Safaa Ramadan1,2, Giusy Ceparano1, Alessandro Cignetti3, Simona Sammassimo1, Vincenzo Bagnardi4, Eleonora Pagan4, Daniela Gottardi3, Stefano Fiori5, Rita Passerini6, Tommaso Radice1, Giuseppe Saglio3 and Corrado Tarella1,7.
1 Division of Onco-Hematology, European Institute of Oncology, IRCCS, Milan, Italy.
2 NCI-Cairo University, Egypt, Cairo, Egypt.
3 Divisione Universitaria di Ematologia e Terapie Cellulari, A.O. Ordine Mauriziano, Torino, Italy.
4 Department of Statistics and Quantitative Methods, University of Milan-Bicocca, Milano, Italy.
5 Haemolymphopathology Unit, European Institute of Oncology IRCCS, Milan, Italy.
6 Divisione di Medicina di Laboratorio, European Institute of Oncology, Milano, Italy.
7 Dipartimento Universitario di Scienze della Salute (DISS), Università di Milano, Italy.
Correspondence to:
Prof. Corrado Tarella. Onco-hematology Division, European Institute of
Oncology IRCCS, Milan, Italy; & Dip. Universitario Scienze della
Salute (DISS), Universita’ di Milano, Italy, Via Ripamonti 435, 20141
Milan, Italy. Tel: +39 02-574896. E-mail:
corrado.tarella@unimi.it
Published: March 1, 2021
Received: October 26, 2020
Accepted: February 6, 2021
Mediterr J Hematol Infect Dis 2021, 13(1): e2021018 DOI
10.4084/MJHID.2021.018
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Host
immune homeostasis as an independent prognostic indicator has been
inadequately evaluated in aggressive non-Hodgkin's lymphomas (NHL). The
present study addresses the prognostic significance in aggressive NHLs
of the immunologic profile evaluated by pretreatment serum levels of
immunoglobulins (Ig) and lymphocyte-monocyte ratio (LMR). In this
series of 90 patients with aggressive lymphoma, the median level for
IgG was 1,024mg/dl (range 436-2236), and for LMR was 2.2 (range
0.2-13.8). CR rate was higher with IgG levels ≥1,024mg/dL (91% vs 77%
p=0.059). LMR ≤ 2.2 was associated with lower 1-year PFS (73% vs. 92%,
p 0.016). Patients with good/very good R-IPI showed a reduced PFS if
IgG or LMR was low, while patients with poor R-IPI did better if LMR or
IgG levels were high. We combined both parameters with the R-IPI and
produced a four-risk prognostic score showing one-year PFS of 95% (95%
CI 68%-99%), 100% (95% CI 100%-100%), 73% (95% CI 52%-86%), and 59%
(95% CI 31%-79%), in patients with zero, one, two and three risk
factors, respectively. The results indicate for the first time
the value of baseline serum Ig levels in the prognostic assessment of
aggressive lymphoma.
|
Introduction
Several
immune biomarkers have been recognized as having a prognostic impact on
the clinical outcome in patients with Hodgkin and non-Hodgkin
lymphomas. Some of them are the absolute lymphocyte (ALC) and monocyte
count (AMC) as well as the lymphocyte-monocyte ratio (LMR) at
diagnosis.[1,2] The latter was documented as an
independent prognostic factor from the IPI prognostic score in patients
with DLBCL treated with chemo-immunotherapy.[3]
Serum
immunoglobulin (Ig) levels can mirror immune homeostasis and may be of
prognostic relevance in hematologic malignancies. Low levels of serum
Ig have a well-documented adverse prognostic role in various indolent
lymphomas.[4,5] Interestingly, pre-transplant
hypogammaglobinemia had a negative impact on RFS in patients with DLBCL
undergoing autologous transplant, with 18-month RFS 44% if the levels
of IgG <600mg/dl, and 63% if higher.[6] To our
knowledge, there are no other reports on the prognostic impact of
immunoglobulin levels at diagnosis in patients with aggressive NHL.
Therefore, we evaluated the prognostic role of LMR and pretreatment
levels of immunoglobulins in aggressive NHL patients. In addition, we
investigated the role of these factors in the current standard
prognostic score system, the R-IPI score. Finally, we developed a
scoring system that includes patients' immunologic profile and R-IPI to
optimize their outcome prediction.
Methods
A
retrospective analysis has been performed in aggressive B-cell NHL
patients diagnosed and managed at two Hematology Centers, at the
Mauriziano Hospital in Torino and the European Institute of Oncology in
Milan, Italy, between April 2014 and October 2018. Eligible for this
study were newly diagnosed patients with a histologically proven
aggressive B-cell lymphoma, including diffuse large B-cell lymphoma
(DLBCL, n=71), plasmablastic lymphoma (n=1), primary mediastinal large
B-cell lymphoma (PMLCL, n=17), and Burkitt's lymphoma (n=1).
All
patients were treated with chemo-immunotherapy according to the Center
guidelines, after giving informed consent. The study has been approved
by the local Ethical Committee in Milan. PFS and O.S. were
assessed using the Kaplan-Meier method and compared between groups
using the log-rank test. Hazard ratios were calculated using univariate
and multivariate Cox proportional hazard models. Analyses were
performed with SAS software, version 9.4 (SAS Institute, Cary, North
Carolina, USA).
Results
Overall,
90 patients were eligible for the study, but 89 were evaluable for
response (one patient died due to sepsis immediately after the first
cycle). At baseline, the median serum immunoglobulin levels was
1,024mg/dL (range 436-2,236) for IgG, 191mg/dL (range 15-510) for IgA
of and 91mg/dL (range 15-1462) for IgM. Median ALC was 1,300/mmc (range
230-3,990), median AMC was 600/mmc (range 100-1,440) and median LMR was
2.2 (range 0.2-13.8).IgM
levels were higher in females compared to males (median 102mg/dL, vs
81mg/dL, respectively, P=0.038). Patients with no bone marrow (B.M.)
infiltration showed higher IgG and IgA levels than patients with B.M.
involvement. There was no association between Ig levels and LMR.A
trend of higher CR rates was seen in patients with IgG levels
≥1,024mg/dL (91.3% vs 76.7%, respectively p=0.059) and in patients with
LMR > 2.2 (91.1% vs 77.3%, p=0.073). At
a median follow up of 16 months, the 1-year O.S. and PFS of the whole
series were 92% (95% CI: 83%-96%) and 83% (95% CI: 73%-89%),
respectively. All
risk factors included in the revised IPI (R-IPI) score (i.e., age >
60, advanced stage, high ECOG PS, 2 or more extranodal sites, high LDH)
were associated with a worse prognosis (Table 1). PFS was significantly lower in patients with poor R-IPI score compared to the other two groups (Figure 1A,
73% vs. 91%, p=0.018). Patients with IgG lower than 1,024mg/dl had
lower 1-year PFS (73% vs 91%, respectively p=0.135), and patients with
LMR <= 2.2 had also inferior 1-year PFS (73% vs 92%, respectively
p=0.016). The
prognostic role of IgG and LMR on PFS was further investigated
according to the R-IPI score. Having a low IgG at diagnosis
(<1,024mg/dl) worsened the 1-year PFS in good or very good R-IPI
patients (84% vs. 96%). Interestingly, the poor R-IPI risk group with
high IgG levels had 1-year PFS similar to the good risk group with low
IgG levels (87% vs. 84%). The lowest PFS (63%, 95% CI 40%-80%) was seen
in the poor R-IPI group with low IgG (Figure 1B). Similarly, a low LMR ratio strongly worsened the PFS of all R-IPI risk groups (Figure 1C). Poor
R-IPI, low IgG, and low LMR were then assessed in a new 4-level risk
score, taking 1 point for each adverse factor. The 1-year PFS was 95%
(95% CI 68%-99%) in patients with zero risk factors, and 100% (95% CI
100%-100%), 73% (95% CI 52%-86%), 59% (95% CI 31%-79%) in patients with
one, two and three risk factors, respectively (Figure 1D).
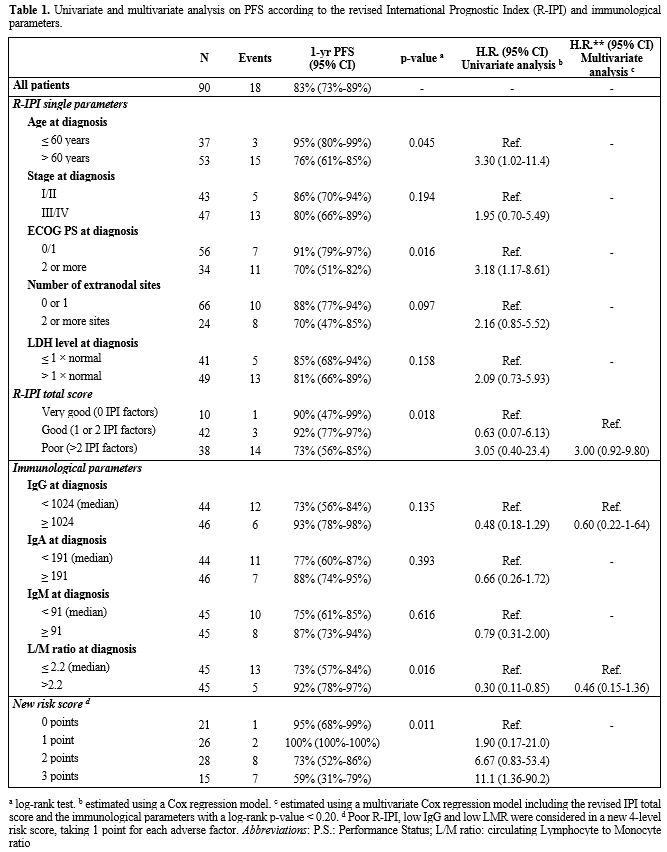 |
Table 1. Univariate and multivariate
analysis on PFS according to the revised International Prognostic Index
(R-IPI) and immunological parameters. |
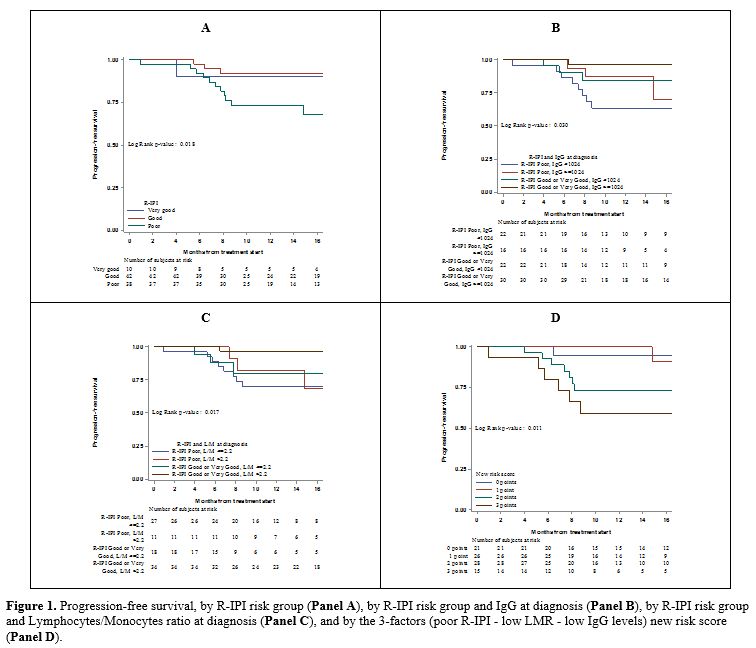 |
Figure
1. Progression-free survival, by R-IPI risk group (Panel A), by R-IPI risk group and IgG at diagnosis (Panel B), by R-IPI risk group and Lymphocytes/Monocytes ratio at diagnosis (Panel C), and by the 3-factors (poor R-IPI - low LMR - low IgG levels) new risk score (Panel D).
|
Discussion
Besides
cell-mediated immunity, humoral factors, mainly antibody-mediated
immunity, have a role in controlling neoplastic cell development and
expansion.[7,8] In the present study, the prognostic
role and the clinical implications of serum Ig levels at baseline,
along with the pattern of circulating lymphocytes and monocytes, were
investigated in aggressive NHL patients. Although the follow-up period
was limited to 16 months, the estimated 1-year O.S. (92%) and PFS (83%)
look quite promising.Overall,
hypogammaglobulinemia was recorded at baseline in only eight patients
(9%), which is less than the 15% reported in a previous study.[9]
Interestingly, four out of these eight patients had primary refractory
disease with rapidly fatal outcome. Among the remaining 82
patients, more than half had Ig values towards the low normal range,
according to the reference laboratory values, and that is in line with
similar results previously reported in NHLs.[10]
Therefore, we chose to use the median immunoglobulin values at
diagnosis corresponding to IgG 1,024mg/dL to categorize patients with
high or low IgG levels in correlative analysis. In univariate analysis,
patients with IgG value <1024mg/dL had a worse 1-year PFS than
patients with higher levels. In
the present series, patients with LMR <=2.2 had an inferior 1-year
PFS. Baseline LMR was matched with IgG levels, and the two parameters
were not correlated. This
finding could be explained by the fact that low LMR was due to high
absolute monocytic count in 11 out of 45 (24.5%) patients. Besides, IgG
levels were low only in 60% of patients with lymphopenic
versus 40% of those with lymphocytes in the normal range. Due
to the lack of correlation between IgG levels and LMR at baseline, both
parameters were assessed in combination with the R-IPI. Patients with
good/very good R-IPI showed a reduced PFS if associated with either low
IgG or low LMR. Patients with poor R-IPI did better if they had either
high LMR or high IgG levels. Dismal outcome was seen in those patients
with poor IPI and low levels of either IgG or LMR. Based on these
observations, a novel clinical and immunological prognostic score was
developed. The score distinguished four groups with different 1-year
PFS ranging from 95% if no or one risk factor was present to 59% if the
three factors were present. Our
study introduces the baseline serum immunoglobulin levels in the
prognostic assessment of aggressive lymphoma patients. We are aware of
some weak points of the study: in particular, the retrospective
analysis and the relatively small number of patients, with the
inclusion of different aggressive lymphoma subtypes, although most of
them were DLBCL; lastly, the follow up time is short. However, C.R.
achievement and the 1-year PFS are considered reliable surrogate
endpoints to predict the ultimate outcome in DLBCL.[11,12]
Conclusions
Our
results suggest that the addition of baseline immunologic profile to
R-IPI optimizes the prognostic stratification of patients with
aggressive B-NHL and identifies more distinctly patients at high risk
of poor outcome. Additional studies on the role of Ig levels in the
development of aggressive B-cell lymphoma are advised.
Acknowledgments
This
work was supported in part by grants for research programs to C.T. by
Banca del Piemonte (Torino, Italy) and by Piaggio and C. SpA
(Pontedera, Italy).
References
- Porrata LF, Ristow K, Colgan JP, Habermann TM,
Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski
GS, Thompson C, Markovic SN. Peripheral blood lymphocyte/monocyte ratio
at diagnosis and survival in classical Hodgkin's lymphoma. Haematol.
2012; 97(2): 262-269. https://doi.org/10.3324/haematol.2011.050138 PMid:21993683 PMCid:PMC3269488
- Wilcox
RA, Ristow K, Habermann TM, Inwards DJ, Micallef INM, Johnston PB,
Colgan JP, Nowakowski GS, Ansell SM, Witzig TE, Markovic SN, Porrata
LF. The absolute monocyte and lymphocyte prognostic score predicts
survival and identifies high-risk patients in diffuse large-B-cell
lymphoma. Leukemia. 2011; 25(09): 1502-1509. https://doi.org/10.1038/leu.2011.112 PMid:21606957
- Rambaldi
A, Boschini C, Gritti G, Delaini F, Oldani, E, Rossi A, Barbui AM,
Caracciolo D, Ladetto M, Gueli A, De Crescenzo A, Passera R, Devizzi L,
Patti C, Gianni AM, Tarella C. The lymphocyte to monocyte ratio
improves the IPI-risk definition of diffuse large B-cell lymphoma when
rituximab is added to chemotherapy. Am J Hematol. 2013; 88(12):
1062-1067. https://doi.org/10.1002/ajh.23566 PMid:23940056
- Atilla
E, Atilla PA, Civriz Bozdag S, Toprak SK, Topcuoglu P, Ilhan O, Ozcan
M, Arslan O. Does hypogammaglobulinemia at diagnosis effects survival
and infection risk in chronic lymphocytic leukemia (CLL)?. Blood. 2016;
128: 5577. https://doi.org/10.1182/blood.V128.22.5577.5577
- Fischer
T, Ni A, Soumerai JD, Alperovich A, Batlevi CL, Younes A, Zelenetz AD.
Natural history of hypogammaglobulinemia in patients with follicular
lymphoma and the impact of anti-CD20-based therapy. Blood. 2017; 130:
4054.
- Bolwell BJ, Kalaycio M, Sobecks R,
Andresen S, Rybicki L, Kuczkowski E, Bates J, Summers K, Bernhard L,
Cherni K, Baker J, Brown S, Pohlman B. Autologous transplantation for
Diffuse Large B Cell Lymphoma: pre-transplant hypogammaglobulinemia is
a predictor for early toxicities. Blood. 2004; 104(11): 908. https://doi.org/10.1182/blood.V104.11.908.908
- Upadhyay
R, Hammerich L, Peng P, Brown B, Merad M, Brody JD. Lymphoma: immune
evasion strategies. Cancers (Basel). 2015; 7(2): 736-62. https://doi.org/10.3390/cancers7020736 PMid:25941795 PMCid:PMC4491682
- Lo
Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC.
NK-mediated antibody-dependent cell-mediated cytotoxicity in solid
tumors: biological evidence and clinical perspectives. Ann Transl Med.
2019; 7(5): 105. https://doi.org/10.21037/atm.2019.01.42 PMid:31019955 PMCid:PMC6462666
- Casulo
C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in
patients receiving Rituximab and the use of intravenous immunoglobulin
for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;
13(2):106-11. https://doi.org/10.1016/j.clml.2012.11.011 PMid:23276889 PMCid:PMC4035033
- Biggar
RJ, Christiansen M, Rostgaard K, Smedby KE, Adami HO, Glimelius B,
Hjalgrim H, Melbye M. Immunoglobulin subclass levels in patients with
non-Hodgkin lymphoma. Int J Cancer. 2009; 124(11): 2616-20. https://doi.org/10.1002/ijc.24245 PMid:19235925
- Shi
Q, Schmitz N, Ou FS, Dixon JG, Cunningham D, Pfreundschuh M, Seymour
JF, Jaeger U, Habermann TM, Haioun C, Tilly H, Ghesquieres H, Merli F
Ziepert M, Herbrecht R Flament J, Fu T Coiffier B, Flowers CR.
Progression-free survival as a surrogate end point for overall survival
in first-line diffuse large B-cell lymphoma: an individual
patient-level analysis of multiple randomized trials (SEAL). JCO. 2018;
36(25): 2593-2602. https://doi.org/10.1200/JCO.2018.77.9124 PMid:29975624 PMCid:PMC6532366
- Tarella
C, Gueli A, Delaini F, Rossi A, Barbui AM, Gritti G, Boschini C,
Caracciolo D, Bruna R, Ruella M, Gottardi D, Passera R, Rambaldi A.
Rate of primary refractory disease in B and T-cell non-Hodgkin's
lymphoma: correlation with long-term survival. PLoS One. 2014; 9(9):
e106745. https://doi.org/10.1371/journal.pone.0106745 PMid:25255081 PMCid:PMC4177839
,
[TOP]

