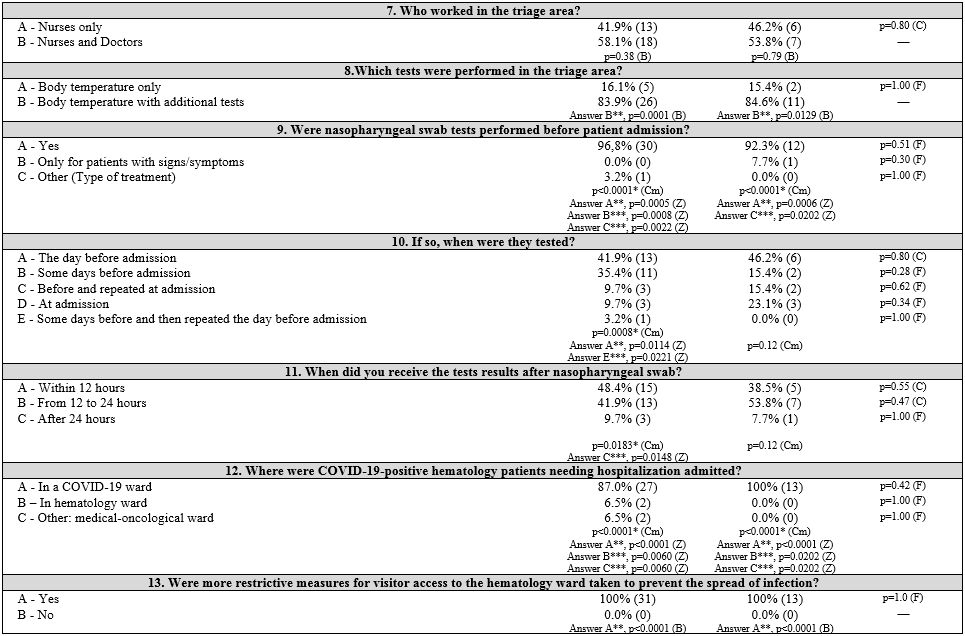
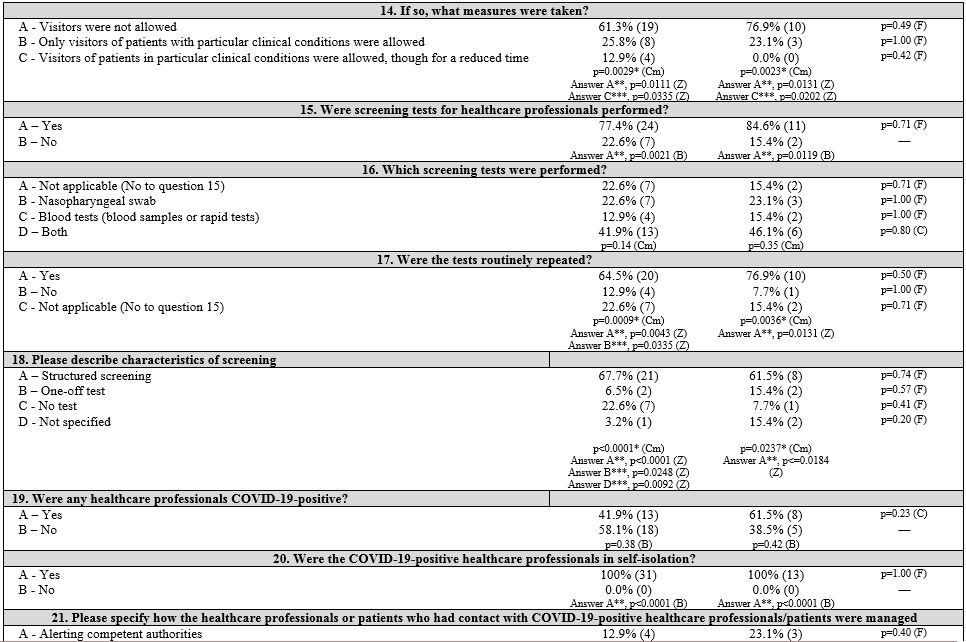
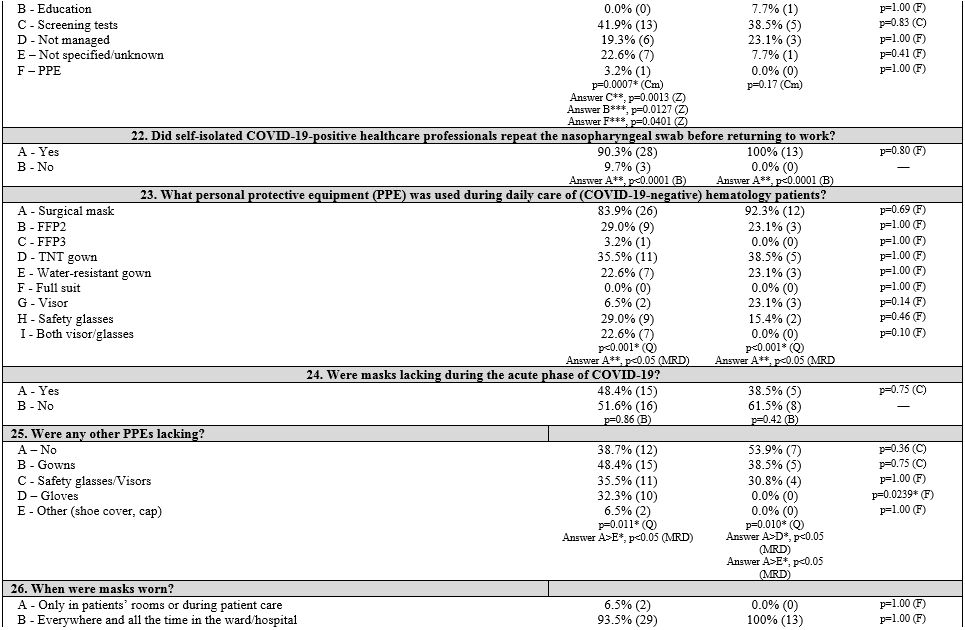
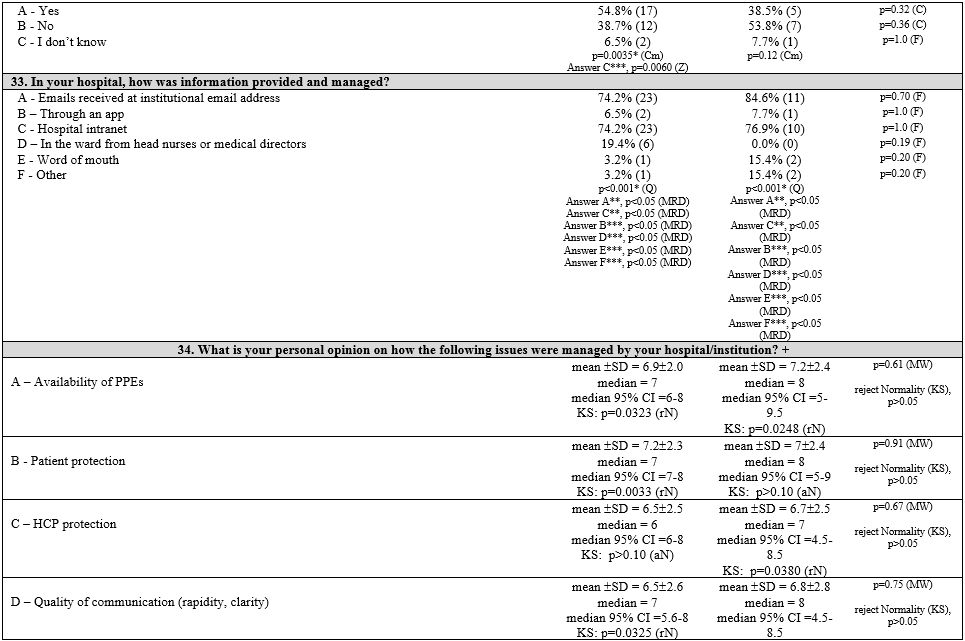
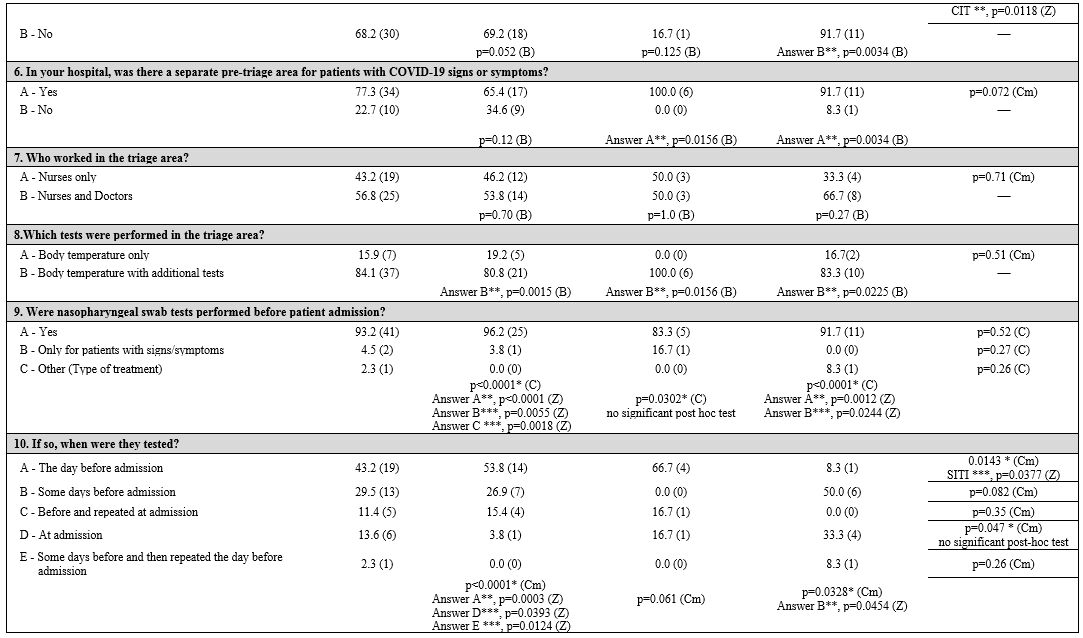
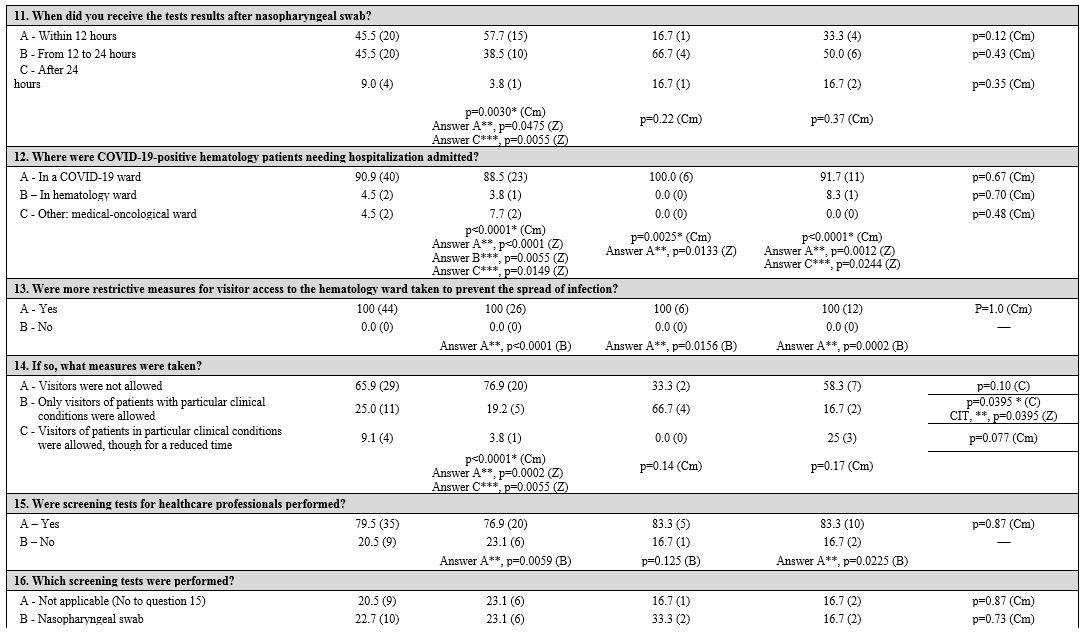
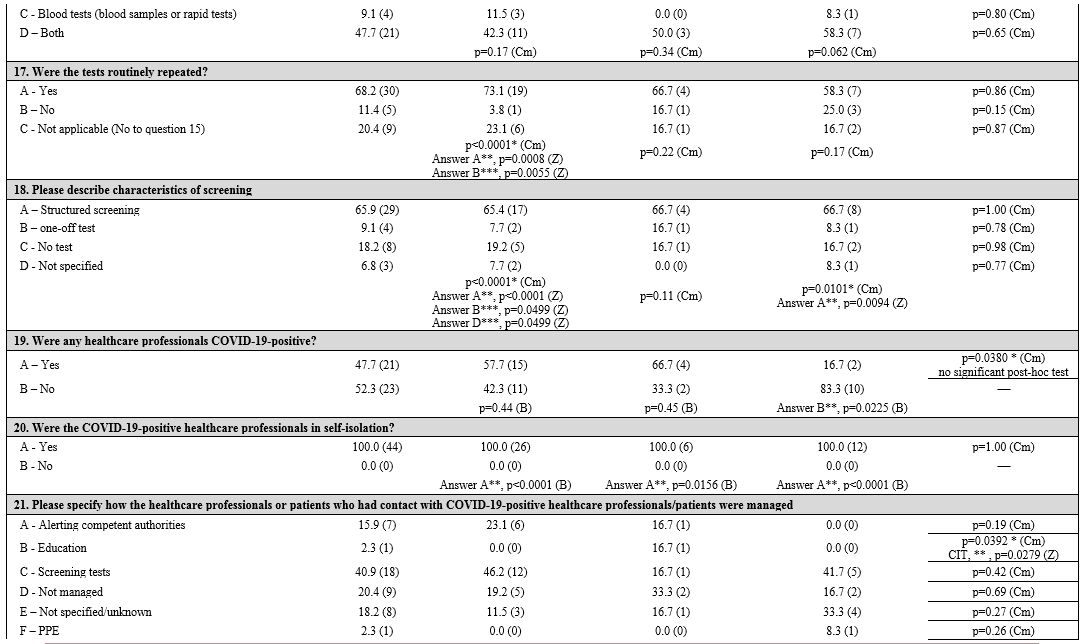
First, three groups corresponding to the three macro areas of Italy (North, Central, and South and Islands) were considered. Table 1
summarizes the survey items, answer frequencies, and univariate and
multivariate analyses both in and between groups of each item. The
participating centers were then grouped as follows: adult patients (AP)
centers, pediatric centers, and those who treat both adults and
pediatrics (PAP). Results are shown in Table 2 (Supplementary materials).
 |
Table 1. Geographic areas: univariate and multivariate analyses in and between subgroups. |
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
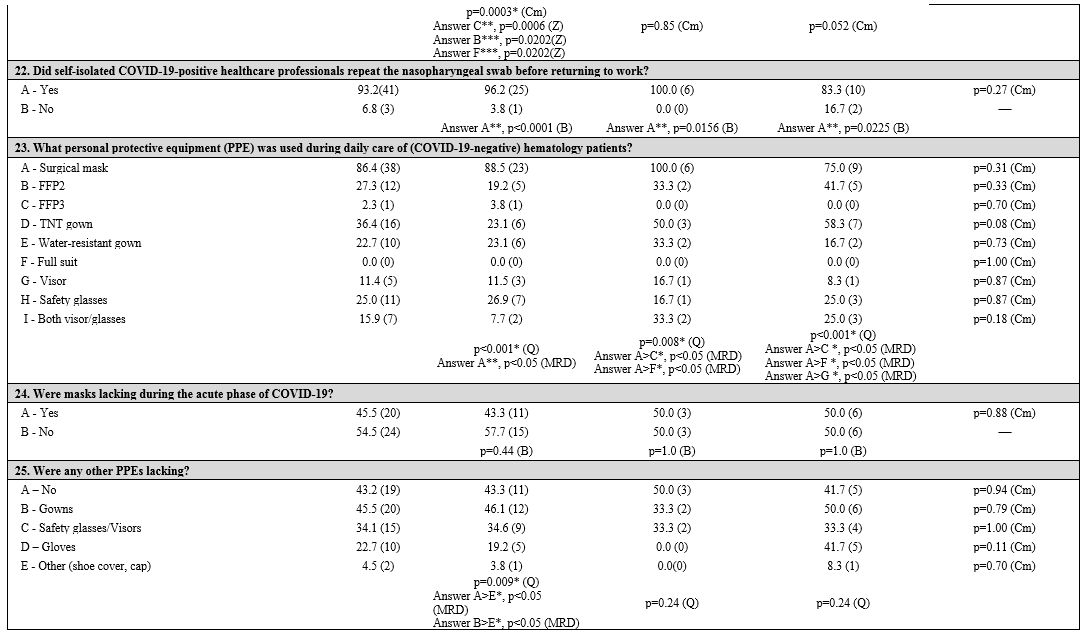
General information. Almost all the participating hematology centers (Table 3 supplementary materials)
were located in hospitals with a dedicated COVID-19 ward (96.1%, 100%,
and 83.3% in NIT, CIT, SITI, respectively). Hematology patients
positive to SARS-CoV-2 were generally admitted in these wards (90.9%),
with no differences between groups. Many hospitals organized COVID-19
pre-triage pathways (77.3%) performed by physicians and/or nurses in
order to filter patients' access at hospital gates. These pre-triage
pathways were less present in NIT (65.4%) than in CIT (100%) and SITI
(91.7%), although the differences were not statistically significant
(p=0.072). Body temperature, respiratory frequency, and oxygen
saturation measurements, and rapid diagnostic tests for COVID-19 were
included in these pathways.
Infection control measures.
The number of beds was reduced in 22.7% of hematology centers, with no
statistically significant difference between groups. Three centers
stopped stem cell transplant activity.
The proportion of
hematology wards who have had COVID-19-positive patients differed
between geographic groups (46.2% in NIT vs. 83.3% in CIT vs. 16.7% in
SITI centers); PAP centers with COVID-19-positive cases were
significantly more (p = 0.0239). In addition, CIT had a higher
percentage of centers reporting patients with COVID-19 clinical signs
or symptoms (83.3%; p=0.0118) without laboratory confirmation.
Almost
all centers (93.2%) performed swab tests before patient admission: the
day before admission in 43.2% of centers, more frequently in NIT and
CIT centers (53.8% and 66.7%, respectively), less frequently in SITI
centers (8.3%, p = 0.0377). Both AP and PAP centers performed swabs the
day before admission (41.9% and 46.2%), or, less frequently, some days
before (35.4% and 15.4%). The test results were available within 12
hours (45.5%) or between 12 to 24 hours (45.5%) or after 24 hours
(9.0%). Protective measures were locally adopted: limiting access to
the hematology wards (100%), not allowing visitors in 65.9% of centers
(especially but not significantly in NIT centers, p = 0.10). CIT
centers preferred to allow visitors based on the patient's clinical
condition (66.7%), differing significantly from the other geographic
areas (p = 0.0395). COVID-19 testing was performed on HCPs in 79.5% of
centers, most commonly with both nasopharyngeal swab and blood tests
(47.7%); tests were repeated routinely in 68.2% of centers. There were
no statistical differences by geographical area or type of patient
assisted.
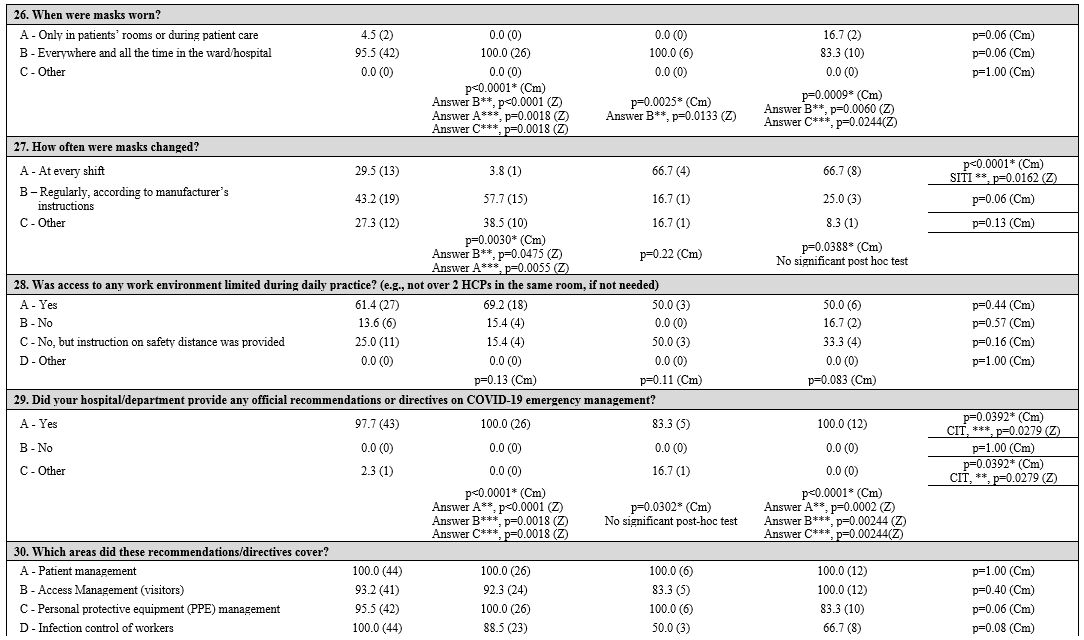
Patient and health care professional protection.
Personal protective equipment (PPEs) are routinely used in the
hematology setting during patient care. Surgical masks (86.4%), TNT
gowns (36.4%), and safety glasses (25.0%) were the first choices. HCPs
wore masks at all times, whether during patient care or not (95.5%),
especially in NIT (100%) and CIT (100%) than SITI centers (83.3%; p =
0.06); masks were changed at every shift, especially in SITI centers
(66.7%, p = 0.0162), while they were more frequently changed according
to manufacturer's instructions in NIT centers (57.7%), without any
significant differences (p = 0.06).
A shortage of PPEs during the
initial phase of the COVID-19 pandemic in Italy was observed: 45.5% of
centers reported a lack of masks, while 43.2% reported there was no
problem with PPE availability (gowns, visors, safety glasses, gloves,
etc.). There were no differences between groups except for lack of
gloves in AP centers than the PAP ones (p = 0.0239).
Regarding
infected HCPs, there was a significant difference among the three
geographic areas (p = 0.0380). In particular, Northern and Central
Italy had more centers with diagnosed cases among HCPs (57.7% and
66.7%, respectively); home isolation (varying from 10 days to 5-6
weeks) and various strategies of readmission to work (after 1 to 3
negative swab tests, or after symptom resolution, without a test) were
adopted. Swab test negativity was required before work readmission in
93.2% of centers. The management of patients and HCPs who had contact
with COVID-19-positive individuals was based on alerting the competent
authorities and performing diagnostic tests (56.8%). However, in 38.6%
of centers, this issue was not managed or not specified/unknown. In
Northern Italy, management's first choice was performing a diagnostic
test (46.2%; p = 0.0003). Other infection control strategies were
reported; for example, limiting access to the work environment (e.g.,
no more than 2 HCPs in a patient room if not needed) was adopted by
61.4% of centers, and 25.0% provided only safety distance norms.
Information management.
All centers referred to the official instructions or recommendations
for situation management provided by their local institutions
(hospital/department directives); only one center in the CIT group
reported a delay in receiving any instructions. These recommendations
mainly concerned “patient management” (100%), “access management”
(92.3%, 83.3%, and 100% in NIT, CIT, and SITI, respectively), “PPE
management” (100% in NIT and CIT, 83.3% SITI), and “infection
protection of HCPs” (88.5%, 50.0%, 66.7%, respectively). Instructions
for patients and families aimed at preventing the spread of the virus
were provided by local health authorities, mainly in NIT (73.1%), but
with no significant differences between the three areas. Hematology
units provided specific recommendations for hematology patients and
their relatives in 61.6% of NIT centers and in 33.3% of both CIT and
SITI centers.
A significant difference was seen in the modality of
providing updated information. The majority of centers provided updated
information in real-time through the official institutional email
service (80.8%, 83.3%, and 66.7% in NIT, CIT, and SITI, respectively)
and the local hospital intranet (92.3%, 50.0%, and 50.0%), especially
in NIT centers (p = 0.0062). Information transmission by word of mouth
was significantly adopted in CIT centers (p = 0.0195).
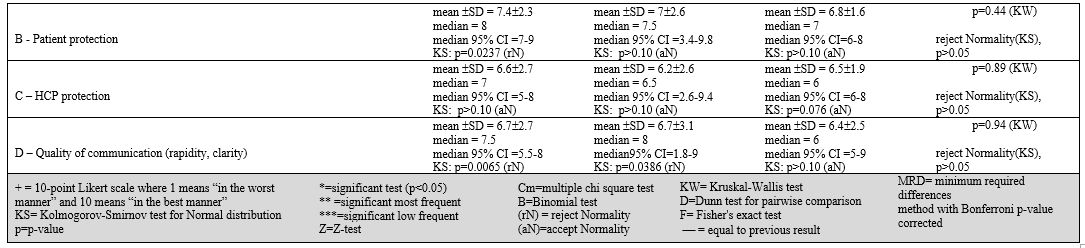
Nurses' perception.
Responders' subjective opinion on PPE availability, patient protection,
HCP protection, and communication quality was generally better in NIT
centers than in CIT and SITI centers. However, there were no
significant differences between groups.
Discussion
Our
work suggests that local health institutions approached the problem in
agreement with Italian health authorities' recommendations and
Government laws, without any significant differences between geographic
areas or type of patients cared for. More restrictive measures on
outpatient and visitor accesses, for example, not allowing visitors any
access at all or only in particular cases, were adopted in all centers.
In pediatric centers, one parent or caregiver was always permitted. A
reduction in beds was applied in a few centers while maintaining the
recommended routine activity on malignant diseases.[24,25]
However,
differences in specific issues and some critical aspects emerged. There
were fewer hematology centers with COVID-19-positive patients in
Southern regions, in accordance with the lower incidence of the
infection. CIT centers reported higher percentages of COVID-19-positive
patients, but the low number of collected answers from this area is
likely to be a confounding factor. Considering the virus'
aggressiveness, hematology patients' immunocompromised status, and the
severity of the pandemic, especially in Northern Italy, the number of
hematology centers with no SARS-CoV-2-positive patients suggest that
safety procedures were applied and adhered to. Fewer pre-triage zones
were organized in the NIT hospitals than in the other geographic
groups. However, this did not seem to affect the infection's spread,
probably due to other strategies adopted such as remote working or
screening procedures. In addition, at the time this survey was
conducted, the severity of the pandemic in the Northern regions may
have delayed the implementation of some containment measures.[41]
Patients
admitted to hematology wards were tested before or at admission and
were considered infected and thus isolated until test results were
available, as required by the SIE/GITMO and EBMT
recommendations;[24,25] almost all centers were in line with the
recommendations. However, in this context, a laboratory response time
of more than 12 hours for swab tests does not appear appropriate.
Different
strategies were adopted regarding the management of infected HCPs and
their contacts. Rapid identification and isolation of infected subjects
as well as contact tracing appear to be crucial,[42,43,44] although
there are still some unresolved issues, such as the management of the
return to work of previously infected HCPs. The timing of viral
shedding after symptom onset in infected subjects appears variable and
likely depends on many factors, including the host's immunological
features, the severity of illness, and viral load.[45,46] A limited
number of centers based on their approach to patient and HCP safety on
a time frame only, reporting that COVID-19 positive HCPs returned to
work without performing any further tests. This approach appeared
incautious, given what has been stated above and the variability in
readmission timing as reported by the centers in this survey.
Significant
difficulties were registered in managing both patients and HCPs who
have had contact with COVID-19-positive patients or HCPs. Tracing and
managing contacts should be considered one of the critical measures to
contain the spread of SARS-CoV-2 infection.[43,44] Our results showed
that screening tests were performed in just over a third of hematology
centers on this population of contacts and that compliance with
alerting the competent authorities of these exposed contacts was scarce.
The
lack of PPEs, including face masks and gloves, was reported in the
survey. The lockdown measures adopted affected the circulation of
materials and commerce, resulting in supply problems. International
guidelines have recommended both extensive adoption of at least
standard precautions and optimizing the use of PPEs in order to ensure
their availability;[43,44,47] strategies to make more efficient use of
PPEs may have been perceived as a lack of them, especially by HCPs
working in hematology settings, where PPEs are commonly used.
HCPs
in almost all hospitals were provided with recommendations and
directives on patient management, visitor access, PPEs, and infection
prevention and control among workers. However, specific recommendations
for hematology patients and their families on preventing the spread of
the virus and aimed to reduce misinformation exposure [48] were less
frequently available. As hematology HCPs apply infection control
measures in their daily practice, this may have allowed them to contain
the spread of the virus in their setting more effectively. The
strategies described by almost all centers, such as the maximum length
of time a mask could be worn, the surgical masks as the first choice,
and applying strict measures to limit environmental contact between
HCPs, may be considered a reasonable translation of recommended
measures into practice.
Our study has some limitations. As the
sample represents just over half of the invited centers, the results
cannot, therefore, be said to represent all hematology centers in
Italy. Some specific issues, such as HCPs exposure without PPEs, home
isolation follow-up practices, environmental hygiene practices, and
waste management, were not investigated. The survey provided insight
into practices put in place during the acute phase of the COVID-19
pandemic (lockdown), and qualitative feedback assessed issues felt to
be pertinent to nursing. However, this research methodology does not
provide an in-depth understanding of these issues. Qualitative research
could be conducted to complement this investigation. However,
substantial compliance of Italian hematology centers with National
health authorities' recommendations and Government laws to control the
spread of SARS-CoV-2 was observed, and hematology HCPs' skills in
infection control may have contributed to having many COVID-19-free
hematology centers. Our data do not allow us to evaluate the impact of
acted measures on patients' outcomes or on the epidemic.[48,49]
However, they could provide gained information on what occurred in our
hematology ward during the COVID-19 pandemic, such as activities
limitation, variability in swab practices, reduced PPEs availability as
well as outpatients, visitors, and contacts management. Our findings
could be useful to face a better further round of infection.
Acknowledgments
The
Authors wish to thank Professor Robin Foà for the interest shown in our
work. Thanks also to the “Nursing Campus in Hematology” members:
Michela Colalelli, Daniela Manzo, Daniela Trentin, Roberto Ricci,
Jessica Germanò, Marco Calcini (LLA Group); Valentina De Cecco, Chiara
Cannici, Emanuela Samarani, Marco Cioce, Elena Rostagno, and Sarah
Liptrott (GvHD Group); Assunta Guillari, Anna Bressan, Francesca
Palmisano, Fabio Lamberti, Cristiana Caffarri, Federica Olivazzi, (CML
Group). We would also like to thank Jacqueline M. Costa for the English
language editing.
Special
thanks to the Italian hematology centers colleagues who voluntarily
participated in this survey and for their dedication to patients care.
References
- Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19) outbreak in China:
summary of a report of 72 314 cases from the Chinese Center for Disease
Control and Prevention. JAMA 2020; 323: 1239-42. https://doi.org/10.1001/jama.2020.2648 PMid:32091533
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Published February 16, 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed June 18, 2020.
- Hewitt
J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, Pearce L,
Stechman M, Short R, Price A, Collins JT, Bruce E, Einarsson A, Rickard
F, Mitchell E, Holloway M, Hesford J, Barlow-Pay F, Clini E, Myint PK,
Moug SJ, McCarthy K; COPE Study Collaborators. The effect of frailty on
survival in patients with COVID-19 (COPE): a multicentre, European,
observational cohort study. Lancet Public Health. 2020
Aug;5(8):e444-e451. https://doi.org/10.1016/S2468-2667(20)30146-8
- Laing
AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L,
Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S,
Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu
Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman J, Gee S, Chan
JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L,
Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K,
Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason
J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J,
Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes
associations with poor prognosis. Nat Med. 2020 August 17. https://doi.org/10.1038/s41591-020-1038-6 PMid:32807934
- World
Health Organization. Coronavirus disease (COVID-2019) situation
reports. Situation report - 34. February 23, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed May 17, 2020.
- Onder
G, Rezza, Brusaferro S. Case-Fatality Rate and Characteristics of
Patients Dying in Relation to COVID-19 in Italy. JAMA.
2020;323(18):1775-1776. https://doi.org/10.1001/jama.2020.4683
PMid:32203977
- Presidenza del Consiglio
dei Ministri, Dipartimento di Protezione Civile e Ministero della
Salute. Bollettino COVID-19. 1 Marzo 2020. Available at: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioVideoNuovoCoronavirus.jsp?lingua=italiano&menu=multimedia&p=video&id=2048. Accessed May 14, 2020.
- Grasselli
G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19
outbreak in Lombardy, Italy: early experience and forecast during an
emergency response. JAMA. Published online March 13, 2020. https://doi.org/10.1001/jama.2020.4031 PMid:32167538
- Istituto
Superiore di Sanità. Epidemiology for Pubblic Health (Epicentro).
COVID-19 integrated surveillance: key National data. Available at: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data. Accessed May 15, 2020.
- Presidenza
della Repubblica Italiana. Decreto Legge 23 febbraio 2020, n. 6. Misure
urgenti in materia di contenimento e gestione dell'emergenza
epidemiologica da COVID-19. (20G00020). Gazzetta Ufficiale della
Repubblica Italiana. Anno 161° - Numero 45 del 23-2-2020.
- Presidenza
del Consiglio dei Ministri. Decreto del Presidente del Consiglio Dei
Ministri, 1 Marzo 2020. Ulteriori disposizioni attuative del
decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia
di contenimento e gestione dell'emergenza epidemiologica da COVID-19.
(20A01381) Gazzetta Ufficiale della Repubblica Italiana. Anno 161° -
Numero 52 del 1-3-2020.
- Presidenza del
Consiglio dei Ministri. Decreto del Presidente del Consiglio Dei
Ministri, 1 Marzo 2020. Ulteriori disposizioni attuative del
decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia
di contenimento e gestione dell'emergenza epidemiologica da COVID-19
applicabili sull'intero territorio Nazionale. (20A01475) Gazzetta
Ufficiale della Repubblica Italiana. Anno 161° - Numero 55 del 4-3-2020.
- World
Health Organization. Coronavirus disease (COVID-2019) situation
reports. Situation report - 48. March 8, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200308-sitrep-48-covid-19.pdf?sfvrsn=16f7ccef_4. Accessed May 17, 2020.
- Istituto
Superiore di Sanità. Report sulle caratteristiche dei pazienti deceduti
positivi a COVID-19 in Italia del 20 Marzo 2020. Available at: https://www.iss.it/documents/20126/0/Report+per+COVID_20_3_2019.pdf/f4d20257-53d5-eb89-087e-285e2cadf44f?t=1584724121898. Accessed May 8, 2020.
- Zhou
G, Chen S. & Chen Z. Advances in COVID-19: the virus, the
pathogenesis, and evidence-based control and therapeutic strategies.
Front. Med. 14, 117-125 (2020). https://doi.org/10.1007/s11684-020-0773-x PMid:32318975 PMCid:PMC7171433
- Wang
D, Hu B, HU C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong
Y, Zhao Y, Li Y, Wang X, Peng Z Clinical Characteristics of 138
Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in
Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585 PMid:32031570 PMCid:PMC7042881
- Guan W, Ni Z, Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. New England Journal of Medicine https://doi.org/10.1056/NEJMoa2002032 PMid:32109013 PMCid:PMC7092819
- Yang
K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong
Y, Pan D, Shu C, Li J, Wei J, Huang Y, Peng L, Wu M, Zhang R, Wu B, Li
Y, Cai L, Li G, Zhang T, Wu G. Clinical characteristics, outcomes, and
risk factors for mortality in patients with cancer and COVID-19 in
Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol.
2020 Jul;21(7):904-913. https://doi.org/10.1016/S1470-2045(20)30310-7
- Russell
B, Moss C, Papa S, Irshad S, Ross P, Spicer J, Kordasti S, Crawley D,
Wylie H, Cahill F, Haire A, Zaki K, Rahman F, Sita-Lumsden A, Josephs
D, Enting D, Lei M, Ghosh S, Harrison C, Swampillai A, Sawyer E,
D'Souza A, Gomberg S, Fields P, Wrench D, Raj K, Gleeson M, Bailey K,
Dillon R, Streetly M, Rigg A, Sullivan R, Dolly S, Van Hemelrijck M.
Factors Affecting COVID-19 Outcomes in Cancer Patients: A First Report
From Guy's Cancer Center in London. Front Oncol. 2020 July 22;10:1279. https://doi.org/10.3389/fonc.2020.01279 PMid:32903324 PMCid:PMC7396540
- Gosain
R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and
Cancer: a Comprehensive Review. Curr Oncol Rep. 2020 May 8;22(5):53. https://doi.org/10.1007/s11912-020-00934-7 PMid:32385672 PMCid:PMC7206576
- He
W, Chen L, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y,
Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with
haematological cancers. Leukemia 2020; 34: 1637-45. https://doi.org/10.1038/s41375-020-0836-7 PMid:32332856 PMCid:PMC7180672
- Mahmoudjafari
Z, Alexander M, Roddy J, Shaw R, Shigle TL, Timlin C, Culos K. American
Society for Transplantation and Cellular Therapy Pharmacy Special
Interest Group Position Statement on Pharmacy Practice Management and
Clinical Management for COVID-19 in Hematopoietic Cell Transplantation
and Cellular Therapy Patients in the United States. Biol Blood Marrow
Transplant. 2020 Jun;26(6):1043-1049. https://doi.org/10.1016/j.bbmt.2020.04.005 PMid:32305359 PMCid:PMC7162779
- Zeidan
AM, Boddu PC, Patnaik MM, Bewersdorf JP, Stahl M, Rampal RK, Shallis R,
Steensma DP, Savona MR, Sekeres MA, Roboz GJ, DeAngelo DJ, Schuh AC,
Padron E, Zeidner JF, Walter RB, Onida F, Fathi A, DeZern A, Hobbs G,
Stein EM, Vyas P, Wei AH, Bowen DT, Montesinos P, Griffiths EA, Verma
AK, Keyzner A, Bar-Natan M, Navada SC, Kremyanskaya M, Goldberg AD,
Al-Kali A, Heaney ML, Nazha A, Salman H, Luger S, Pratz KW, Konig H,
Komrokji R, Deininger M, Cirici BX, Bhatt VR, Silverman LR, Erba HP,
Fenaux P, Platzbecker U, Santini V, Wang ES, Tallman MS, Stone RM,
Mascarenhas J. Special considerations in the management of adult
patients with acute leukaemias and myeloid neoplasms in the COVID-19
era: recommendations from a panel of international experts. Lancet
Haematol. 2020 Jun 18:S2352-3026(20)30205-2. https://doi.org/10.1016/S2352-3026(20)30205-2
- Ljungman
P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S,
Duarte R, Dolstra H, Lankester AC, Mohty M, Montoto S, Murray J,
Peffault de Latour R, Snowden JA, Yakoub-Agha I, Verhoeven B, Kröger N,
Styczynski J; European Society for Blood and Marrow Transplantation.
The challenge of COVID-19 and hematopoietic cell transplantation; EBMT
recommendations for management of hematopoietic cell transplant
recipients, their donors, and patients undergoing CAR T-cell therapy.
Bone Marrow Transplant. 2020 May 13:1-6. https://doi.org/10.1038/s41409-020-0919-0 PMid:32404975 PMCid:PMC7220575
- Società
Italiana di Ematologia (SIE) e Gruppo Italiano Trapianto di Midollo
Osseo, Cellule Staminali e Terapia Cellulare (GITMO). Raccomandazione
SIE-GITMO circa epidema COVID-19 del 20 Marzo 2020. Published March 23,
2020. Available at: http://www.siematologia.it/files/COVID19-Raccomandazioni-SIE-GITMO.pdf . Accessed April 28, 2020.
- Istituto
Superiore di Sanità, Centro Nazionale Trapianti. Aggiornamento delle
misure di prevenzione della trasmissione dell'infezione da nuovo
Coronavirus (SARS‐CoV‐2) in Italia attraverso il trapianto di organi,
tessuti e cellule. Nota CNT n° 652 del 30 Marzo 2020. Available at: http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_238_0_file.pdf. Accessed April 26, 2020.
- Istituto
Superiore di Sanità. Centro Nazionale Sangue. Aggiornamento delle
misure di prevenzione della trasmissione dell'infezione da nuovo
Coronavirus (SARS‐CoV‐2) mediante la trasfusione di emocomponenti
labili. Nota CNS n° 653 del 9 Marzo 2020. Available at: https://www.centronazionalesangue.it/sites/default/files/Prot.%20n.%200653.CNS_.2020_Aggiornamento%20misure%20di%20prevenzione%20nuovo%20Coronavirus%20%28SARS-CoV-2%29.pdf. Accessed March 20, 2020.
- Istituto
Superiore di Sanità. Centro Nazionale Trapianti, Gruppo Italiano
Trapianto di Midollo Osseo, Italian Bone Marrow Donor registry.
Aggiornamento delle misure di prevenzione della trasmissione
dell'infezione da nuovo Coronavirus (SARS-CoV-2) in Italia attraverso
CSE. Nota CNT-GITMO-IBMDR del 9 Giugno 2020. Published June 24, 2020.
Available at: http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_249_0_file.pdf . Accessed June 26, 2020.
- Bai
Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, Wang, M. Presumed
Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;
323(14):1406-1407. https://doi.org/10.1001/jama.2020.2565 PMid:32083643 PMCid:PMC7042844
- Kampf
G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on
inanimate surfaces and their inactivation with biocidal agents. J Hosp
Infect. 2020;104(3):246-251. https://doi.org/10.1016/j.jhin.2020.01.022 PMid:32035997 PMCid:PMC7132493
- Shereen
MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin,
transmission, and characteristics of human coronaviruses. J Adv Res.
2020 Mar 16;24:91-98. https://doi.org/10.1016/j.jare.2020.03.005 PMid:32257431 PMCid:PMC7113610
- Terpos
E, Engelhardt M, Cook G, Gay F, Mateos MV, Ntanasis-Stathopoulos I, van
de Donk NWCJ, Avet-Loiseau H, Hajek R, Vangsted AJ, Ludwig H, Zweegman
S, Moreau P, Einsele H, Boccadoro M, San Miguel J, Dimopoulos MA,
Sonneveld P. Management of patients with multiple myeloma in the era of
COVID-19 pandemic: a consensus paper from the European Myeloma Network
(EMN). Leukemia. 2020 May 22:1-12. https://doi.org/10.1038/s41375-020-0876-z PMid:32444866 PMCid:PMC7244257
- European Hematology Association. Covid-19 recommendations. Available at: https://ehaweb.org/covid-19/covid-19-recommendations/recommendations-for-specific-hematologic-malignancies/. Accessed June 10, 2020.
- Scarfò
L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C,
Garcia-Marco JA, Hernández-Rivas JÁ, Mirás F, Baile M, Marquet J,
Niemann CU, Reda G, Munir T, Gimeno E, Marchetti M, Quaglia FM,
Varettoni M, Delgado J, Iyengar S, Janssens A, Marasca R, Ferrari A,
Cuéllar-García C, Itchaki G, Špaček M, De Paoli L, Laurenti L, Levin
MD, Lista E, Mauro FR, Šimkovič M, Van Der Spek E, Vandenberghe E,
Trentin L, Wasik-Szczepanek E, Ruchlemer R, Bron D, De Paolis MR, Del
Poeta G, Farina L, Foglietta M, Gentile M, Herishanu Y, Herold T,
Jaksic O, Kater AP, Kersting S, Malerba L, Orsucci L, Popov VM,
Sportoletti P, Yassin M, Pocali B, Barna G, Chiarenza A, Dos Santos G,
Nikitin E, Andres M, Dimou M, Doubek M, Enrico A, Hakobyan Y,
Kalashnikova O, Ortiz Pareja M, Papaioannou M, Rossi D, Shah N,
Shrestha A, Stanca O, Stavroyianni N, Strugov V, Tam C, Zdrenghea M,
Coscia M, Stamatopoulos K, Rossi G, Rambaldi A, Montserrat E, Foà R,
Cuneo A, Ghia P. COVID-19 severity and mortality in patients with
chronic lymphocytic leukemia: a joint study by ERIC, the European
Research Initiative on CLL, and CLL Campus. Leukemia. 2020
Sep;34(9):2354-2363. https://doi.org/10.1038/s41375-020-0959-x PMid:32647324 PMCid:PMC7347048
- Cuneo
A, Scarfò L, Reda G, Varettoni M, Quaglia FM, Marchetti M, De Paoli L,
Re F, Pietrasanta D, Rigolin GM, Orsucci L, Ibatici A, Gattei V, Mauro
FR, Trentin L, Laurenti L, Marasca R, Foà R. Chronic lymphocytic
leukemia management in Italy during the COVID-19 pandemic: a Campus CLL
report. Blood. 2020 Aug 6;136(6):763-766. https://doi.org/10.1182/blood.2020006854 PMid:32559271 PMCid:PMC7414586
- Breccia
M, Abruzzese E, Bocchia M, Bonifacio M, Castagnetti F, Fava C,
Galimberti S, Gozzini A, Gugliotta G, Iurlo A, Latagliata R, Luciano L,
Pregno P, Rege-Cambrin G, Rosti G, Stagno F, Tiribelli M, Foà R, Saglio
G; Campus CML working group. Chronic myeloid leukemia management at the
time of the COVID-19 pandemic in Italy. A campus CML survey. Leukemia.
2020 Aug;34(8):2260-2261. https://doi.org/10.1038/s41375-020-0904-z PMid:32555369 PMCid:PMC7301058
- Foà
R, Bonifacio M, Chiaretti S, Curti A, Candoni A, Fava C, Ciccone M,
Pizzolo G, Ferrara F. Philadelphia-positive acute lymphoblastic
leukaemia (ALL) in Italy during the COVID-19 pandemic: a Campus ALL
study. Br J Haematol. 2020 Jul;190(1):e3-e5. https://doi.org/10.1111/bjh.16758 PMCid:PMC7267647
- Istituto Superiore di Sanità. Epidemia Covid-19. Aggiornamento Nazionale 16 Giugno 2020. Published June 19, 2020. Available at: http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed June 26, 2020.
- Xia
W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in
pediatric patients with COVID-19 infection: Different points from
adults. Pediatr Pulmonol. 2020 May;55(5):1169-1174. https://doi.org/10.1002/ppul.24718 PMid:32134205 PMCid:PMC7168071
- Ludvigsson
JF. Systematic review of COVID-19 in children shows milder cases and a
better prognosis than adults. Acta Paediatr. 2020 Jun;109(6):1088-1095.
https://doi.org/10.1111/apa.15270 PMid:32202343 PMCid:PMC7228328
- The Lancet. COVID-19: protecting health-care workers. Lancet. 2020 March 21;395(10228):922. https://doi.org/10.1016/S0140-6736(20)30644-9
- Corman
VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T,
Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der
Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J,
Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C.
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR.
Euro Surveill. 2020 Jan;25(3):2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
- Center
for Disease Control and Prevention. HEALTH DEPARTMENTS: Interim
Guidance on Developing a COVID-19 Case Investigation & Contact
Tracing Plan. Updated May 26, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/case-investigation-contact-tracing.pdf. Accessed June 20, 2020.
- European
Centre for Disease Prevention and Control. Infection prevention and
control for COVID-19 in health-care settings - Second update. March 31
2020. ECDC: Stockholm; 2020.
- Ling Y, Xu
SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, Hu
BJ, Wang S, Mao EQ, Zhu L, Zhang WH, Lu HZ. Persistence and clearance
of viral RNA in 2019 novel coronavirus disease rehabilitation patients.
Version 2. Chin Med J (Engl). 2020 May 5;133(9):1039-1043. https://doi.org/10.1097/CM9.0000000000000774 PMid:32118639 PMCid:PMC7147278
- He
X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan
X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L,
Zhang F, Cowling BJ, Li F, Leung GM. Temporal dynamics in viral
shedding and transmissibility of COVID-19. Nat Med. 2020
May;26(5):672-675. https://doi.org/10.1038/s41591-020-0869-5 PMid:32296168
- World
Health Organization. Infection prevention and control during health
care when coronavirus disease (COVID-19) is suspected or confirmed.
Interim guidance. June 29, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications. Accessed June 30, 2020.
- Lee
JJ, Kang KA, Wang MP, Zhao S, Wong JYH, O'Connor S, Yang SC, Shin SH.
Associations between COVID-19 misinformation exposure and belief with
COVID-19 knowledge and preventive behaviors: A cross-sectional online
study. J Med Internet Res. 2020 October 8. https://doi.org/10.2196/22205 PMid:33048825 PMCid:PMC7669362
- Atay
S, Cura ŞÜ. Problems Encountered by Nurses Due to the Use of Personal
Protective Equipment During the Coronavirus Pandemic: Results of a
Survey. Wound Manag Prev. 2020 Oct;66(10):12-16. https://doi.org/10.25270/wmp.2020.10.1216
Supplementary data
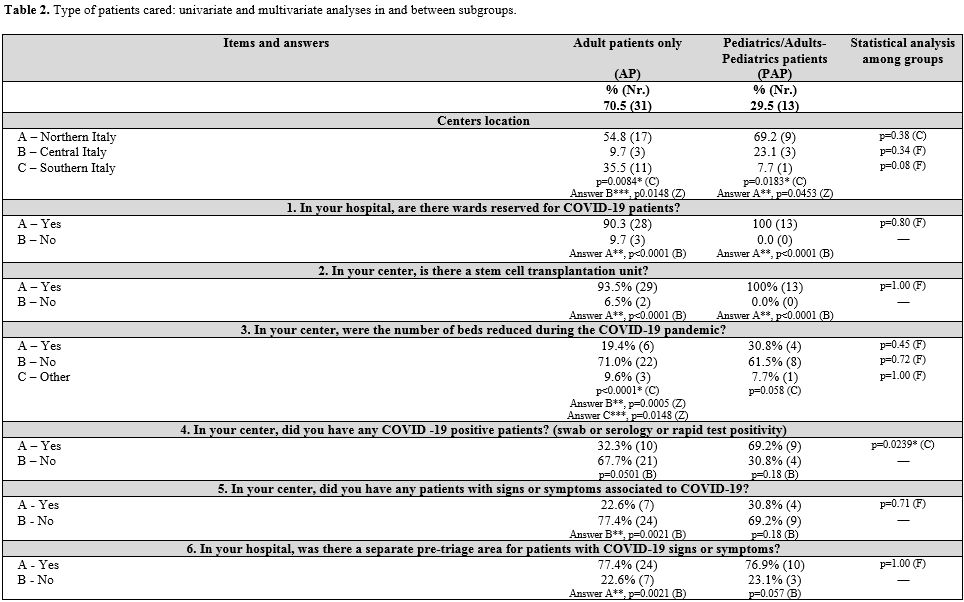 |
Table 2. Type of patients cared: univariate and multivariate analyses in and between subgroups. |
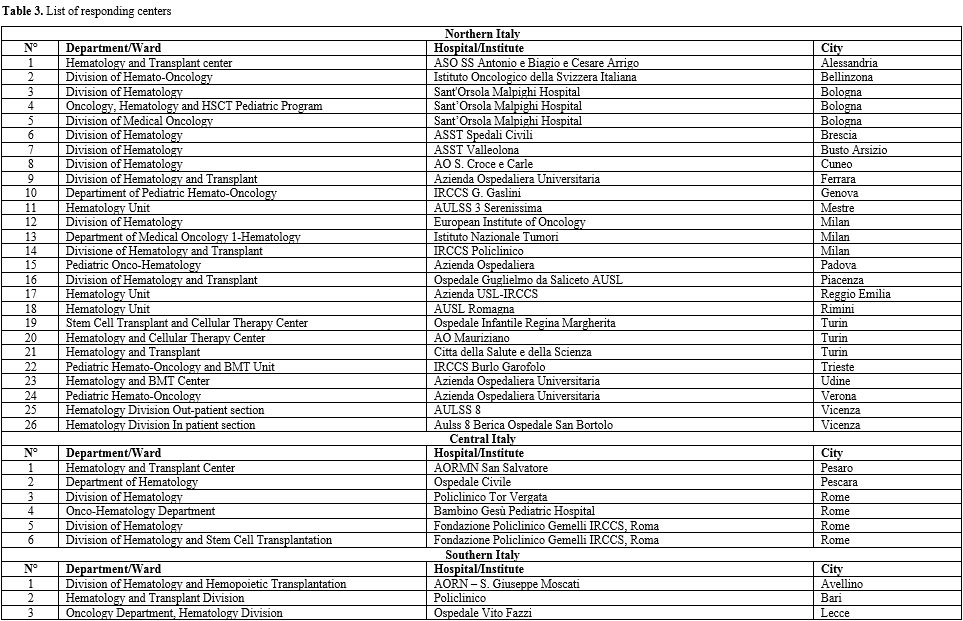 |
Table 3. List of responding centers |
[TOP]